Back to Journals » Journal of Inflammation Research » Volume 16
Research Progress on Application of Inonotus obliquus in Diabetic Kidney Disease
Authors Wang S, Wang R, Li R, Li Y
Received 22 July 2023
Accepted for publication 3 December 2023
Published 25 December 2023 Volume 2023:16 Pages 6349—6359
DOI https://doi.org/10.2147/JIR.S431913
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Shuyue Wang,1 Ruihua Wang,2 Rongshan Li,1 Yafeng Li1
1Department of Nephrology, Shanxi Provincial People’s Hospital, Taiyuan, Shanxi, 030012, People’s Republic of China; 2The Third Clinical College, Shanxi University of Chinese Medicine, Taiyuan, Shanxi, 030002, People’s Republic of China
Correspondence: Yafeng Li, Department of Nephrology, Shanxi Provincial People’s Hospital, No. 29, Shuangtasi Street, Yingze District, Taiyuan, Shanxi, 030012, People’s Republic of China, Email [email protected]
Abstract: Diabetic kidney disease (DKD) is one of the prime causes of end-stage renal disease. At present, the treatment of DKD is mainly confined to inhibiting the renin-angiotensin-aldosterone system, but the therapeutic effects is not satisfactory. As a kind of very rare and precious medicinal fungi, Inonotus obliquus has a very high medicinal value. Due to its special hypoglycemic and pharmacological effect, researchers currently have attached great importance to it. In this paper, the biological activities, pharmacological effects and application status in the treatment of DKD-related diseases of Inonotus obliquus and the latest progress of metabolites isolated from it in DKD were summarized, thus providing detailed insights and basic understanding of the potential application prospects in DKD.
Keywords: diabetic kidney disease, Inonotus obliquus, triterpenoid, polysaccharide, hypoglycemic effect
Introduction
The Inonotus obliquus (I. obliquus), a plant parasitic fungus growing in cold zone (40°~68° of the North latitude), is extensively distributed in northern North America, Finland, Poland, Russia, Japan as well as Heilongjiang Province and Jilin Province of China.1 As an extremely cold-resistant fungus, it can tolerate low temperature of −40°C. It is often boiled and drunk as tea by the folk because of the natural properties of medicinal fungi. Researchers in Russia and other Eastern European countries in the 16th century began to use the extract of I. obliquus to treat diabetes, cardiovascular diseases, glandular dysplasia, colon cancer, Hodgkin’s lymphoma, gastric cancer, liver cancer, and gastrointestinal cancer.2 Besides, the I. obliquus fine powder produced by Komsomlski Pharmaceuticals is also used as an effective medication for type 2 diabetes.
Diabetic kidney disease (DKD) is one of the most common and serious complications of diabetes, DKD affects about 20% of patients with diabetes and is associated with increased risks of morbidity and mortality, making it one of the leading causes of end-stage renal disease (ESRD).3,4 Besides, it is also the most common cause of proteinuria and non-proteinuria ESRD. The global prevalence of diabetes has increased significantly, with estimates indicating a global prevalence of 9.3%, and about one in two people with diabetes are undiagnosed. The International Diabetes Federation projects that global prevalence will continue to increase, especially in developing countries, if prevention methods and treatment programs remain unchanged.5 It has been proved that oxidative stress, insulin resistance, angiotensin II and proinflammatory cytokines play important roles in the pathogenesis of renal injury in type 2 diabetes.6
Currently, a substantial number of small molecule biological agents have been identified and most of them have been evaluated in preclinical and clinical trials for the treatment of type 2 diabetes.7 I. obliquus has a significant treatment effect in kidney diseases, so extensive attention has been paid to its chemically active components and subsequent pharmacological effects. However, there are few reports on the mechanism of action of I. obliquus on DKD. In this paper, based on original textual research, and the summary of existing research results, the chemical constituents, pharmacological effects and clinical application of I. obliquus in the treatment of DKD were introduced.
Current Understanding of I. obliquus
Based on original textual research, the biological characters of I. obliquus were studied by combining the herbal medicine works of past generations, as well as modern pharmaceutical monographs and literature.
I. obliquus, also called Inonotus obliquus (Fr.) pilat and chaga mushroom, belongs to basidiomycotina, agaricomycetidae, hymenochaetaceae, thelephoraceae and Inonotus.
It is a kind of plant parasitic fungus in the frigid zone (40°~68° of the North latitude), and mainly grows under the barks of white birches, silver birches, elms and alders. The fungus usually gathers and takes into shape after infecting birches for 3~4 years, and then forms Inonotuso bliquus (Fr.) Pilat. Because it is rich in melanin, the sclerotia and mycelium are mostly black and look like charred charcoal in the process of growing, which is also known as Chaga mushroom.8 In 1801, it was first discovered and described by Persoon, who named it Boletus obliquus.9 In 1955, the Ministry of Health of the former Soviet Union recognized the therapeutic value of I. obliquus decoction and included it in the Soviet pharmacopoeia under the name Befunginum.10
There have been a lot of reports on the chemical components of I. obliquus, mainly including polysaccharide (IOP), Inonotus extract, inotodiol, various kinds of oxidized triterpenoids, trametenolic acid, various kinds of lanosterol triterpenoids, folic acid derivatives, aromatic vanillic acid, syringic acid and γ-hydroxybenzoic acid. In addition, some reports also show that Tannins, steroids, alkaloids, melanins, low molecular polyphenols and lignins are isolated from it. These substances have been proven to have anti-tumor activity, anti-inflammatory, antioxidant, hypoglycemic and immunomodulatory effects,11 among which triterpenoids and IOP are not only the main components in the fruiting bodies of I. obliquus, but also the best natural substances with hypoglycemic effect.
