Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Resection Margin Width Does Not Influence the Prognosis of Solitary Hepatocellular Carcinoma After Anatomic Resection: A Real-World Study from China
Authors Ke Q, Guo Z, He J, Lai Z, Xin F , Zeng Y, Wang L , Liu J
Received 25 May 2023
Accepted for publication 29 July 2023
Published 16 August 2023 Volume 2023:10 Pages 1353—1365
DOI https://doi.org/10.2147/JHC.S420828
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Qiao Ke,1,2,* Zhiting Guo,3,* Jian He,1,* Zisen Lai,1 Fuli Xin,1,2 Yongyi Zeng,1 Lei Wang,4 Jingfeng Liu1,2
1Department of Hepatopancreatobiliary Surgery, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, Fujian, People’s Republic of China; 2Department of Hepatopancreatobiliary Surgery, Clinical Oncology School of Fujian Medical University, Fujian Cancer Hospital, Fuzhou, Fujian, People’s Republic of China; 3College of Biological Science and Engineering, Fuzhou University, Fuzhou, Fujian, People’s Republic of China; 4Department of Radiation Oncology, Clinical Oncology School of Fujian Medical University, Fujian Cancer Hospital, Fuzhou, Fujian, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lei Wang, Department of Oncology, the Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, 360000, People’s Republic of China, Tel +86 133 2825 2899, Fax +86 791 8612 0120, Email [email protected] Jingfeng Liu, Clinical Oncology School of Fujian Medical University, Fujian Cancer Hospital, Fuzhou, Fujian, 350014, People’s Republic of China, Tel +86 139 0502 9580, Fax +86 591 8370 2529, Email [email protected]
Purpose: The influence of resection margin (RM) width on the prognosis of solitary hepatocellular carcinoma (HCC) following anatomical resection (AR) has yet to be determined. Therefore, we conducted a real-world study to identify the optimal RM width and assess its impact on the outcomes of solitary HCC patients undergoing AR.
Methods: The data pertaining to patients diagnosed with solitary HCC who underwent AR between December 2012 and December 2015 were retrospectively collected. The optimal cutoff value for the width of the RM was determined using X-tile software. The Kaplan-Meier method was utilized to compare the overall survival (OS) and disease-free survival (DFS) between the narrow and wide RM groups. Additionally, propensity score matching (PSM) was performed to minimize potential bias in the data.
Results: Of the 1033 patients who met the inclusion criteria, 293 (28.4%) were categorized into the narrow RM group (≤ 4 mm) and 740 (71.6%) into the wide RM group (> 4mm). Before and after PSM, there were no significant differences in OS and DFS between the two groups (before PSM: OS, HR=0.78, P=0.071; DFS, HR=0.95, P=0.620; after PSM: OS, HR=0.77, P=0.150; DFS, HR=0.90, P=0.470). Multivariate analysis demonstrated that RM width was not an independent risk factor for DFS and OS both before and after PSM (all P> 0.05). However, subgroup analyses revealed that patients with ALBI grade 1, absence of cirrhosis, and AJCC stage II significantly benefited from wide RM in OS (all P< 0.05). Similarly, patients without HBV infection and absence of cirrhosis also exhibited significant benefits from wide RM in DFS (both P< 0.05).
Conclusion: In patients with solitary HCC undergoing AR, the width of the RM does not appear to have a significant impact on their prognosis. However, in certain selected patients, a wider RM may confer benefits.
Keywords: hepatocellular carcinoma, anatomical resection, resection margin, prognosis
Introduction
Surgical resection is considered the most cost-effective curative treatment for hepatocellular carcinoma (HCC).1–3 Anatomical resection (AR) and non-anatomical resection (NAR) are the two commonly employed modalities.2,4 AR involves the complete removal of liver segments according to Couinaud’s classification, which is typically accompanied by segmentectomy, hemihepatectomy, or trisectionectomy.5 Compared to NAR, AR allows for the complete removal of the tumor-bearing portal territory, decreasing the risk of tumor dissemination and metastasis in the involved liver segment through the portal vein blood flow.6,7 In the concept of modern precision surgery, AR is increasingly valued. Nevertheless, the 5-year postoperative recurrence rate of HCC remains high at approximately 70%,8 presenting significant challenges to both surgeons and patients.
