Back to Journals » Drug Design, Development and Therapy » Volume 17
Research Progress on Mechanism and Management of Adverse Drug Reactions of Anlotinib
Received 20 June 2023
Accepted for publication 20 October 2023
Published 15 November 2023 Volume 2023:17 Pages 3429—3437
DOI https://doi.org/10.2147/DDDT.S426898
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Shiting Li, Hongqin Wang
Department of Pharmacy, Inner Mongolia Baogang Hospital, Baotou City, Inner, Mongolia, People’s Republic of China
Correspondence: Hongqin Wang, Department of Pharmacy, Inner Mongolia Baogang Hospital, No. 20, Shaoxian Road, Kun District, Baotou City, Inner, Mongolia, 014010, People’s Republic of China, Tel +86-472-5992956, Email [email protected]
Abstract: Anti-angiogenesis therapy plays a vital role in the treatment of tumors, with anlotinib as its representative targeted drug. Anlotinib is a novel oral tyrosine kinase inhibitor (TKI) with inhibitory effects on tumor growth tumor angiogenesis. In Phase III clinical trials, anlotinib demonstrated better overall survival and progression-free survival than placebo in patients with advanced non-small cell lung cancer (NSCLC), and was approved for the first time as a third-line treatment for refractory advanced NSCLC. Going far beyond that, anlotinib has shown encouraging results in a variety of malignancies, including medullary thyroid carcinoma, renal cell carcinoma, gastric cancer and esophageal squamous cell carcinoma. Nevertheless, anlotinib has been subject to some controversy in terms of adverse events due to its widespread use. In this review, the mechanism of action, pharmacokinetic characteristics, adverse reactions in clinical use and management of anlotinib were summarized.
Keywords: anlotinib, anti-angiogenesis, non-small cell lung cancer, anti-cancer mechanism, adverse drug reaction
Graphical Abstract:
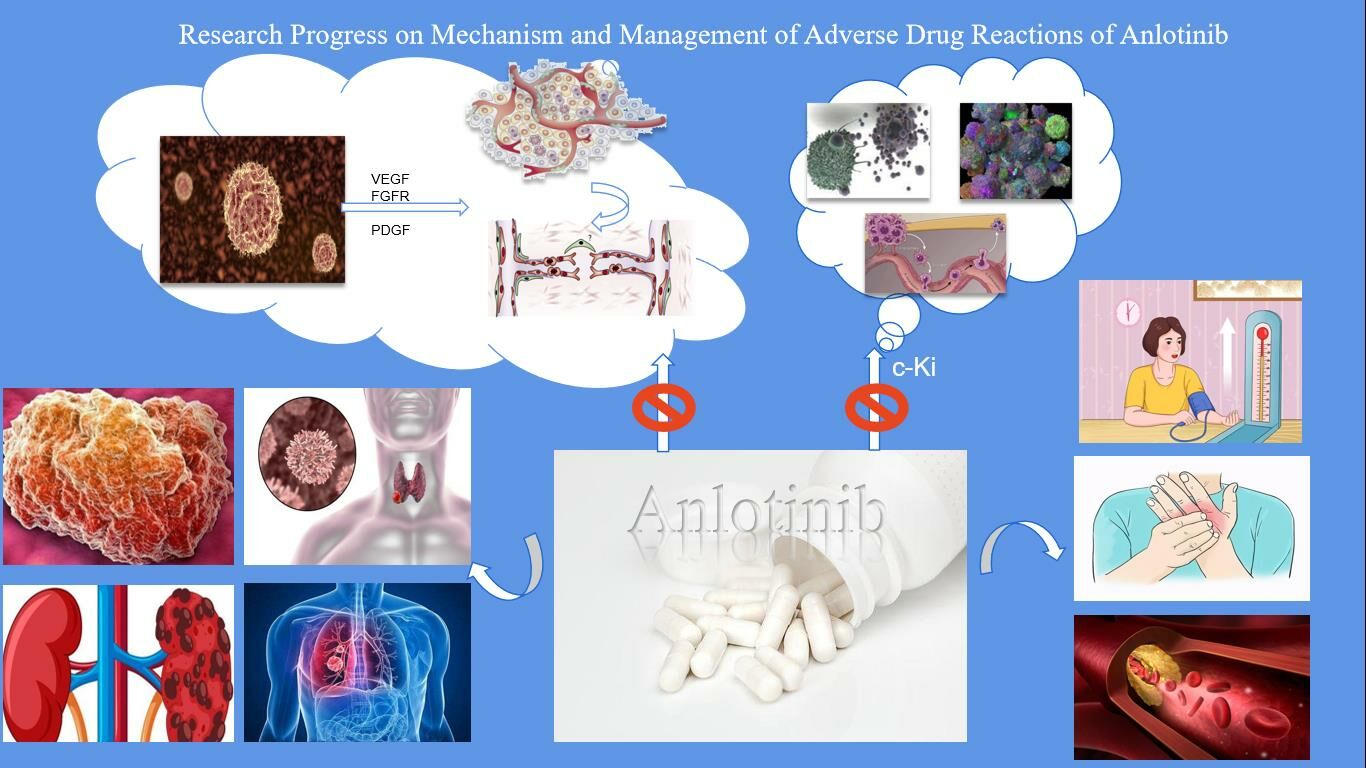
Introduction
Cancer has always been a major public health issue requiring close attention in countries around the world, making inroads on 19.29 million new patients and causing 9.96 million deaths worldwide in 2020 alone, according to to the statistics of the International Agency for Research on Cancer (IARC) of the World Health Organization.1 There are many kinds of treatment methods for malignant tumors. The traditional methods mainly include surgery, chemotherapy, radiotherapy, interventional therapy, etc., while the emerging malignant tumor treatment methods also include targeted therapy, immunotherapy, etc. Traditionally, chemotherapy with cytotoxic drugs has been used as the cornerstone of tumor treatment, especially for those with advanced cancer. However, chemotherapy drugs will produce systemic toxicity and drug resistance, so better treatment strategies are urgently needed to prolong the life span of patients. Targeted therapy refers to the use of standardized biomarkers to identify whether there is a disease-specific gene or