Polysaccharide
Among all the active components of I. obliquus, IOP has the most extensive biological activities, including anti-tumor activity, hypoglycemic activity, anti-inflammatory activity and antioxidant activity. A series of proinflammatory cytokines, including prostaglandin mediators, cytokines (TNF-α, IL-1β, IL-6) and nitric oxide (NO) will be released after lipopolysaccharide (LPS) stimulation. IOP can achieve anti-inflammatory effect by inhibiting the induction of carbon monoxide and other similar cytokines.12 Monosaccharides, as the structural units of IOP are linked by glycosidic bonds, which are significantly correlated with the activity of IOP. β-(1,3) -D glycosidic bonds in the glucose backbone are required for their anti-tumor effects. IOP, especially exopolysaccharides, has significant hydroxyl radical and 2, 2-diphenyl-1-picrohydrazide (DPPH) radical scavenging activity, while low molecular weight IOP (29 kDa) (LIOP) has relatively high antioxidant activity,13 which can decrease insulin tolerance14 through NF-κB/TGF-β1 signaling pathway. Liu et al15 isolated and purified two polysaccharides, HIOP1-S and HIOP2-S, from Porus obliquus by DEAE-52 cellulose and Sephadex G-100 column chromatography. HIOP1-S and HIOP2-S had a strong inhibitory effect on α-glucosidase activity, increasing glucose consumption in HepG2 cells and exerting a hypoglycemic effect. At presents, a lot of scholars have evaluated the IOP content in Chaga mushroom extracts and also summarized the IOP content under various extraction conditions such as freezing, hot air or vacuum drying methods.15 Currently, Chinese people extract IOP by the following methods: water extraction,16 ultrasonic-microwave collaborative extraction,17 30% ethanol extraction,18 0.6mol/L sodium hydroxide extraction,17 and ultrasonic method.18 Ultrasonic method and traditional water extraction methods greatly enhance the extraction efficiency. Great progress has been made in the development of IOP over the past decade. However, due to different habitat environments and extraction methods, the obtained composition and content of IOP are different.
Triterpenoid
I. obliquus contains rich contents and complex types of triterpenes, including tramadol acid (TA), indole diol and betulinic acid (BA). Unlike IOP which is extracted often under high temperature and pressure, triterpenes should be extracted with warm water because high temperature and pressure cause damage to it. Based on extensive studies, BA has been proved to have hypoglycemic effects, and various immunomodulatory activities. For example, BA can inhibit various enzymes related to carbohydrate/fat absorption and metabolism, such as α-amylase and several other enzymes.19 Moreover, BA also promotes the secretion of leptin and insulin. The existing signaling pathway studies related to BA are summarized in Table 1. Spiroinonotsuoxotriols A and B are two highly-rearranged pentacyclic triterpenoids newly extracted from I. obliquus by Yin Youming, and they exhibit stronger α-glucosidase inhibitory activity than acarbose.20
 |
Table 1 Signaling Pathway Studies Related to BA |
II. IOP also has a renoprotective effect. Liu et al demonstrated that IOP could alleviate the abnormal body weight, kidney value increased and kidney enlarged in diabetic nephropathy mouse model induced by streptozocin combined with high-sugar and high-fat feed, which may involve in increasing renal oxidase activity and inhibiting malondialdehyde production, thus exerting a renoprotective effect in diabetic nephropathy mice.58
Other
Besides IOP and triterpenoids, Inotodiol and trametenolic acid isolated from the ethyl acetate fraction of I. obliquus have an inhibitory effect on α-amylase. It can remove the effects of 1,1-diphenyl-2-picrylhydrazyl radicals to lower blood glucose levels in diabetic mice. Water-soluble melanin complex extracted from I. obliquus can improve insulin sensitivity and anti-hyperglycemia and promote lipid metabolism in obese mice fed with a high-fat diet.59 The extraction method is usually water extraction.60
The pathogenesis of DKD is complex, and the inflammatory response is an important cause. Hyperglycemia causes renal cells to produce various inflammatory and growth factors. The increase in inflammatory factors can stimulate the production of oxygen-free radicals and increase oxidative stress. Oxidative stress occurs when there is an imbalance between free radicals and antioxidants in the body. Inflammatory activity and free radical production in the immune response can lead to oxidative stress, causing chronic inflammation and potentially contributing to the development of various chronic conditions, including diabetes, cancer, and heart disease.61,62 In turn, oxidative stress can also promote the release of inflammatory factors and exacerbate damage to renal function.63 The lignin complex, phenolic compounds, and melanin in I. obliquus have antioxidant activities. Among them, the lignin-carbohydrate complexes showed significant reducing power and strong scavenging activity against DPPH and hydroxyl radicals. Oblique polyphenol could be extracted with 50% ethanol. It showed stronger ferric-reducing antioxidant power, which can minimize the activity of DPPH and hydroxyl radicals.64 The study by Lu et al also showed that Inotodiol and trametenolic acid have a scavenging effect on 1,1-diphenyl-2-picrylhydrazyl radicals.59
Pharmacological Effects of I. obliquus in the Treatment of DKD
Hypoglycemic Effect
Existing studies reveal that I. obliquus has a better hypoglycemic effect on type 2 diabetes, and its mechanism mainly includes related pathways of glucose metabolism, correction of lipid metabolism disorders, regulation of enzyme activities, intestinal flora, protection and repair of islet cells, and improvement of insulin sensitivity.65,66 A recent literature also mentioned the protective role of betulinic acid, an essential component I. obliquus, in DKD, help potentially increase insulin and C-peptide and decrease fasting blood sugar, kidney lesions, TNF-α, IFN-γ, and IL-1.67
Early studies have proved that IOP can reduce postprandial blood glucose and treat diabetes by inhibiting intestinal α-glucosidase and reducing the absorption of carbohydrates in food.68 Meanwhile, I. obliquus has an obvious hypoglycemic effect on alloxan-induced diabetic mice. As for its main mechanism, it is mediated by the interaction between insulin and gluconeogenesis, which further affects glucose metabolism in the liver and other tissues in the body, thus achieving a hypoglycemic effect.69 Streptozotocin-induced type 2 diabetic mice were mostly used as the study model in a series of subsequent studies. Based on related studies, Wang et al concluded that oral administration of IOP (900mg/kg) could increase the expression of GLUT4 and up-regulate PI3K/Akt pathway in adipose tissue of streptozotocin-induced type 2 diabetic mice, thereby reducing the fasting blood glucose level, improving glucose tolerance, increasing liver glycogen level and enhancing insulin resistance.70,71 Zhang et al72 also confirmed the result and concluded that I. obliquus extract could also achieve hypoglycemic effect by up-regulating the expression of AMPK/ACC pathway in diabetic mice based on additionally studies of the influence of I. obliquus extract on AMPK/ACC signaling pathway. Xu et al73 investigated the hypoglycemic activity and the underlying molecular mechanism of IOP in streptozotocin-induced diabetic mice using metabolomics based on UPLC-Q-Exactive-MS method, they identified a total of 15 differential metabolites between normal control group and diabetes model group. Among them, L-tryptophan, L-leucine, uric acid, 12-HETE and arachidonic acid showed important variations, and could be used as potential biomarkers of diabetes. With the intervention of 11.2g/kg IOP, the differential metabolites leucine and proline in diabetic mice were reversed; phytosphingosinol was further reduced; blood glucose decreased. According to another study, IOP increases the levels of insulin and pyruvate kinase in serum and improves glycogen synthesis, especially IOP5. It restores the levels of superoxide dismutase (SOD), catalase, glutathione peroxidase, and malondialdehyde in serum, thereby alleviating symptoms of type 2 diabetes. It is expounded that the metabolic regulation of IOP at the molecular level can provide a scientific basis for the hypoglycemic effect.74 In conclusion, I. obliquus can repair damaged islet β-cells, promote insulin secretion, and increase liver glycogen content in diabetic mice.