The width of the RM plays a significant role in the recurrence and metastasis of HCC after radical resection.9,10 Although there is no consensus on the optimal RM width, many studies have suggested that a wider RM, based on achieving R0 resection, can reduce postoperative recurrence and improve long-term survival due to the detection of more micrometastases.11,12 However, there is still controversy regarding the width of RM and the choice of surgical modalities. A systematic review of 12,429 samples from 43 studies revealed patients received AR were accompanied by wider RM (mean difference: +0.29cm, 95% CI: 0.15–0.44, P<0.001).5 However, the results of a study by Su et al13 demonstrated that the width of RM did not influence the prognosis of patients with solitary HCC ≤2cm in diameter who received AR. Conversely, for patients undergoing NAR, a wide margin (>1cm) may improve postoperative recurrence-free survival. Nevertheless, some studies suggest that a wider RM may improve outcomes in patients undergoing AR, particularly those with microvascular invasion (MVI).14,15
This study aimed to recruit 1033 patients with solitary HCC who had undergone AR from two tertiary specialized hepatopancreatobiliary (HPB) centers to 1) determine the optimal RM width for the resection of solitary HCC, and 2) evaluate the influence of RM width on the prognosis of patients with solitary HCC.
Materials and Methods
Patient Selection
This study was in accordance with the guidance of Declaration of Helsinki and approved by Mengchao Hepatobiliary Hospital of Fujian Medical University’s Ethics Committee (2021_004_01). Informed consent was obtained from all participants prior to the initiation of the study. All patients diagnosed with solitary HCC who underwent AR were considered eligible for inclusion in this study. However, patients who had undergone preoperative treatments, the presence of macrovascular or bile duct invasion or lymph node metastasis, did not undergo radical resection, experienced perioperative mortality, or had incomplete clinical or follow-up data were excluded from the study.
Data Collection
Data of HCC patients who underwent radical resection between December 2012 and December 2015 from two tertiary specialized HPB centers were collected in a predefined form, including age, gender, HBV infection (yes or no), preoperative white blood cell count, hemoglobin, platelet count, preoperative serum levels of total bilirubin (TBil), albumin, albumin-bilirubin score (ALBI, >-2.60 or ≤-2.60), alpha-fetoprotein (AFP, >20 or ≤20 ng/mL), gamma-glutamyl transferase (GGT, >72 or ≤72 U/L) and alkaline phosphatase (ALP, >104 or ≤104 U/L), cirrhosis (present or absent), hospital stays (>10 or ≤10 days), RM width (>4 or ≤4 mm), tumor diameter (>5 or ≤5 cm), Edmondson-Steiner (ES) grade (I–II or III–IV), satellite (yes or no), capsule (absent or present), MVI (yes or no), adjuvant transarterial chemoembolization (TACE, yes or no) and follow-up.
In accordance with the previous formula [0.66 x log10 (bilirubin, μmol/L) −0.085 x (albumin, g/L), grade 1: ≤-2.60, grade 2: >-2.60 to ≤-1.39, grade 3: >-1.39], the ALBI score and grade were calculated.16 Due to the small sample size, ALBI grade 2 and ALBI grade 3 were grouped together. The RM width was defined as the shortest distance between the liver section and the tumor edge.17 The optimal cut-off value for AFP level was determined by previous reports,13 while those for GGT, ALP, hospital stays, and RM width were determined by X-tile software.
Follow-Up
Patients were followed up as recommended by the Chinese guideline for HCC.2 Briefly, a comprehensive evaluation including AFP levels, and contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) at one month after surgery. Then, patients received routine examination every three months in the first 2 year after surgery, every six months from 2–5 years, and every 12 months after 5 years. Once a suspected recurrence or metastasis is confirmed by contrast enhanced CT or MRI, salvage treatment should be initiated immediately.
Study Endpoints
The primary endpoints were overall survival (OS) and disease-free survival (DFS). OS time was defined as the time between the date of the operation and either the date of death or the latest follow-up, while DFS time was defined as the time between the date of the operation and the date of recurrence or the latest follow-up.