gene spectrum that controls tumor growth, so as to determine the treatment method for specific targets. In tumor tissues, nutrients and oxygen are supplied and metabolites are excreted through a complex tumor microvascular system.2,3 It was widely believed until the early 1970s that tumor angiogenesis was an inflammatory response to necrotic tumor cells. But everything changed with Judah Folkman’s research into tumor angiogenesis, which led to new insights into tumor biology. Folkman believed that tumors can be limited to a size of 1–2 mm and enter a dormant state in the absence of angiogenesis, and at the same time proposed the concept of anti-angiogenesis.4 Folkman’s hypothesis of tumor angiogenesis as a potential therapeutic target has shifted the focus from traditional tumor cell-centric therapeutic strategies to anti-angiogenesis approaches, pioneering a new field in oncology.5 Tyrosine kinase inhibitor (TKI) is currently one of the most common targeted therapeutic drugs with high selectivity and few side effects.6 The listed drugs have shown the superiority of traditional therapeutic drugs in the treatment of chronic myeloid leukemia, non-small cell lung cancer, renal cell carcinoma and other diseases, and some of them have become the first-line drugs for the treatment of tumors.7,8 Larotrectinib for solid tumor with NTRK-positive rearrangements, imatinib/sunitinib for leukemia and GIST, pazopanib for renal and lung cancer and for sarcoma.9,10
Anlotinib is a novel oral tyrosine kinase inhibitor developed independently in China. For one thing, it inhibits the VEGFR2/3-mediated signaling pathway by blocking the phosphorylation of vascular endothelial growth factor receptor (VEGFR) 2/3, thus inhibiting angiogenesis and tumor progression. For another, it exhibits strong inhibitory effects on platelet-derived growth factor receptor (PDGFR) α/β and fibroblast growth factor receptor (FGFR) 1–4 pathways, which can also inhibit angiogenesis and treat tumors.11 Moreover, anlotinib showed a strong inhibitory activity on the stem cell factor receptor c-Kit,12 suppressing the growth of tumor. It can not only inhibit tumor angiogenesis in an all-round way, but also interfere with the dual functions of tumor cells through kinases such as c-Kit. In other words, anlotinib boasts a distinct advantage over single-target anti-tumor drugs, ie, it achieves tumor suppression via multiple pathways while avoiding possible target resistance that may lead to treatment failure during treatment. Despite the diversity of targets of this multi-target receptor tyrosine kinase inhibitor and its higher survival benefits, the safety risks brought by anlotinib cannot be ignored.13 In this review, the mechanism of action and pharmacokinetic characteristics of adverse drug reactions (ADRs) of anlotinib were summarized, and more importantly, the common adverse events and their management in clinical use of anlotinib in cancer treatment were fully described.