Protective Effect on Kidney
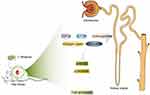
Renal fibrosis is a symptom of ESRD developed from chronic kidney disease (CKD). In this case, abnormal accumulation of extracellular matrix results in loss of kidney tissue and function. The protective effect of BA on kidney is manifested in the following aspects: in CKD-induced rats, BA treatment significantly reverses the changes of histological molecules and dysregulation of metabolic pathways induced by CKD; BA treatment inhibits the expression of pro-fibrotic proteins such as transforming growth factor-β (TGF-β), connective tissue growth factor (C-TGF), hydroxyproline, collagen type I and fibronectin in renal tissue of CKD rats; BA treatment reduces renal tubular dilatation, glomerular degeneration, vacuolation and collagen fiber deposition.73 BA can also restrain high glucose-induced cell proliferation and fibronectin expression in glomerular mesangial cells by inhibiting ERK1/2 and p38MAPK pathways, and reverse the expression of p21Waf1/Cip1 and p27Kip1 inhibited by high glucose at the same time.75 Based on the established C57BL/6 diabetic mouse model induced by streptozotocin, Liu et al76 found that ergosterol reduced mesangial cell proliferation and subsequent extracellular matrix deposition by regulating TGF-β1/Smad2 signaling pathway, indicating that ergosterol could treat DKD. According to the DN mouse model established by intraperitoneal injection of 30mg/kg streptozotocin, it is found that ergosterol significantly reduces fasting blood glucose level, inflammatory cytokine level and renal injury, and increase insulin level, so it can be used for clinical treatment of DKD. Based on histopathological studies, our team77 added the evidence of renal Doppler ultrasound (RDUS) on the structure, function and physiology of the animal kidney. As the results showed, the peak systolic velocity (PSV), mean velocity (MV) and end-diastolic velocity (EDV) of the right renal artery in the Chagas group were higher than those in the DKD group. The PSV and EDV of the internal renal artery were positively correlated with renal functions. It was observed from scanning electron microscopy (SEM) that the average thickness of renal basement membrane, the segmental fusion of podocytes and the average width of foot process were all improved after Chaga-treatment, revealing the protective effect of Chaga-treatment on kidney for the first time. The proposed scheme of the effect is described in Figure 1.
Antioxidant Effect
Hyperglycemia has been shown to induce an augmented formation of ROS, contributing to the development of diabetic nephropathy. The increase in renal cortical oxygen consumption and the impaired oxygen tension in the tissue are related to oxidative stress, which could be prevented by treatment with ROS-scavengers such as α-tocopherol. These oxidative stress modifications can change the metabolic pathways of the kidney and disrupt the hemodynamics associated with DN, eventually causing renal fibrosis.78,79 The oxidative activity in vitro is evaluated usually by establishing Raw 264.7 cell model. NF-κB and Nrf2 are key signaling pathways in cells that regulate oxidative stress and inflammation. IκB/NF-κB and Keap1/Nrf2 signaling pathways can interact with each other. To be specific, NF-κB can regulate the transcription and activity of Nrf2, and the deficiency of Nrf2 up-regulates the activity of NF-κB and results in the expression of proinflammatory factors. Moreover, the abnormalities of IκB/NF-κB and Keap1/Nrf2 signaling pathways are strongly linked with renal fibrosis. IOP has antioxidant activity; the higher the content of uronic acid and protein, the stronger the antioxidant activity of IOP. Antioxidant activity, by inhibiting NF-κB/TGF-β1 signaling pathways, can reduce insulin tolerance, reduce triglyceride amount, increase HDL/LDL ratio and decrease urinary albumin/creatinine ratio (ACR), restore the integrity of glomerular capsule, increase the number of glomerular mesangial cells, reduce the expression of TGF-β in mouse renal cortex, and improve glycolipid toxicity renal fibrosis in DKD mice.14 Mediating the transcription of downstream antioxidant genes can remove ROS and inhibit the accumulation of toxic substances. Previous reports have revealed Nrf2 activation can protect renal functions. However, recent literature has reported it has negative effects in rat models of autoimmune nephritis and DKD, where Nrf2 activation worsens DN in rats. BA of I. obliquus can protect streptozotocin-induced DN rats by inhibiting AMPK/NF-κB/Nrf2 pathway.80 As a lanosterane-type tetracyclic triterpene, TA is one of the main active components extracted from the natural product I. obliquus. Our previous study81 has confirmed that it can significantly reduce the right kidney weight/body weight ratio and the content of urea nitrogen, serum creatinine and urinary albumin in db/db mice. It was observed that the glomerular mesangial matrix expansion and collagen deposition were significantly decreased in kidney tissue. As for the specific mechanism, TA reduces oxidative stress by activating Nrf2/HO-1 pathway in DKD, reduces inflammation by inhibiting NF-κB signaling in DKD, and cuts down podocyte injury and fibrosis. As reported by Ma et al.82 I. obliquus extract contains many LIOP, which are considered to be the antioxidants of these mentioned extracts with this activity. According to the study of Wang et al,72 severe symptoms such as glomerular basement membrane thickening, mesangial hyperplasia, inflammatory infiltration and ECM deposition injury in diabetic mice were alleviated after 4 weeks of IOP, and urinary albumin levels also dropped sharply. Hence, it is argued that IOP can protect the kidney of diabetic mice by inhibiting oxidation products.