Statistical Analysis
Data are shown as prevalence and means (standard deviation). Continuous variables were compared by the Student’s t-test between groups, while categorical variables were by the chi-squared test. Kaplan-Meier curves of OS and DFS were compared using Log rank test with hazard ratio (HR) and 95% confidence interval (CI). Prognostic factors were identified using the forward method of the Cox regression model, and the assumption of proportional hazards was assessed using Schoenfeld residuals.18 Factors with a significance level of P<0.10 in univariate analysis were subsequently included in the multivariate Cox regression analysis. Notably, RM width (wide vs narrow) were automatically incorporated in multivariate analysis, regardless of their differences in univariate analysis. Propensity score matching (PSM) was adopted to decrease the selection bias. Variables with a P<0.05 between groups were used to be matched using a 1:1 nearest neighbor method with a caliber of 0.01 as depicted previously.19
The statistical analysis was conducted using Rstudio including packages of “Table 1”, “MatchIt”, “survminer”, and “survival”. For all statistical tests, statistical significance was defined as two-sided P<0.05 in this study.
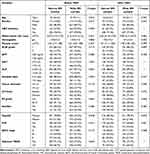 |
Table 1 Clinicopathological Characteristics Before and After PSM |
Results
Patient’s Characteristics
Initially, a total of 1552 patients with solitary HCC who received AR were enrolled for this study. Based on the pre-defined inclusion and exclusion criteria, 1033 patients were eligible for the analysis. The patient’s selection process is depicted in Figure 1. The optimal cut-off value for the RM was determined using the X-tile software. Supplementary Figure 1 shows that the RM was skewed distribution, and a cut-off value of 4mm was chosen, which corresponded to the maximum X2 value for both OS and DFS analyses (Figure 1A: OS, P=0.071, X2=3.54; Figure 1B: DFS, P=0.620, X2=0.245).
 |
Figure 1 The flow chart in patient’s selection. |
Using the current cut-off value, 293 patients (28.4%) were classified into the narrow RM group (RM ≤4mm), while 740 patients (71.6%) were categorized into the wide RM group (RM >4mm). As shown in Table 1, the narrow RM group had a significantly higher age and percentages of GGT >72U/L and ALP >104U/L than the wide RM group (all P<0.05, Table 1). However, after conducting 1:1 PSM, the baseline characteristics between the two groups were comparable (all P>0.05, Table 1).
Survival Analysis Between Wide and Narrow RM
Before PSM, the 1-, 3-, and 5-year OS rates for the wide RM group were 94.1%, 76.8%, and 59.1%, respectively. In contrast, the corresponding OS rates for the narrow RM group were 91.3%, 71.9%, and 49.6%, respectively (Figure 2A). Regarding DFS, the 1-, 3-, and 5-year rates for the wide RM group were 81.4%, 58.9%, and 43.4%, respectively, while for the narrow RM group, they were 78.2%, 59.2%, and 39.1%, respectively (Figure 2B). There was no significant survival advantage observed for the wide RM group compared to the narrow RM group in terms of both OS and DFS (OS: HR=0.78, 95% CI: 0.60–1.02, P=0.071; DFS: HR=0.95, 95% CI: 0.76–1.17, P=0.620, Figure 2A and B).
After PSM, the 1-, 3-, and 5-year OS rates in the wide RM group were 93.6%, 86.3%, and 57.6%, respectively, whereas in the narrow RM group, the corresponding rates were 93.3%, 72.9%, and 53.7%, respectively (Figure 2C). Similarly, the 1-, 3-, and 5-year DFS rates in the wide RM group were 83.0%, 61.2%, and 45.6%, respectively, whereas in the narrow RM group, the corresponding rates were 79.1%, 59.3%, and 43.7%, respectively (Figure 2D). Statistical analysis revealed no significant survival advantage for the wide RM group compared to the narrow RM group in terms of both OS and DFS (OS: HR=0.77, 95% CI: 0.54–1.10, P=0.150; DFS: HR=0.90, P=0.470, Figure 2C and D).
Independent Factors Associated with OS and DFS
The assessment of the assumption of proportional hazards was conducted in both the pre- and post-PSM cohorts, with the results presented in Supplementary Table 1. Upon careful examination of the test outcomes, it is apparent that none of the covariates exhibit statistical significance (P > 0.05), and the global test also fails to achieve statistical significance. As a consequence, we can infer that the Cox model employed in this study satisfies the assumption of proportional hazards.