Anti-Tumor Mechanism of Anlotinib
Angiogenesis in the human body is basically controlled by two substances to maintain stability: pro-angiogenic molecular substances and anti-angiogenesis substances.14 In malignant tumor tissues, there is a steady transition from angiogenesis to pro-angiogenesis, a process termed the “angiogenic switch”.15,16 It is currently believed that hypoxia of cancer cells, which increases the formation of pro-angiogenesis factors, is a crucial pathological root that mediates these changes.17 Vascular endothelial growth factor (VEGF) family is the most important and typical molecular factor to promote angiogenesis in malignant tumor.18,19 VEGF can fully exert its function through the combination of vascular endothelial growth factor receptors (VEGFR) related to its function. With the combination of VEGF and VEGFR, the activity of tyrosine kinase at the intracellular receptor site can be increased to cause the phosphorylation of tyrosine residues, thereby stimulating specific intracellular signaling pathways.20,21
Anlotinib is a kind of multi-target tyrosine kinase inhibitor with small molecular structure, with a variety of targets such as vascular endothelial growth factor receptor (VEGFR)1/2/3, platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR) and c-Kit as its functional targets.12 Yu et al22 carried out in vitro kinase function tests and molecular dynamics simulations, showing the dual functions of anlotinib hydrochloride in comprehensively controlling malignant tumor capillary regeneration and interfering with various biological functions of malignant tumor cells by using active kinases such as c-Kit. Anlotinib hydrochloride, compared with active kinases such as EGFR and HER2, inhibits not only the phosphorylation activity of active kinases such as VEGFR2, c-Kit, and PDGFRβ, but also the phosphorylation of active kinases such as ERK and Akt in its downstream signaling pathways, thus effectively cutting off its related signaling pathways. It is further explained that anlotinib is highly selective against angiogenic receptors and c-Kit, boasting an anti-angiogenic effect by specifically integrating receptors involved in angiogenesis to effectively inhibit their transmission of information. Therefore, anlotinib can effectively inhibit the angiogenesis caused by the three information channels of VEGF/PDGFRβ/FGFR2, both in vivo or in vitro, and then tumor angiogenesis.23 Anlotinib inhibits the activation of signaling pathways downstream of the relevant receptors, blocking cell proliferation, migration and other biological processes. There is a broad crosstalk between the three major signaling pathways related to the regulation of neovascularization, and each signaling pathway can be crosstalk through the downstream signaling process by the modulation of kinases such as ERK, Akt, and PI3K, which implies that the inhibition of the three signaling pathways mediated by VEGFR, PDGFRβ, and FGFR is less likely to have a bypass activation and restart neovascularization than simply inhibiting one of them.11 This means that simultaneous inhibition of the three signaling pathways mediated by VEGFR, PDGFRβ, and FGFR has a lower likelihood of bypass activation and restarting neovascularization than inhibition of one of the signaling pathways alone, and has a more comprehensive effect.21 Anlotinib has also achieved good results in the treatment of non-small cell lung cancer, soft tissue sarcoma, medullary thyroid carcinoma, and metastatic renal cell carcinoma. The literature on the treatment of tumors with anlotinib is shown in Table 1.
 |
Table 1 This Study Included Literature on the Treatment of Anlotinib |
Pharmacokinetics of Anlotinib
The pharmacokinetic (PK) characteristics of anlotinib were evaluated in studies involving experimental animals and patients with advanced cancer.24,25 The results confirmed the excellent membrane permeability and digestibility of anlotinib, and its oral bioavailability in dogs and rats was 41–77% and 28–58%, respectively, with significantly different biological conversion rates among different species. In addition, anlotinib had a high apparent volume distribution in rats (27.6±3.1L/kg) and dogs (6.6±2.5L/kg). A better level of fusion of anlotinib to plasma was also demonstrated, whereas in human plasma it is mostly fused to albumin and lipoproteins. In mice and rats, levels of anlotinib significantly exceeded normal levels in human plasma.25 There are many human cytochrome P450 isoforms involved in the metabolism of anlotinib in in vitro scientific studies, among which CYP3A4 and CYP3A5 are of great importance, indicating the possibility that the level and action of anlotinib in vivo are affected by drugs that alter the function of P450 enzyme.25 Although anlotinib has been confirmed to have a significant induction effect on CYP2D1 and CYP3A (1 and 2) in vivo animal studies, it has no significant effect on other metabolic enzymes, including CYP1A2, CYP2D2, and CYP2C6. For this reason, careful consideration should be given when using anlotinib in combination with certain drugs that promote metabolism of CYP2D1 and CYP3A.26 So far, the PK characteristics of anlotinib have been confirmed by two Phase I clinical trials.
According to a Phase I clinical trial in China, anlotinib is rapidly absorbed into the small intestine and significantly increases plasma levels 1h after oral administration, reaching a peak in blood levels within 4–11min with a half-life (t1/2) of 96±17h. Due to the increase of the dosage of anlotinib from 5mg to 16mg, the plasma Cmaxd 15 and the area under the curve of blood drug content-time after 120h of administration also increased accordingly.24 Data show that anlotinib can be widely distributed in human lungs, kidneys, liver and myocardium due to its good membrane permeability, achieving the same content in the central nervous system as in human plasma. In the brain tissue of mice and tumor-bearing animals, its distribution content also significantly exceeds the normal distribution level in the plasma. Anlotinib, like other TKIs which are widely used in clinic at present, also exhibits a long half-life (t1/2) in patients.27 The longer the duration of t1/2, the stronger the accumulation of anlotinib in plasma, with a general accumulation ratio of 12:7. Besides, the 2-week dosing regimen in the experiment resulted in a continuous increase in the blood concentration of anlotinib, reaching a peak at day 14 and gradually decreasing over the next 7 days. Based on these results and toxicological descriptions, a dosing regimen of 12mg daily for 12 weeks followed by 1 week of discontinuation is recommended in future studies.24
Common ADR Mechanisms and Treatment Measures of Anlotinib
As shown in a clinical trial to investigate the safety and efficacy of anlotinib,18 the most common ADRs of anlotinib are hypertension, hand-foot syndrome, hyperlipidemia, etc. In the clinical trial of ALTER0303, the ADRs in the anlotinib group were mainly hypertension (64.6%), fatigue (46.3%), thyroid-stimulating hormone elevation (44.6%), hand-foot skin reaction (43.2%) and so on.28 A study by Abdel-qadir et al29 revealed the short-term efficacy and safety of anlotinib in advanced non-small cell lung cancer, and concluded that among grade I to II ADR, hand-foot syndrome accounted for 41.51%, hypertension for 24.53%, fatigue for 20.75%, and gastrointestinal reaction for 18.87%, but there was no grade IV ADR.