Anti-Inflammatory and Immunoregulatory Effects
There is a complex pathogenesis for DKD. It was considered that DKD is a non-immune, metabolic or hemodynamic glomerular disease caused by hyperglycemia. In fact, hypertension, hyperlipidemia, accumulation of advanced glycosylation end products (AGEs) and proteinuria are also involved in the occurrence and development of DKD. Moreover, more and more clinical and experimental evidence suggests that inflammatory cytokines play a key role in the progression of DKD.
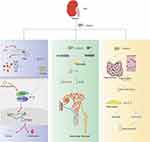
The development of kidney inflammation and fibrosis is a complex process in which many interacting pathways cause chronic inflammatory infiltration, including macrophages and other immune cells that release cytokines and profibrotic factors. I. obliquus can reduce the secretion of proinflammatory cytokines (IL-1β, IFN-γ and TNF-α) in kidney tissue.14 Moreover, it also has anti-inflammatory effects by inhibiting macrophage NO secretion, inhibiting LPS-induced over-expression of iNOS, COX-2, TNF-α and IL-1β in Raw 264.7 cells, and inhibiting inflammatory cytokines and mediators. Currently, there are five known immune-related pathways related to DKD, namely, Toll-like receptor (TLR) signaling pathway; NLRP3 inflammasome, nucleotide binding oligomeric domain (NOD) -like receptor (NLR) signaling; Kinin-kinin system (KKS); protease-activated receptor (PAR) signaling; and complement cascades.82 Most pathways related to the studies of I. obliquus are only involved in the first two ones. TLRs can be expressed by a variety of immune cells (including macrophages, dendritic cells, T cells, B cells, and natural killer cells), as well as by non-immune cells (including renal tubular epithelial cells, endothelial cells, podocytes, and mesangial cells). Xu et al83 proved that birch IOP could inhibit the expression of TLR4/NF-κB signaling pathway proteins in placenta caused by Toxoplasma gondii infection, thereby blocking the TLR4/NF-κB signaling pathway. NF-κB is a key transcription factor mediating the excessive inflammatory response in the occurrence and development of DN. Blood glucose induced NF-κB activation makes TGF-β increase. Tgf-β-dependent signaling in turn promotes fibrosis and inhibits inflammation, which is the characteristic of DKD. Therefore, I. obliquus may also be involved in the inflammatory suppression and fibrosis of DKD through TLR4/NF-κB signaling pathway, but few scholars study it from this aspect. NLRP3 inflammasome participates in the occurrence and development of many human diseases, such as cancer, liver and kidney diseases, and it is the most widely studied inflammasome complex. Human podocytes induced by high glucose level can activate NLRP3 inflammasome.83 Significant up-regulation of inflammasome related proteins such as NLRP3, ASC, CASP1 and IL-18 can also be observed in DN. NLRP3 inflammasome activation can result in angiotensin II–induced podocellular apoptosis and mitochondrial dysfunctions, thus aggravating albuminuria in patients with DKD.84 Inhibiting NLRP inflammasome is a kind of new therapeutic strategy for many inflammatory diseases. NLRP3 inhibitor MCC950 can improve podocyte injury, renal fibrosis and renal dysfunctions in db/db mice. However, existing studies have revealed that IOP inhibits the development of colon cancer by activating the NLRP3 inflammasome in the colon of mice.85 Similarly, according to Western blot of the cells, the levels of nuclear NF-κB and phosphorylated NF-κB increased after the administration of BA.86 As for DKD, there has been no report of how I. obliquus acts on NLRP3 inflammasome to inhibit renal fibrosis. The production of AGEs plays a key role in DN because AGEs can significantly express TGF-β1 in LLC-PK1 and aggravate renal injury. In the study of Chou et al,14 glucose toxicity induced by STZ+ AGEs could also be inhibited by reducing the expression of NF-κB and TGF-β in LLC-PK1 cells after 72h of treatment. Obviously, 10–100kDa LIOP can significantly improve renal dysfunctions and secondary renal fibrosis induced by glycolipidosis in DKD mice.The proposed scheme of the effect is described in Figure 2.
Anti-Hypertensive Effect
Hypertension is not only common in DKD, but also an important factor causing the occurrence and development of DKD. I. obliquus has a vasoprotective effect through anti-inflammation. The ApoE KO mice treated by BA show lower systolic pressure, because BA can improve the expression of endothelial nitric oxide synthase (ENOS) and inhibit the expression of ICAM-1 and ET-1 in the cells.87
Researches of I. obliquus in the Treatment of DKD-Related Diseases
Liquid submerged fermentation is featured by short period, high yield and low price, so it is widely used in the cultivation of I. obliquus. However, due to the rarity of fruiting bodies and the low efficiency of the current submerged fermentation methods, the yield of IOP is relatively low. Based on a large number of repeated experiments, Dr. Jia Chunbao et al verified taking I. obliquus extract could significantly treat diabetes. Since then, Dr. Jia Chunbao led the team to use the “TCM based” cultivation and fermentation technology to obtain fungal drugs that had more refined molecular structures, were more suitable for intestinal absorption and had more obvious efficacy. Thus, the application of I. obliquus in China is further promoted.
Summary and Prospect
Traditional Chinese Medicine (TCM) has been developing for thousands of years in China and has accumulated considerable and valuable experience in treating various diseases. TCM has lower risks of adverse reactions than western medicine. The chemical synthetic drugs that are currently applied in treating diabetes have many serious adverse reactions, including gastrointestinal reactions, liver and kidney damage, and also negatively affect the treatment of complications. Therefore, the development of novel hypoglycemic agents with minimal side effects and superior efficacy from natural products is still a challenge for clinical medicine. I. obliquus has shown great potential in enhancing immunity, anti-tumor, anti-oxidation, anti-fatigue, hypoglycemic activity and anti-hyperlipidemic effect. Hence, it will certainly show great pharmacological effects and potential application value in the treatment of DKD-related diseases. A huge number of studies have shown that I. obliquus has protective effects on DKD. However, it is still necessary to further explore the toxic and side effects, effective therapeutic dose and drug standardization so as to provide solid scientific basis and theoretical guidance for the synthesis of I. obliquus derivatives with higher bioactivity and fewer side effects.