Prior to PSM, the results presented in Table 2 indicated that several variables were independent risk factors for OS, including ALP >104U/L (HR=1.55, 95% CI=1.07–2.26), hospital stays >10 days (HR=1.77, 95% CI=1.27–2.47), tumor diameter >5cm (HR=1.65, 95% CI=1.26–2.17), presence of cirrhosis (HR=1.47, 95% CI=1.12–1.96), and MVI (HR=1.94, 95% CI=1.46–2.57). Additionally, HBV infection (HR=1.32, 95% CI=1.02–1.72), GGT>72U/L (HR=1.30, 95% CI=1.03–1.63), ALP >104U/L (HR=1.42, 95% CI=1.05–1.92), hospital stays >10 days (HR=1.54, 95% CI=1.16–2.04), tumor diameter >5cm (HR=1.46, 95% CI=1.17–1.82), and presence of MVI (HR=1.47, 95% CI=1.15–1.87) were identified as independent risk factors for DFS.
 |
Table 2 Univariate and Multivariate Analysis of Overall Survival and Disease-Free Survival Before PSM |
After PSM, the results presented in Table 3 showed that ALBI grade 2 (HR=1.97, 95% CI=1.25–3.11), hospital stays >10 days (HR=1.79, 95% CI=1.13–2.85), presence of cirrhosis (HR=1.56, 95% CI=1.05–2.33), and MVI (HR=2.30, 95% CI=1.57–3.37) were identified as independent risk factors for OS. Hospital stays >10 days (HR=1.65, 95% CI=1.11–2.44), tumor diameter >5cm (HR=1.38, 95% CI=1.01–1.89), and presence of MVI (HR=1.63, 95% CI=1.21–2.18) were identified as independent risk factors for DFS.
 |
Table 3 Univariate and Multivariate Analysis of Overall Survival and Disease-Free Survival After PSM |
Of note, as presented in both Table 2 and 3, wide RM was not found to be independent factor for either OS or DFS.
Subgroup Analysis
Subgroup analyses were conducted on the PSM cohort, stratified by different factors, and the findings are presented in Figures 3 and 4. It was observed that patients with ALBI grade 1, the absence of cirrhosis, and AJCC stage II benefited from wide RM in terms of OS (all P<0.05, Figure 3). Furthermore, patients without HBV infection and absence of cirrhosis exhibited a significant benefit from wide RM in terms of DFS (all P<0.05, Figure 4).
 |
Figure 3 Overall survival of wide RM and narrow RM groups stratified by different potential confounders in PSM cohort. |
 |
Figure 4 Disease-free survival of wide RM and narrow RM groups stratified by different potential confounders in PSM cohort. |
Discussion
Despite being a subject of controversy, the prevailing view maintains that AR surpasses NAR in terms of reducing postoperative recurrence.6,20 Moreover, the necessity of achieving extensive RM in patients undergoing AR remains underreported. In this study, a total of 1033 patients who were diagnosed with solitary HCC and underwent AR at two specialized HPB centers were analyzed. The X-tile software was utilized to determine the optimal cut-off value for RM, which was found to be 4mm. Subsequent analysis demonstrated that there was no statistically significant difference in OS and DFS between the wide RM group (RM >4mm) and narrow RM group (RM ≤4mm) in both the unmatched and matched cohorts. Furthermore, the width of RM was not identified as an independent risk factor for either OS or DFS (both P>0.05).
The objective of HPB surgeons is to minimize potential lesions as much as possible. Previous studies have demonstrated that wide RM can reduce the risk of postoperative recurrence.12,21 Therefore, wide RM has been recommended for patients with good liver function and sufficient residual liver volume.2 However, the optimal cut-off value for wide RM remains controversial. Several commonly used cut-off values for RM width include 1cm, 2cm, 5mm, and 2mm, but these values are not often supported by high-level evidence-based medicine.10,12,13,22 Nitta et al23 proposed a cut-off value of 7mm for RM width based on the minimum P value. Other studies suggested that the optimal RM width is determined by the pretreatment AFP levels.24,25 In the current study, X-tile software was utilized to identify the optimal cut-off value for RM width, and a width of 4mm was chosen as the optimal RM width.