ADRs Related to Cardiovascular System
VEGF, the primary target of anlotinib, has been scientifically linked to an increased incidence of many cardiovascular diseases (including hypertension, hypoxic heart disease, heart failure, and thromboembolism),30–32 of which hypertension is the most typical and common ADR.21 Hypertension is a typical ADR with VEGF inhibitor and has a certain drug concentration dependence, but with an unclear mechanism.33 Hypertension found during the application of anlotinib should be treated early. Angiotensin converting enzyme inhibitors (ACEI) or angiotensin II receptor inhibitors (ARB) are most commonly used to regulate blood pressure, and if they are not effective, some antihypertensive drugs (such as calcium antagonists, diuretics, and beta blockers) should be used in combination. It is worth noting that the simultaneous use of CYP3A4 enzyme inhibitors (such as verapamil or diltiazem) should be avoided as much as possible because anlotinib needs to be metabolized by CYP3A4 enzymes.34 A study35 showed that those with a history of hypertension, advanced age and overweight were the main factors for VEGF inhibitor-induced hypertension. Therefore, patients with high-risk factors who have been diagnosed with the type of drug and used anlotinib should be closely monitored by medical staff and clinicians for their blood pressure and various symptoms induced, and the intricate correlation between the above-mentioned various factors should be deeply explored.36
VEGF inhibitors are risk factors for reversible posterior leukoencephalopathy syndrome (PRES).37 Despite usually being manageable, PRES may cause permanent central nervous system damage and even death if not detected in time. Since no relatively complete treatment measures for PRES have been proposed so far, when anlotinib is used clinically, some manifestations caused by adverse reactions of PRES should be alerted and symptomatic treatment should be carried out.
ADRs Related to Urinary System
It has been confirmed that renal function damage is mainly related to VEGF inhibitors, and the common symptoms are proteinuria and hematuria, and occasionally serum creatinine increases.38 It is currently unclear about the main mechanism of VEGF inhibitors leading to hypoproteinuria, which is considered to be caused by inhibiting vascular endothelial growth factor in podocytes, causing detachment and hypertrophy of glomerular endothelial cells, and causing thrombotic microangiopathy with low glomerular volume.39 In the animal experiment results of ALTER-0303 (phase III clinical trial of the treatment of NSCLC with anlotinib), the prevalence of proteinuria was only 28.9%. The animal experiment results suggested that after discontinuing anlotinib in patients with urinary protein ≥2.0 g/L, their urinary protein level was <1.0 g/L, and then anlotinib was continued at a lower therapeutic dose.33 Zhang et al40 observed in a study that the incidence of proteinuria after application of anlotinib was about 17.3%. Continuous and large-scale proteinuria will affect renal function, especially for those with renal dysfunction and cardiovascular diseases. Regular detection and effective management of urinary protein levels are critical. It is recommended that physicians estimate renal dysfunction in patients who have decided to start anlotinib and perform quantitative monitoring of urinary protein before each cycle of treatment. In case of high levels, ACEI and ARB can be used to reduce proteinuria. In addition, reducing the intake of edible salt and using glucocorticoids will also fix the cytoskeleton of podocytes, thus reducing the amount of proteinuria.33
ADRs Related to Skin and Its Subcutaneous Tissue
In the clinical trial of ALTER-0303, several patients had reduced medication due to hand-foot syndrome among the cases of skin and subcutaneous ADRs caused by anlotinib.25 The exact mechanism by which tyrosine kinase inhibitors (TKIs) cause hand-foot syndrome is currently unknown, but its potential causes39 are speculated to include: ① increased drug content in capillaries; ② apoptosis and inflammation of nerve cells caused by capillary repair damage; ③ effects on endothelial cell survival. In case of more serious hand-foot syndrome, it will cause severe pain, infection, and even interfere with normal life. In view of this, patients with hand-foot syndrome should be treated appropriately according to their course of disease. It is recommended that patients use soft insoles to reduce damage to the pressure parts of the feet when applying anlotinib. For patients with grade 1–2 of hand-foot syndrome, emollients, antibiotics or cortisone ointment can be used locally, while those with grade ≥3 can choose to stop taking drugs.33,39
AEs of Anlotinib
AEs against anlotinib has been studied in different cancer research areas, as indicated by the pharmacokinetic data of anlotinib. In phase I trials, AEs were almost entirely manageable, with incidences greater than 30% including: hand-foot skin reaction (53%), hypertension (34%), proteinuria (67%), triglyceride increase (62%), total cholesterol increase (62%), hypothyroidism (57%), elevated alanine aminotransferase (48%), elevated aspartate aminotransferase (43%), elevated total bilirubin (38%), serum amylase (43%), abnormal myocardial enzymes (38%), leukopenia (33%), and neutropenia (33%).24 Patients treated with anlotinib have a higher overall incidence of AEs, with 29% having a history of grade III/IV AEs, including severe hand-foot skin reactions (5%), hypertension (10%), elevated triglycerides (10%), and lipase (10%).24 Notably, anlotinib results in less diarrhea than other anti-VEGFR TKI drugs,40–43 but has a higher risk of triggering an increase in triglycerides and cholesterol. Although these AEs do not cause corresponding symptoms, regular monitoring of patients taking anlotinib is still of great importance, especially considering that some AEs are associated with arterial thromboembolism. However, such cases are very common in patients taking anti-VEGFR TKI drugs.44