Acknowledgments
We would like to acknowledge the reviewers for their helpful comments on this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Niu H, Song D, Mu H, Zhang W, Sun F, Duan J. Investigation of three lignin complexes with antioxidant and immunological capacities from Inonotus obliquus. Int J Biol Macromol. 2016;86:587–593. doi:10.1016/j.ijbiomac.2016.01.111
2. Hu Y, Sheng Y, Yu M, et al. Antioxidant activity of Inonotus obliquus polysaccharide and its amelioration for chronic pancreatitis in mice. Int J Biol Macromol. 2016;87:348–356. doi:10.1016/j.ijbiomac.2016.03.006
3. Anil DA, Aydin BO, Demir Y, Turkmenoglu B. Design, synthesis, biological evaluation and molecular docking studies of novel 1H-1,2,3-Triazole derivatives as potent inhibitors of carbonic anhydrase, acetylcholinesterase and aldose reductase. J Mol Struct. 2022;1257:132613. doi:10.1016/j.molstruc.2022.132613
4. Antonio G-P, Maria S, David V, Marcus L, Luis Garcia R. Incidence and risk factors for mortality and end-stage renal disease in people with type 2 diabetes and diabetic kidney disease: a population-based cohort study in the UK. BMJ Open Diabetes Res Care. 2021;9(1):e002146. doi:10.1136/bmjdrc-2021-002146
5. Misra A, Gopalan H, Jayawardena R, et al. Diabetes in developing countries. J Diabetes. 2019;11(7):522–539. doi:10.1111/1753-0407.12913
6. Warren AM, Knudsen ST, Cooper ME. Diabetic nephropathy: an insight into molecular mechanisms and emerging therapies. Expert Opin Ther Targets. 2019;23(7):579–591. doi:10.1080/14728222.2019.1624721
7. Sever B, Altıntop MD, Demir Y, et al. Identification of a new class of potent aldose reductase inhibitors: design, microwave-assisted synthesis, in vitro and in silico evaluation of 2-pyrazolines. Chem. Biol. Interact. 2021;345:109576. doi:10.1016/j.cbi.2021.109576
8. Lee MW, Hur H, Chang KC, Lee TS, Ka KH, Jankovsky L. Introduction to distribution and ecology of sterile conks of Inonotus obliquus. Mycobiology. 2008;36(4):199–202. doi:10.4489/MYCO.2008.36.4.199
9. Persoon CH. Synopsis Methodica Fungorum. Pars Secunda. Göttingen, German: Henricum Dieterich; 1801.
10. Gery A, Dubreule C, Andre V, et al. Chaga (Inonotus obliquus), a future potential medicinal fungus in oncology? A chemical study and a comparison of the cytotoxicity against human lung adenocarcinoma cells (A549) and Human Bronchial Epithelial Cells (BEAS-2B). Integr Cancer Ther. 2018;17(3):832–843. doi:10.1177/1534735418757912
11. Kim J, Yang SC, Hwang AY, Cho H, Hwang KT. Composition of triterpenoids in Inonotus obliquus and their anti-proliferative activity on cancer cell lines. Molecules. 2020;25(18). doi:10.3390/molecules25184066
12. Ma L, Chen H, Dong P, Lu X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013;139(1–4):503–508. doi:10.1016/j.foodchem.2013.01.030
13. Van Q, Nayak BN, Reimer M, Jones PJ, Fulcher RG, Rempel CB. Anti-inflammatory effect of Inonotus obliquus, Polygala senega L., and Viburnum trilobum in a cell screening assay. J Ethnopharmacol. 2009;125(3):487–493. doi:10.1016/j.jep.2009.06.026
14. Xiang Y, Xu X, Li J. Chemical properties and antioxidant activity of exopolysaccharides fractions from mycelial culture of Inonotus obliquus in a ground corn stover medium. Food Chem. 2012;134(4):1899–1905. doi:10.1016/j.foodchem.2012.03.121
15. Liu P, Xue J, Tong S, Dong W, Wu P. Structure characterization and hypoglycaemic activities of two polysaccharides from Inonotus obliquus. Molecules. 2018;23(8). doi:10.3390/molecules23081948
16. Chou YJ, Kan WC, Chang CM, et al. Renal protective effects of low molecular weight of Inonotus obliquus polysaccharide (LIOP) on HFD/STZ-induced nephropathy in mice. Int J Mol Sci. 2016;17(9):1535. doi:10.3390/ijms17091535
17. Zheng W, Zhang M, Zhao Y, et al. Analysis of antioxidant metabolites by solvent extraction from sclerotia of Inonotus obliquus (Chaga). Phytochem Anal. 2011;22(2):95–102. doi:10.1002/pca.1225
18. Zs JX, Jiang J, Luo P, Duan F, Zhang J. Research on the optimization of exaction parameter for polysaccharide of Inonotus obliquus and its antioxidant activity. J Nat Sci Heilongjiang Univ. 2017;2017:1.
19. Zong S, Yu Z, Zhao D, et al. Optimization of extraction technology for polysaccharides in Inonotus obliquus. Chin J Mod Appl Pharm. 2014;2014:167–172.