However, the efficacy of a wide RM in improving the prognosis of patients receiving AR for HCC remains a controversial topic. To explore the impact of RM width on patient outcomes, Aoki et al26 conducted a multicenter study utilizing a nationwide survey database in Japan. The study revealed that OS and DFS were comparable for patients with negative-0mm RM and those with negative>0mm RM, as long as AR was performed. Conversely, two studies by Shi et al14 and Zhang et al15 in China concluded that for patients undergoing AR, a wide RM led to better prognosis than a narrow RM. However, in the present study, which included patients with solitary HCC who underwent AR, there was no significant improvement in OS and DFS when comparing wide RM to narrow RM, both before and after PSM, consistent with Aoki et al’s findings.26 Previous research has established that portal vein invasion and/or intrahepatic metastases tend to spread through the portal territories, as opposed to radially in all directions.27 This finding may help to shed light on the present study results, which indicate that the width of the RM does not have a significant impact on the prognosis of patients undergoing AR for solitary HCC.
Since liver function or cirrhosis is crucial in determining whether AR or wide RM can be performed, excessive resection of liver tissue may lead to complications, such as postoperative liver failure.22 Additionally, cirrhosis is an independent prognostic factor in patients with solitary HCC.28 Our study found that none of the patients with solitary HCC who underwent AR developed postoperative liver failure. Based on the results of subgroup analysis, we observed that wide RM improved the prognosis of patients with ALBI grade I, the absence of cirrhosis, or hepatitis B infection. Therefore, selected patients may benefit from wide RM among solitary HCC patients undergoing AR.
MVI is a well-known invasive characteristic of HCC and an independent risk factor for intrahepatic and distant metastasis.29 The prevalence of MVI in HCC patients varies from 15–57% and can be detected at different stages of the disease.30 Previous studies have indicated that portal vein invasion and intrahepatic micrometastasis occur predominantly within 1 cm of the main tumor and seldom extend beyond 2 cm.11,31 Therefore, a wide RM (≥1 cm) may be more effective in eradicating MVI from adjacent tumors following surgical resection. Evidence suggests that wide RM can improve the prognosis of HCC patients with MVI.10,32 In current study, the benefits of wide RM were evaluated in HCC patients with MVI who underwent AR. The findings suggested that wide RM may not benefit these patients since the included studies were solitary HCC cases with a relatively favorable prognosis. Furthermore, AR involves the complete removal of the tumor-bearing portal territory, including the MVI along the distribution of portal vein branches within the peritumoral liver tissue. Therefore, the width of the RM may have little impact on the prognosis of these patients.
Furthermore, postoperative management is crucial in reducing the risk of recurrence and improving the long-term prognosis of HCC, which includes rigorous follow-up and efficient adjuvant treatment. TACE and targeted therapy represent viable and effective options for the comprehensive management of HCC.33 These therapeutic modalities are suitable for both first-line treatment in intermediate to advanced stages of HCC and as adjuvant treatment following radical hepatectomy, yielding promising outcomes.34–36 Furthermore, the utilization of radiotherapy and immune checkpoint inhibitors has also been reported in the postoperative management of HCC.37,38 In this study, we observed that narrow RM patients were more likely to receive adjuvant TACE, as previously reported.21 This finding suggests that a wide RM may not be the sole optimal approach, as subsequent adjuvant treatment can be utilized to complement the potential risks associated with a narrow RM.
The strength of this study lies in its large multicenter design, utilizing data from two specialized HPB centers located in China. Additionally, PSM and subgroup analysis were conducted to minimize potential biases. However, certain limitations of the study should be acknowledged. Firstly, selection and recall biases are challenging to avoid in retrospective studies, even with the implementation of PSM and multivariate Cox models. Secondly, operator-related factors, such as the use of open versus laparoscopic approaches, Pringle maneuver, and intraoperative navigation, may impact the width of the RM and subsequently affect the outcomes of hepatectomy. Lastly, there exist evident differences between the epidemiology, tumor characteristics, and management of HCC in the East and West, indicating the need for further validation of the study’s conclusions in Western series.
Conclusion
In patients with solitary HCC undergoing AR, the width of the RM does not appear to have a significant impact on their prognosis. However, in certain selected patients, a wider RM may confer benefits. However, further prospective, randomized controlled studies are necessary to validate this conclusion.