Management of AEs of Anlotinib
Anlotinib has been proved to be effective in various advanced tumors, but its incidental AEs may affect the quality of life of patients and their compliance. The survey confirmed that the incidence of AEs in patients treated with anlotinib was approximately 87.70–100%, and that of grade III or higher AEs was 21.67–61.90%.24,28,45,46 Other common AEs associated with anlotinib treatment include blood pressure (BP) syndrome, hand-foot syndrome reaction (HFSR), fatigue, diarrhea, and anorexia. Therefore, prevention and control of AEs during anlotinib therapy is of great importance to improve treatment effectiveness and reduce unnecessary drug discontinuation. Most of them were grade I–II mild ADR, which could be alleviated and recovered by suspension of administration, adjustment of dosage and symptomatic treatment, without affecting the overall course of treatment. Anlotinib is currently widely used in clinical practice, and there are many super-indication drugs. Therefore, while paying attention to the clinical efficacy, it is also necessary to closely monitor related adverse events, which is of great significance for improving the therapeutic effect and reducing unnecessary drug withdrawal.
Hypertension
Hypertension can also be considered as a common AE of angiogenesis inhibitors, especially for VEGFR TKI.41 Clinical data indicate that 42–55% of patients with advanced tumors treated with anlotinib develop hypertension, and 4.80–13.60% develop grade III or higher hypertension,28,36,45–47 demonstrating the importance of controlling hypertension during treatment. The following methods will help reduce the probability of hypertension.
Firstly, blood pressure assessment must be done regardless of normal blood pressure, and blood pressure must be closely monitored when starting treatment.33,48 It is very critical to detect blood pressure several times in the first few months for early intervention and relief of hypertension. Patients treated with antihypertensive drugs should have their blood pressure measured twice a day at the nearest hospital or at home, and at the same time, strengthen patient education.49 Patients should be aware of dietary restrictions to help control their blood pressure and report to their doctor about headaches, shortness of breath and other symptoms such as blushing.
Drugs that relieve hypertension,33,49 such as thiazide diuretics, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, beta receptor blockers and calcium channel blockers, are beneficial for patients to control hypertension when using anlotinib. All the above drugs can be used independently or in combination, but angiotensin converting enzyme inhibitors should be avoided in combination with angiotensin II receptor blockers as far as possible. Anlotinib is metabolized by cytochrome CYP3A4. Diltiazem and verapamil are inhibitors of liver drug enzyme CYP3A4, which should be avoided in combination with anlotinib due to possible drug effects. It has also been reported that nifedipine has the effect of enhancing VEGF release, which should also be avoided.50 In view of the danger of treating diarrhea, thiazide diuretics should be used cautiously. Drug therapy should be based primarily on the patient’s medical history and clinical condition. Usually, BP may recover after long-term use of anlotinib, so it is essential to reduce or stop using related antihypertensive drugs.
Hand-Foot Syndrome
HFSR, also known as painful erythema palmaris or hand-foot syndrome, is a typical AE caused by some biochemical therapies and targeted therapies, which may cause physical and psychological discomfort to patients.51 The incidence of HFSR in patients undergoing anlotinib therapy ranges from 28.33 to 79.31%, with 3.33 to 8.62% presenting with grade 3 or higher.24,36,45–47 It is characterized by erythema under pressure and bending parts, slow perception, pain, cracking and desquamation, mostly on the palms and soles of feet. Although HFSR is generally reversible, it causes a significant reduction in the patient’s quality of life during the healing process. The following methods can be used to prevent and reduce the occurrence of HFSR caused by anlotinib.33,48,49,52,53
Firstly, appropriate precautions should be taken, such as advising patients to reduce mechanical stress on the body and to protect their hands and feet by wearing thick cotton socks, gloves, and suitable boots. Meanwhile, skin protection should be strengthened, and it is recommended to use moisturizers containing urea. Hot and cold stimuli should be avoided, and hands should be washed frequently and kept dry. If HFSR persists during the treatment period, the above-mentioned prophylaxis can be further carried out. For patients with grade 1 HFSR, emollients containing 20–40% urea can be used and long-term follow-up can be carried out. For those with grade 2 to 3 HFSR, cortisone or 0.05% polychlorinated betasol can be used for treatment, or painkillers and antibiotics can be used to reduce or prevent HFSR. In the event of failure of the above schemes, adjustment of the dosage and/or withdrawal of the drug should be considered to relieve the symptoms of HFSR before continuing the drug. If the grade of HFSR is reduced to ≤1, the full dosage can be restored. However, once there is a change in HFSR during use, the dosage may need to be reduced or suspended in the following course of treatment.