20. Chen X, Zhu Z, Li X, Yao X, Luo L. The ferroptosis-related noncoding RNA signature as a novel prognostic biomarker in the tumor microenvironment, immunotherapy, and drug screening of gastric adenocarcinoma. Front Oncol. 2021;11:778557. doi:10.3389/fonc.2021.778557
21. Sharma S, Kulkarni SK, Chopra K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2006;33(10):940–945. doi:10.1111/j.1440-1681.2006.04468.x
22. Kim HK, Park Y, Shin M, Kim JM, Go GW. Betulinic acid suppresses de novo lipogenesis by inhibiting insulin and IGF1 signaling as upstream effectors of the nutrient-sensing mTOR pathway. J Agric Food Chem. 2021;69(42):12465–12473. doi:10.1021/acs.jafc.1c04797
23. Ajala-Lawal RA, Aliyu NO, Ajiboye TO. Betulinic acid improves insulin sensitivity, hyperglycemia, inflammation and oxidative stress in metabolic syndrome rats via PI3K/Akt pathways. Arch Physiol Biochem. 2020;126(2):107–115. doi:10.1080/13813455.2018.1498901
24. Jin KS, Oh YN, Hyun SK, Kwon HJ, Kim BW. Betulinic acid isolated from Vitis amurensis root inhibits 3-isobutyl-1-methylxanthine induced melanogenesis via the regulation of MEK/ERK and PI3K/Akt pathways in B16F10 cells. Food Chem Toxicol. 2014;68:38–43. doi:10.1016/j.fct.2014.03.001
25. Jiao S, Zhu H, He P, Teng J. Betulinic acid protects against cerebral ischemia/reperfusion injury by activating the PI3K/Akt signaling pathway. Biomed Pharmacother. 2016;84:1533–1537. doi:10.1016/j.biopha.2016.11.028
26. Xu T, Pang Q, Wang Y, Yan X. Betulinic acid induces apoptosis by regulating PI3K/Akt signaling and mitochondrial pathways in human cervical cancer cells. Int J Mol Med. 2017;40(6):1669–1678. doi:10.3892/ijmm.2017.3163
27. Savova MS, Vasileva LV, Mladenova SG, et al. Ziziphus jujuba Mill. leaf extract restrains adipogenesis by targeting PI3K/AKT signaling pathway. Biomed Pharmacother. 2021;141:111934. doi:10.1016/j.biopha.2021.111934
28. Majeed R, Hussain A, Sangwan PL, et al. PI3K target based novel cyano derivative of betulinic acid induces its signalling inhibition by down-regulation of pGSK3beta and cyclin D1 and potentially checks cancer cell proliferation. Mol Carcinog. 2016;55(5):964–976. doi:10.1002/mc.22339
29. Wu C, Chen H, Zhuang R, et al. Betulinic acid inhibits pyroptosis in spinal cord injury by augmenting autophagy via the AMPK-mTOR-TFEB signaling pathway. Int J Biol Sci. 2021;17(4):1138–1152. doi:10.7150/ijbs.57825
30. Lee D, Lee SR, Kang KS, et al. Betulinic acid suppresses ovarian cancer cell proliferation through induction of apoptosis. Biomolecules. 2019;9(7). doi:10.3390/biom9070257
31. Mioc M, Mioc A, Prodea A, et al. Novel triterpenic acid-benzotriazole esters act as pro-apoptotic antimelanoma agents. Int J Mol Sci. 2022;23(17):9992. doi:10.3390/ijms23179992
32. Kim SY, Hwangbo H, Kim MY, et al. Betulinic acid restricts human bladder cancer cell proliferation in vitro by inducing caspase-dependent cell death and cell cycle arrest, and decreasing metastatic potential. Molecules. 2021;26(5). doi:10.3390/molecules26051381
33. El-Baba C, Baassiri A, Kiriako G, et al. Terpenoids’ anti-cancer effects: focus on autophagy. Apoptosis. 2021;26(9–10):491–511. doi:10.1007/s10495-021-01684-y
34. Zhao Y, Shi X, Wang J, Mang J, Xu Z. Betulinic acid ameliorates cerebral injury in middle cerebral artery occlusion rats through regulating autophagy. ACS Chem Neurosci. 2021;12(15):2829–2837. doi:10.1021/acschemneuro.1c00198
35. Zheng LY, Zou X, Wang YL, et al. Betulinic acid-nucleoside hybrid prevents acute alcohol -induced liver damage by promoting anti-oxidative stress and autophagy. Eur J Pharmacol. 2022;914:174686. doi:10.1016/j.ejphar.2021.174686
36. Zhao H, Wu L, Zhang Y, et al. Betulinic acid prevents liver fibrosis by binding Lck and suppressing Lck in HSC activation and proliferation. J Ethnopharmacol. 2022;296:115459. doi:10.1016/j.jep.2022.115459
37. Zhan XK, Li JL, Zhang S, Xing PY, Xia MF. Betulinic acid exerts potent antitumor effects on paclitaxel-resistant human lung carcinoma cells (H460) via G2/M phase cell cycle arrest and induction of mitochondrial apoptosis. Oncol Lett. 2018;16(3):3628–3634. doi:10.3892/ol.2018.9097
38. Chen S, Bai Y, Li Z, et al. A betulinic acid derivative SH479 inhibits collagen-induced arthritis by modulating T cell differentiation and cytokine balance. Biochem Pharmacol. 2017;126:69–78. doi:10.1016/j.bcp.2016.12.006
39. Liu Y, Bi Y, Mo C, et al. Betulinic acid attenuates liver fibrosis by inducing autophagy via the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway. J Nat Med. 2019;73(1):179–189. doi:10.1007/s11418-018-1262-2