Funding
This work was supported by Fujian Provincial Clinical Research Center for Hepatobiliary and Pancreatic Tumors, Fujian, P.R.C (2020Y2013), the Key Clinical Specialty Discipline Construction Program of Fuzhou, Fujian, P.R.C (201912002), the Startup Fund for Scientific Research, Fujian Medical University, Fujian, P.R.C (2020QH1241, 2020QH1242).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Benson AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(5):541–565. doi:10.6004/jnccn.2021.0022
2. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
3. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
4. Liao K, Yang K, Cao L, et al. Laparoscopic anatomical versus non-anatomical hepatectomy in the treatment of hepatocellular carcinoma: a randomised controlled trial. Int J Surg. 2022;102:106652. doi:10.1016/j.ijsu.2022.106652
5. Moris D, Tsilimigras DI, Kostakis ID, et al. Anatomic versus non-anatomic resection for hepatocellular carcinoma: a systematic review and meta-analysis. Eur J Surg Oncol. 2018;44(7):927–938. doi:10.1016/j.ejso.2018.04.018
6. Jiao S, Li G, Zhang D, Xu Y, Liu J, Li G. Anatomic versus non-anatomic resection for hepatocellular carcinoma, do we have an answer? A meta-analysis. Int J Surg. 2020;80:243–255. doi:10.1016/j.ijsu.2020.05.008
7. Shindoh J, Kobayashi Y, Umino R, Kojima K, Okubo S, Hashimoto M. Successful anatomic resection of tumor-bearing portal territory delays long-term stage progression of hepatocellular carcinoma. Ann Surg Oncol. 2021;28(2):844–853. doi:10.1245/s10434-020-08927-3
8. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
9. Zhou Z, Qi L, Mo Q, et al. Effect of surgical margin on postoperative prognosis in patients with solitary hepatocellular carcinoma: a propensity score matching analysis. J Cancer. 2021;12(15):4455–4462. doi:10.7150/jca.57896
10. Yang P, Si A, Yang J, et al. A wide-margin liver resection improves long-term outcomes for patients with HBV-related hepatocellular carcinoma with microvascular invasion. Surgery. 2019;165(4):721–730. doi:10.1016/j.surg.2018.09.016
11. Shi M, Guo RP, Lin XJ, et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245(1):36–43. doi:10.1097/01.sla.0000231758.07868.71
12. Wang H, Yu H, Qian YW, Cao ZY, Wu MC, Cong WM. Impact of surgical margin on the prognosis of early hepatocellular carcinoma (≤5 cm): a propensity score matching analysis. Front Med. 2020;7:139. doi:10.3389/fmed.2020.00139
13. Su CM, Chou CC, Yang TH, Lin YJ. Comparison of anatomic and non-anatomic resections for very early-stage hepatocellular carcinoma: the importance of surgical resection margin width in non-anatomic resection. Surg Oncol. 2021;36:15–22. doi:10.1016/j.suronc.2020.11.009
14. Shi C, Zhao Q, Liao B, et al. Anatomic resection and wide resection margin play an important role in hepatectomy for hepatocellular carcinoma with peritumoural micrometastasis. Anz J Surg. 2019;89(11):E482–E486. doi:10.1111/ans.15396
15. Zhang XP, Xu S, Lin ZY, et al. Significance of anatomical resection and resection margin status in patients with HBV-related hepatocellular carcinoma and microvascular invasion: a multicenter propensity score-matched study. Int J Surg. 2023;109(4):679–688. doi:10.1097/JS9.0000000000000204
16. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-The ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
17. Lai EC, You KT, Ng IO, Shek TW. The pathological basis of resection margin for hepatocellular carcinoma. World J Surg. 1993;17(6):786–791. doi:10.1007/BF01659097
18. Hyun J, Ha MS, Oh SY, et al. Urinary tract infection after radiation therapy or radical prostatectomy on the prognosis of patients with prostate cancer: a population-based study. BMC Cancer. 2023;23(1):395. doi:10.1186/s12885-023-10869-4
19. Wang L, Ke Q, Lin K, et al. Not all hepatocellular carcinoma patients with microvascular invasion after R0 resection could be benefited from prophylactic transarterial chemoembolization: a propensity score matching study. Cancer Manag Res. 2020;12:3815–3825. doi:10.2147/CMAR.S251605