Diarrhea
Diarrhea, one of the most common side effects in TKI therapy, may cause a decrease or even suspension of dosage during treatment. Studies have revealed that broad-target TKI therapy is associated with a greater risk of diarrhea than single-target TKI therapy.54 Despite the uncertain underlying mechanism of TKI causing diarrhea, gastrointestinal inflammation, mucosal damage and ion transport dysfunction are all risk factors for diarrhea.55 The incidence of diarrhea during anlotinib treatment ranged from 23.33 to 34.48%, compared with 1.10% for those with grade 3 and above.24,45–47
Monitoring and intervention at the early stage of treatment can effectively avoid diarrhea, and for severe diarrhea, dietary habits must be changed.48,53 Patients should be advised to take less drugs, such as caffeine, ethanol, foods containing lactose, and insoluble multi-fiber foods, and to increase water intake to prevent dehydration. If diarrhea persists despite dietary improvements, pharmacologic intervention should be initiated for patients. Antidiarrheal drugs such as loperamide and atropine can be considered.48,54 For patients who do not respond to drug treatment and those with severe diarrhea, the use of anlotinib should be reduced or suspended. However, anlotinib should be started immediately at full dose if diarrhea resolves.
Anorexia
Anorexia tendency occurs in about 13.33–34.48% of patients receiving anlotinib treatment, but grade 3 or higher anorexia rarely occurs45,46,48 Many patients develop anorexia due to medication or extreme psychological anxiety or depression. In terms of diet, nutritious and high-calorie foods, such as protein food, eggs, meat and milk products, are recommended. Some drugs that stimulate appetite can also be used appropriately, such as dronabinol or megestrol acetate.49 Besides, patients are encouraged to eat less and eat more meals instead of three meals a day. Moderate physical exercise, such as walking, exercising or riding a bicycle, will help to increase appetite. Furthermore, attention should be paid to controlling treatment or other related AEs, such as vomiting and diarrhea, which also helps to reduce anorexia. Cancer patients need appropriate social activities because they are prone to anxiety or depression. In the event of anorexia, lowering the dose of anlotinib should only be considered in severe emergencies.
Conclusion
Anlotinib, currently the most commonly used anti-tumor drug, is a class of multi-target receptor tyrosine kinase inhibitors that can achieve favourable therapeutic effects due to the diversity of its targets. But at the same time, it may also cause security risks. In this paper, the mechanism and pharmacokinetic characteristics of adverse drug reactions (ADR) to anlotinib were introduced, and the common adverse conditions and treatment measures in clinical application of anlotinib in preventing and treating tumors were also described. AEs related to anlotinib can be solved through patient management, prevention, therapeutic intervention and drug adjustment. To this end, attention should be paid to the management of AEs when using anlotinib, which is of great importance improve patient compliance and quality of life.
Data Sharing Statement
Data not directly reported in this publication can be obtained from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
An ethics statement is not applicable because this study is based exclusively on published literature.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest related to this study.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Palma D, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457–474. doi:10.1038/nrc.2017.51
3. Kabiraj A, Jaiswal R, Singh A, Gupta J, Singh A, Samadi FM. Immunohistochemical evaluation of tumor angiogenesis and the role of mast cells in oral squamous cell carcinoma. J Cancer Res Ther. 2018;14(3):495–502. doi:10.4103/0973-1482.163693
4. Bagley RGCOF. Tumor Angiogenesis Factor. Cancer Res. 2016;76(7):1673–1674. doi:10.1158/0008-5472.CAN-16-0675
5. Grothey A, Galanis E. Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nat Rev Clin Oncol. 2009;6(9):507–518. doi:10.1038/nrclinonc.2009.110
6. Pottier C, Fresnais M, Gilon M, et al. Tyrosine kinase inhibitors in cancer: breakthrough and challenges of targeted therapy. Cancers. 2020;12(3):731. doi:10.3390/cancers12030731
7. Shyam Sunder S, Sharma UC, Pokharel S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: pathophysiology, mechanisms and clinical management. Signal Transduct Target Ther. 2023;8(1):262. doi:10.1038/s41392-023-01469-6
8. Recine F, De Vita A, Fausti V, et al. Case report: adult NTRK-rearranged spindle cell neoplasm: early tumor shrinkage in a case with bone and visceral metastases treated with targeted therapy. Front Oncol. 2022;11:740676. doi:10.3389/fonc.2021.740676
9. Dermawan JKTK, Kelly C, Gao Z, et al. Novel Genomic Risk Stratification Model for Primary Gastrointestinal Stromal Tumors (GIST) in the Imatinib and Adjuvant Therapy Era. Clin Cancer Res. 2023;29(19):3974–3985. doi:10.1158/1078-0432.CCR-23-1184
10. Tommasi C, Scartabellati G, Giannarelli D, et al. The role of mean corpuscular volume and red cell distribution width in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors: the MARECAP retrospective study. Ther Adv Urol. 2023;15:17562872231187216. doi:10.1177/17562872231187216