40. Ou Z, Zhu L, Huang C, et al. Betulinic acid attenuates cyclophosphamide-induced intestinal mucosa injury by inhibiting the NF-kappaB/MAPK signalling pathways and activating the Nrf2 signalling pathway. Ecotoxicol Environ Saf. 2021;225:112746. doi:10.1016/j.ecoenv.2021.112746
41. Wei J, Li Y, Liu Q, et al. Betulinic acid protects from bone loss in ovariectomized mice and suppresses RANKL-associated osteoclastogenesis by inhibiting the MAPK and NFATc1 pathways. Front Pharmacol. 2020;11:1025. doi:10.3389/fphar.2020.01025
42. Zhu L, Yi X, Zhao J, et al. Betulinic acid attenuates dexamethasone-induced oxidative damage through the JNK-P38 MAPK signaling pathway in mice. Biomed Pharmacother. 2018;103:499–508. doi:10.1016/j.biopha.2018.04.073
43. Lin X, Zhu L, Gao X, et al. Ameliorative effect of betulinic acid against zearalenone exposure triggers testicular dysfunction and oxidative stress in mice via p38/ERK MAPK inhibition and Nrf2-mediated antioxidant defense activation. Ecotoxicol Environ Saf. 2022;238:113561. doi:10.1016/j.ecoenv.2022.113561
44. Jeong DH, Kwak SC, Lee MS, Yoon KH, Kim JY, Lee CH. Betulinic acid inhibits RANKL-induced osteoclastogenesis via attenuating Akt, NF-kappaB, and PLCgamma2-Ca(2+) signaling and prevents inflammatory bone loss. J Nat Prod. 2020;83(4):1174–1182. doi:10.1021/acs.jnatprod.9b01212
45. Cheng Z, Yao W, Zheng J, et al. A derivative of betulinic acid protects human Retinal Pigment Epithelial (RPE) cells from cobalt chloride-induced acute hypoxic stress. Exp Eye Res. 2019;180:92–101. doi:10.1016/j.exer.2018.12.011
46. Li X, Liu X, Deng R, et al. Betulinic acid attenuated bleomycin-induced pulmonary fibrosis by effectively intervening Wnt/beta-catenin signaling. Phytomedicine. 2021;81:153428. doi:10.1016/j.phymed.2020.153428
47. Huimin D, Hui C, Guowei S, Shouyun X, Junyang P, Juncheng W. Protective effect of betulinic acid on Freund’s complete adjuvant-induced arthritis in rats. J Biochem Mol Toxicol. 2019;33(9):e22373. doi:10.1002/jbt.22373
48. Ghanadian M, Ali Z, Khan IA, et al. A new sesquiterpenoid from the shoots of Iranian Daphne mucronata Royle with selective inhibition of STAT3 and Smad3/4 cancer-related signaling pathways. Daru. 2020;28(1):253–262. doi:10.1007/s40199-020-00336-x
49. Szuster-Ciesielska A, Plewka K, Daniluk J, Kandefer-Szerszen M. Betulin and betulinic acid attenuate ethanol-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS), cytokine (TNF-alpha, TGF-beta) production and by influencing intracellular signaling. Toxicology. 2011;280(3):152–163. doi:10.1016/j.tox.2010.12.006
50. Nader MA, Baraka HN. Effect of betulinic acid on neutrophil recruitment and inflammatory mediator expression in lipopolysaccharide-induced lung inflammation in rats. Eur J Pharm Sci. 2012;46(1–2):106–113. doi:10.1016/j.ejps.2012.02.015
51. Peng J, Lv YC, He PP, et al. Betulinic acid downregulates expression of oxidative stress-induced lipoprotein lipase via the PKC/ERK/c-Fos pathway in RAW264.7 macrophages. Biochimie. 2015;119:192–203. doi:10.1016/j.biochi.2015.10.020
52. Jiao L, Wang S, Zheng Y, et al. Betulinic acid suppresses breast cancer aerobic glycolysis via caveolin-1/NF-kappaB/c-Myc pathway. Biochem Pharmacol. 2019;161:149–162. doi:10.1016/j.bcp.2019.01.016
53. Zhou Z, Choi JW, Shin JY, et al. Betulinic acid ameliorates the severity of acute pancreatitis via inhibition of the NF-kappaB signaling pathway in mice. Int J Mol Sci. 2021;22(13). doi:10.3390/ijms22136871
54. Shen M, Hu Y, Yang Y, et al. Betulinic acid induces ROS-dependent apoptosis and S-phase arrest by inhibiting the NF-kappaB pathway in human multiple myeloma. Oxid Med Cell Longev. 2019;2019:5083158. doi:10.1155/2019/5083158
55. Mohsen GA, Abu-Taweel GM, Rajagopal R, et al. Betulinic acid lowers lipid accumulation in adipocytes through enhanced NCoA1-PPARgamma interaction. J Infect Public Health. 2019;12(5):726–732. doi:10.1016/j.jiph.2019.05.011
56. Sandeep MRC, Chanotiya CS, Mukhopadhyay P, Ghosh S. Oxidosqualene cyclase and CYP716 enzymes contribute to triterpene structural diversity in the medicinal tree banaba. New Phytol. 2019;222(1):408–424. doi:10.1111/nph.15606
57. Maczynska J, Choromanska A, Kutkowska J, et al. Effect of electrochemotherapy with betulinic acid or cisplatin on regulation of heat shock proteins in metastatic human carcinoma cells in vitro. Oncol Rep. 2019;41(6):3444–3454. doi:10.3892/or.2019.7103
58. Chen C, Liu X, Li L, et al. Protective effect of Inonotus obliquus polysaccharides on kidneys of diabetic kidney disease mice. Sci Technol Food Ind. 2021;42(2):321–325.