20. Dai XM, Xiang ZQ, Wang Q, Li HJ, Zhu Z. Oncological outcomes of anatomic versus non-anatomic resections for small hepatocellular carcinoma: systematic review and meta-analysis of propensity-score matched studies. World J Surg Oncol. 2022;20(1):299. doi:10.1186/s12957-022-02770-4
21. Liu L, Shui Y, Yu Q, et al. Narrow-margin hepatectomy resulted in higher recurrence and lower overall survival for R0 resection hepatocellular carcinoma. Front Oncol. 2021;10:610636. doi:10.3389/fonc.2020.610636
22. Field W, Rostas JW, Philps P, Scoggins CR, Mcmasters KM, Martin RN. Wide versus narrow margins after partial hepatectomy for hepatocellular carcinoma: balancing recurrence risk and liver function. Am J Surg. 2017;214(2):273–277. doi:10.1016/j.amjsurg.2017.06.002
23. Nitta H, Allard MA, Sebagh M, et al. Ideal surgical margin to prevent early recurrence after hepatic resection for hepatocellular carcinoma. World J Surg. 2021;45(4):1159–1167. doi:10.1007/s00268-020-05881-9
24. Marques F, Ghallab M, Vibert E, et al. Prognostic impact of surgical margins for hepatocellular carcinoma according to preoperative alpha-fetoprotein level. HPB. 2022;24(6):848–856. doi:10.1016/j.hpb.2021.10.012
25. Lee JC, Cheng CH, Wang YC, et al. Clinical relevance of alpha-fetoprotein in determining resection margin for hepatocellular carcinoma. Medicine. 2019;98(11):e14827. doi:10.1097/MD.0000000000014827
26. Aoki T, Kubota K, Hasegawa K, et al. Significance of the surgical hepatic resection margin in patients with a single hepatocellular carcinoma. Br J Surg. 2020;107(1):113–120. doi:10.1002/bjs.11329
27. Fukutomi S, Nomura Y, Nakashima O, et al. Evaluation of hepatocellular carcinoma spread via the portal system by 3-dimensional mapping. HPB. 2017;19(12):1119–1125. doi:10.1016/j.hpb.2017.08.011
28. Huang J, Li L, Liu FC, et al. Prognostic analysis of single large hepatocellular carcinoma following radical resection: a single-center study. J Hepatocell Carcinoma. 2023;10:573–586. doi:10.2147/JHC.S404895
29. Zheng Z, Guan R, Jianxi W, et al. Microvascular invasion in hepatocellular carcinoma: a review of its definition, clinical significance, and comprehensive management. J Oncol. 2022;2022:9567041. doi:10.1155/2022/9567041
30. Rodriguez-Peralvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20(1):325–339. doi:10.1245/s10434-012-2513-1
31. Nakashima Y, Nakashima O, Tanaka M, Okuda K, Nakashima M, Kojiro M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res. 2003;26(2):142–147. doi:10.1016/s1386-6346(03)00007-x
32. Lin WD, Ye LN, Song ZS, Wang KP, Feng YF, Pan CY. Wide surgical margins improve prognosis for HCC with microvascular invasion. Eur Rev Med Pharmacol Sci. 2023;27(5):2052–2059. doi:10.26355/eurrev_202303_31576
33. Cai R, Song R, Pang P, et al. Transcatheter arterial chemoembolization plus sorafenib versus transcatheter arterial chemoembolization alone to treat advanced hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2017;17(1):714. doi:10.1186/s12885-017-3707-5
34. Peng Z, Fan W, Zhu B, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a Phase III, randomized clinical trial (LAUNCHl). J Clin Oncol. 2023;41(1):117–127. doi:10.1200/JCO.22.00392
35. Wang L, Ke Q, Deng M, et al. Adjuvant transarterial chemoembolization for patients with hepatocellular carcinoma after radical hepatectomy: a real world study. Scand J Gastroenterol. 2019;54(11):1403–1411. doi:10.1080/00365521.2019
36. Lin K, Wei F, Huang Q, et al. Postoperative adjuvant transarterial chemoembolization plus tyrosine kinase inhibitor for hepatocellular carcinoma: a multicentre retrospective study. J Hepatocell Carcinoma. 2022;9:127–140. doi:10.2147/JHC.S352480
37. Wang L, Qiu L, Ke Q, Ji H, Wu J. Systematic review of adjuvant external beam radiotherapy for hepatocellular carcinoma following radical hepatectomy. Radiother Oncol. 2022;175:101–111. doi:10.1016/j.radonc.2022.08.019
38. Chen W, Hu S, Liu Z, et al. Adjuvant anti-PD-1 antibody for hepatocellular carcinoma with high recurrence risks after hepatectomy. Hepatol Int. 2023;17(2):406–416. doi:10.1007/s12072-022-10478-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