11. Lin B, Song X, Yang D, et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene. 2018;654:77–86.
12. Syed YY. Anlotinib: first global approval. Drugs. 2018;78(10):1057–1062. doi:10.1007/s40265-018-0939-x
13. Gao Y, Liu PF, Shi RH. Anlotinib as a molecular targeted therapy for tumors. Oncol Lett. 2020;2:1001–1014.
14. Marme D. Tumor Angiogenesis: a Key Target for Cancer Therapy. Oncol Res Treat. 2018;41(4):164. doi:10.1159/000488340
15. Hashimoto TS, Shibasaki F. Hypoxia-inducible factor as an angiogenic master switch. Front Pediatr. 2015;3:33. doi:10.3389/fped.2015.00033
16. Payen VL, Brisson L, Dewhirst MW, Sonveaux P. Common responses of tumors and wounds to hypoxia. Cancer J. 2015;21(2):75–87. doi:10.1097/PPO.0000000000000098
17. Rouhi P, Lee SLC, Cao Z, Hedlund E-M, Jensen LD, Cao Y. Pathological angiogenesis facilitates tumor cell dissemination and metastasis. Cell Cycle. 2010;9(5):913–917. doi:10.4161/cc.9.5.10853
18. Yang JG, Wang LL, Ma DC. Effects of vascular endothelial growth factors and their receptors on megakaryocytes and platelets and related diseases. Br J Haematol. 2018;180(3):321–334. doi:10.1111/bjh.15000
19. Barratt SL, Flower VA, Pauling JD, Millar AB. VEGF (Vascular Endothelial Growth Factor) and Fibrotic Lung Disease. Int J Mol Sci. 2018;19(5):1269. doi:10.3390/ijms19051269
20. Dehghani S, Nosrati R, Yousefi M, et al. Aptamer-based biosensors and nanosensors for the detection of vascular endothelial growth factor (VEGF): a review. Biosens Bioelectron. 2018;110:23–37. doi:10.1016/j.bios.2018.03.037
21. Xie C, Wan X, Quan H, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018;109(4):1207–1219. doi:10.1111/cas.13536
22. Yu L, Chen Y, Tooze SA. Autophagy pathway: cellular and molecular mechanisms. Autophagy. 2018;14(2):207–215. doi:10.1080/15548627.2017.1378838
23. Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. 2016;9(1):105. doi:10.1186/s13045-016-0332-8
24. Zhong CC, Chen F, Yang JL, et al. Pharmacokinetics and disposition of anlotinib, anoral tyrosine kinase inhibitor, in experimental animal species. Acta Pharmacol Sin. 2018;39(6):1048–1063. doi:10.1038/aps.2017.199
25. Sun W, Wang Z, Chen R, et al. Influences of anlotinib on cytochrome P450 enzymes in rats using a cocktail method. Biomed Res Int. 2017;2017:3619723. doi:10.1155/2017/3619723
26. Van Erp NP, Gelderblom H, Guchelaar H-J. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev. 2009;35(8):692–706. (). doi:10.1016/j.ctrv.2009.08.004
27. Han B, Li K, Wang Q, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: the ALTER 0303 Phase 3 Randomized Clinical Trial [published correction appears in JAMA Oncol. JAMA Oncol. 2018;4(11):1569–1575.
28. Yu L, Xu J, Qiao R, Han B, Zhong H, Zhong R. Efficacy and safety of anlotinib combined with PD-1/PD-L1 inhibitors as second-line and subsequent therapy in advanced small-cell lung cancer. Cancer Med. 2023;12(5):5372–5383. doi:10.1002/cam4.5360
29. Abdel-qadir H, Ethier J, Lee D, et al. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: a systematic review and meta-analysis. Cancer Treat Rev. 2017;53:120–127. doi:10.1016/j.ctrv.2016.12.002
30. Cameron A, Touyz RM, Lang N. Vascular complications of cancer chemotherapy. Can J Cardio. 2016;32(7):852–862. doi:10.1016/j.cjca.2015.12.023
31. Liu B, Ding F, Liu Y, et al. Incidence and risk of hypertension associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a comprehensive network meta-analysis of 72 randomized controlled trials involving 30013 patients. Oncotarget. 2016;7(41):67661–67673. doi:10.18632/oncotarget.11813
32. Si X, Zhang L, Wang H, et al. Management of anlotinib-related adverse events in patients with advanced non-small cell lung cancer: experiences in ALTER-0303. Thorac Cancer. 2019;10(3):551–556. doi:10.1111/1759-7714.12977
33. Touyz RM, Lang NN, Herrmann J, et al. Recent advances in hypertension and cardiovascular toxicities with vascular endothelial growth factor inhibition. Hypertension. 2017;70(2):220–226. doi:10.1161/HYPERTENSIONAHA.117.08856
34. Hamnvik OR, Choueiri TK, Turchin A, et al. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer. 2015;121(2):311–319. doi:10.1002/cncr.28972
35. Chi Y, Fang ZW, Hong XN, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma. Clin Cancer Res. 2018;24(21):5233–5238. doi:10.1158/1078-0432.CCR-17-3766