59. Lu X, Chen H, Dong P, Fu L, Zhang X. Phytochemical characteristics and hypoglycaemic activity of fraction from mushroom Inonotus obliquus. J Sci Food Agric. 2010;90(2):276–280. doi:10.1002/jsfa.3809
60. Lee JH, Hyun CK. Insulin-sensitizing and beneficial lipid-metabolic effects of the water-soluble melanin complex extracted from Inonotus obliquus. Phytother Res. 2014;28(9):1320–1328. doi:10.1002/ptr.5131
61. Poprac P, Jomova K, Simunkova M, Kollar V, Rhodes CJ, Valko M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol Sci. 2017;38(7):592–607. doi:10.1016/j.tips.2017.04.005
62. Türkeş C, Demir Y, Beydemir Ş. Some calcium-channel blockers: kinetic and in silico studies on paraoxonase-I. J Biomol Struct Dyn. 2022;40(1):77–85. doi:10.1080/07391102.2020.1806927
63. Tang SCW, Yiu WH. Innate immunity in diabetic kidney disease. Nat Rev Nephrol. 2020;16(4):206–222. doi:10.1038/s41581-019-0234-4
64. Wang Y, Ouyang F, Teng C, Qu J. Optimization for the extraction of polyphenols from Inonotus obliquus and its antioxidation activity. Prep Biochem Biotechnol. 2021;51(9):852–859. doi:10.1080/10826068.2020.1864642
65. Ying YM, Yu HF, Tong CP, Shan WG, Zhan ZJ. Spiroinonotsuoxotriols A and B, two highly rearranged triterpenoids from Inonotus obliquus. Org Lett. 2020;22(9):3377–3380. doi:10.1021/acs.orglett.0c00866
66. He P, Zhang Y, Li N. The phytochemistry and pharmacology of medicinal fungi of the genus Phellinus: a review. Food Funct. 2021;12(5):1856–1881. doi:10.1039/d0fo02342f
67. Tian L, Wang Y, Qing J, et al. A review of the pharmacological activities and protective effects of Inonotus obliquus triterpenoids in kidney diseases. Open Chem. 2022;20(1):651–665. doi:10.1515/chem-2022-0168
68. Guo M, Shao S, Wang D, Zhao D, Wang M. Recent progress in polysaccharides from Panax ginseng C. A. Meyer. Food Funct. 2021;12(2):494–518. doi:10.1039/d0fo01896a
69. Chen H, Yan M, Zhu J, Xu X. Enhancement of exo-polysaccharide production and antioxidant activity in submerged cultures of Inonotus obliquus by lignocellulose decomposition. J Ind Microbiol Biotechnol. 2011;38(2):291–298. doi:10.1007/s10295-010-0772-z
70. Sun JE, Ao ZH, Lu ZM, et al. Antihyperglycemic and antilipidperoxidative effects of dry matter of culture broth of Inonotus obliquus in submerged culture on normal and alloxan-diabetes mice. J Ethnopharmacol. 2008;118(1):7–13. doi:10.1016/j.jep.2008.02.030
71. Wang J, Wang C, Li S, et al. Anti-diabetic effects of Inonotus obliquus polysaccharides in streptozotocin-induced type 2 diabetic mice and potential mechanism via PI3K-Akt signal pathway. Biomed Pharmacother. 2017;95:1669–1677. doi:10.1016/j.biopha.2017.09.104
72. Zhang Z, Liang X, Tong L, et al. Effect of Inonotus obliquus (Fr.) Pilat extract on the regulation of glycolipid metabolism via PI3K/Akt and AMPK/ACC pathways in mice. J Ethnopharmacol. 2021;273:113963. doi:10.1016/j.jep.2021.113963
73. Xu T, Li G, Wang X, Lv C, Tian Y. Inonotus obliquus polysaccharide ameliorates serum profiling in STZ-induced diabetic mice model. BMC Chem. 2021;15(1):64. doi:10.1186/s13065-021-00789-4
74. Wang J, Hu W, Li L, et al. Antidiabetic activities of polysaccharides separated from Inonotus obliquus via the modulation of oxidative stress in mice with streptozotocin-induced diabetes. PLoS One. 2017;12(6):e0180476. doi:10.1371/journal.pone.0180476
75. Sharma A, Thakur R, Lingaraju MC, et al. Betulinic acid attenuates renal fibrosis in rat chronic kidney disease model. Biomed Pharmacother. 2017;89:796–804. doi:10.1016/j.biopha.2017.01.181
76. Liu CM, Qi XL, Yang YF, Zhang XD. Betulinic acid inhibits cell proliferation and fibronectin accumulation in rat glomerular mesangial cells cultured under high glucose condition. Biomed Pharmacother. 2016;80:338–342. doi:10.1016/j.biopha.2016.02.040
77. Liu C, Zhao S, Zhu C, et al. Ergosterol ameliorates renal inflammatory responses in mice model of diabetic nephropathy. Biomed Pharmacother. 2020;128:110252. doi:10.1016/j.biopha.2020.110252
78. Fakhruddin S, Alanazi W, Jackson KE. Diabetes-induced reactive oxygen species: mechanism of their generation and role in renal injury. J Diabetes Res. 2017;2017:8379327. doi:10.1155/2017/8379327
79. Palabıyık E, Sulumer AN, Uguz H, et al. Assessment of hypolipidemic and anti-inflammatory properties of walnut (Juglans regia) seed coat extract and modulates some metabolic enzymes activity in triton WR-1339-induced hyperlipidemia in rat kidney, liver, and heart. J Mol Recog. 2023;36(3):e3004. doi:10.1002/jmr.3004
80. Zhang Y, Liao H, Shen D, et al. Renal protective effects of Inonotus obliquus on high-fat diet/streptozotocin-induced diabetic kidney disease rats: biochemical, color Doppler ultrasound and histopathological evidence. Front Pharmacol. 2022;12:743931. doi:10.3389/fphar.2021.743931
81. Xie R, Zhang H, Wang XZ, et al. The protective effect of betulinic acid (BA) diabetic nephropathy on streptozotocin (STZ)-induced diabetic rats. Food Funct. 2017;8(1):299–306. doi:10.1039/c6fo01601d
82. Ma Q, Santhanam RK, Xue Z, Guo Q, Gao X, Chen H. Effect of different drying methods on the physicochemical properties and antioxidant activities of mulberry leaves polysaccharides. Int J Biol Macromol. 2018;119:1137–1143. doi:10.1016/j.ijbiomac.2018.08.023
83. Xu L, Yu Y, Sang R, et al. Inonotus obliquus polysaccharide protects against adverse pregnancy caused by Toxoplasma gondii infection through regulating Th17/Treg balance via TLR4/NF-kappaB pathway. Int J Biol Macromol. 2020;146:832–840. doi:10.1016/j.ijbiomac.2019.10.051
84. Gao P, Meng XF, Su H, et al. Thioredoxin-interacting protein mediates NALP3 inflammasome activation in podocytes during diabetic nephropathy. Biochim Biophys Acta. 2014;1843(11):2448–2460. doi:10.1016/j.bbamcr.2014.07.001
85. Zhao M, Bai M, Ding G, et al. Angiotensin II stimulates the NLRP3 inflammasome to induce podocyte injury and mitochondrial dysfunction. Kidney Dis. 2018;4(2):83–94. doi:10.1159/000488242
86. Li J, Qu C, Li F, et al. Inonotus obliquus polysaccharide ameliorates azoxymethane/dextran sulfate sodium-induced colitis-associated cancer in mice via activation of the NLRP3 inflammasome. Front Pharmacol. 2020;11:621835. doi:10.3389/fphar.2020.621835
87. Shen J, Dai Z, Li Y, Zhu H, Zhao L. TLR9 regulates NLRP3 inflammasome activation via the NF-kB signaling pathway in diabetic nephropathy. Diabetol Metab Syndr. 2022;14(1):26. doi:10.1186/s13098-021-00780-y
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.