36. Eryilmaz MK, Mutlu H, K SD, et al. Fatal posterior revesible leukoencephalopathy syndrome associated Coma induced by bevacizumab in metastatic colorectal cancer and review of literature. J Oncol Pharm Pract. 2016;22(6):806–810. doi:10.1177/1078155215611048
37. Estrada CC, Maldonado A, Mallipattu SK. Therapeutic inhibition of VEGF signaling and associated nephro-toxicities. J Am Soc Nephrol. 2019;30(2):187–200. doi:10.1681/ASN.2018080853
38. Schmidinger M. Understanding and managing toxicities of vascular endothelial growth factor (VEGF) inhibitors. EJC Suppl. 2013;11(2):172–191. doi:10.1016/j.ejcsup.2013.07.016
39. Zhang K, Ma XY, J GAOH, et al. Efficacy and safety of anlotinib in advanced non-small cell lung cancer: a real-world study. Cancer Manag Res. 2020;12:3409–3417. doi:10.2147/CMAR.S246000
40. Beck J, Procopio G, Bajetta E, et al. Final results of the European Advanced Renal Cell Carcinoma Sorafenib (EU-ARCCS) expanded-access study: a large open-label study in diverse community settings. Ann Oncol Offc J Eur Soc Med Oncol. 2011;22(8):1812–1823. doi:10.1093/annonc/mdq651
41. Stadler WM, Figlin RA, McDermott DF, et al. Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer. 2010;116(5):1272–1280. doi:10.1002/cncr.24864
42. Gore ME, Szczylik C, Porta C, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. 2009;10(8):757–763. doi:10.1016/S1470-2045(09)70162-7
43. Qi WX, Shen Z, Tang LN, et al. Risk of arterial thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: an up-to-date meta analysis. Crit Rev Oncol Hematol. 2014;92(2):71–82. doi:10.1016/j.critrevonc.2014.04.004
44. Zhou A-P, Bai Y, Song Y, et al. Anlotinib versus sunitinib as first-line treatment for metastatic renal cell carcinoma: a randomized Phase II clinical trial. Oncologist. 2019;24(8):e702–e708. doi:10.1634/theoncologist.2018-0839
45. Wang W, Shao L, Xu Y, et al. Efficacy and safety of anlotinib with and without EGFR-TKIs or immunotherapy in the treatment of elder patients with non-small-cell lung cancer: a retrospective study. BMC Pulm Med. 2022;22(1):179. doi:10.1186/s12890-022-01981-5
46. Dobbin SJH, Cameron AC, Petrie MC, et al. Toxicity of cancer therapy: what the cardiologist needs to know about angiogenesis inhibitors. Heart. 2018;104(24):1995–2002. doi:10.1136/heartjnl-2018-313726
47. Sun Y, Du F, Gao M, et al. Anlotinib for the treatment of patients with locally advanced or metastatic medullary thyroid cancer. Thyroid Official J Amer Thyroid Assoc. 2018;28(11):1455–1461. doi:10.1089/thy.2018.0022
48. Rimassa L, Danesi R, Pressiani T, et al. Management of adverse events associated with tyrosine kinase inhibitors: improving outcomes for patients with hepatocellular carcinoma. Cancer Treat Rev. 2019;77:20–28. doi:10.1016/j.ctrv.2019.05.004
49. Walko CM, Grande C. Management of common adverse events in patients treated with sorafenib: nurse and pharmacist perspective. Semin Oncol. 2014;41(Suppl 2):S17–28. doi:10.1053/j.seminoncol.2014.01.002
50. Miura S, Fujino M, Matsuo Y, et al. Nifedipine-induced vascular endothelial growth factor secretion from coronary smooth muscle cells promotes endothelial tube formation via the kinase insert domain-containing receptor/fetal liver kinase-1/NO pathway. Hypertens Res. 2005;28(2):147–153. doi:10.1291/hypres.28.147
51. Kim SY, Kim S-M, Chang H, et al. Safety of tyrosine kinase inhibitors in patients with differentiated thyroid cancer: real-world use of lenvatinib and Sorafenib in Korea. Front Endocrinol. 2019;10:384. doi:10.3389/fendo.2019.00384
52. Nikolaou V, Syrigos K, Saif MW. Incidence and implications of chemotherapy related hand-foot syndrome. Expert Opin Drug Saf. 2016;15(12):1625–1633. doi:10.1080/14740338.2016.1238067
53. Srinivas S, Stein D, Teltsch DY, et al. Real-world chart review study of adverse events management in patients taking tyrosine kinase inhibitors to treat metastatic renal cell carcinoma. J Oncol Pharm Pract. 2018;24(8):574–583. doi:10.1177/1078155217719583
54. Rugo HS, Di Palma JA, Tripathy D, et al. The characterization, management, and future considerations for ErbB-family TKI-associated diarrhea. Breast Cancer Res Treat. 2019;175(1):5–15. doi:10.1007/s10549-018-05102-x
55. McCole DF, Barrett KE. Decoding epithelial signals: critical role for the epidermal growth factor receptor in controlling intestinal transport function. Acta physiologica. 2009;195(1):149–159. doi:10.1111/j.1748-1716.2008.01929.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
