Back to Journals » Drug Design, Development and Therapy » Volume 16
Research Progress of Liujunzi Decoction in the Treatment of Tumor-Associated Anorexia
Received 17 March 2022
Accepted for publication 26 May 2022
Published 7 June 2022 Volume 2022:16 Pages 1731—1741
DOI https://doi.org/10.2147/DDDT.S365292
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Xipei Wu, Yongzhao Dai, Ke Nie
School of Chinese Materia Medica, Guangdong Pharmaceutical University, Guangzhou, People’s Republic of China
Correspondence: Ke Nie, School of Chinese Materia Medica, Guangdong Pharmaceutical University, Guangzhou, 510006, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Tumor-associated anorexia, mainly including cancerous anorexia and chemotherapy-induced anorexia, severely reduces the life quality of cancer patients but lacks of effective control until now. Liujunzi decoction (LJZD), a classical tonifying formula in traditional Chinese medicine, has promising effect in preventing and treating many kinds of anorexia. A growing number of evidence showed that LJZD is able to improve tumor-associated anorexia. Up to March 2022, a total of 58 articles studying LJZD or Rikkunshito (the name of LJZD in Japanese herbal medicine) in the treatment of tumor-associated anorexia are searched out in PubMed. This paper summarizes the effect of LJZD in ameliorating tumor-associated anorexia, in order to provide a theoretical basis for the clinical application of LJZD in treating tumor-associated anorexia, laying foundation for further research.
Keywords: Liujunzi decoction, tumor-associated anorexia, review
Background
Cancer is one of the major public health problems around the world, according to the latest data from the International Agency for Research on Cancer, 19.3 million new cancer cases were diagnosed globally and nearly 10 million deaths occurred in 2020.1 Although there are new cancer treatments such as biological therapy and targeted therapy, chemotherapy is one of the most effective and commonly used treatments. However, anorexia is one of the most common complications of advanced cancer patients and one of the most common side effects of antineoplastic agents, which will lead to adverse consequences such as reduced food intake, decreased body weight and affect the therapeutic effect of cancer.2–6 Therefore, tumor-associated anorexia mainly includes cancerous anorexia (CA) caused by cancer itself, and chemotherapy-induced anorexia (CIA) caused by chemotherapeutic drugs.7–10 At present, appetite stimulation, drug intervention and nutritional therapy are often used in the clinical treatment of tumor-associated anorexia, but the effect is unsatisfactory.11,12 For example, progesterone such as megestrol acetate is often used in clinical practice to prevent and treat tumor cachexia. In addition to its efficacy in inhibiting tumor, this drug also has the efficacy of promoting appetite and improving anorexia, but it can lead to side effects such as venous thrombosis, sodium water retention, uterine bleeding, electrolyte disorder, and renal insufficiency.13–15 Studies have shown that anamorelin and thalidomide have positive effects in the treatment of CA, but the mechanisms are not so clear and the high cost deters patients.16,17 In view of the lack of effective drugs for CIA in clinical practice, it is urgent to develop safe and effective drugs for tumor-associated anorexia.18
Liujunzi decoction (LJZD), a classical tonifying prescription in traditional Chinese medicine (TCM), originates from the Yi Xue Zheng Zhuan compiled in the Ming Dynasty. It has the effect of benefiting vital energy and tonifying spleen, removing dampness to reduce phlegm, and mainly treats phlegm-dampness due to deficiency of the spleen syndrome. In 2008, an epoch-making study by Japanese scholars found that Rikkunshito could improve appetite in a cisplatin-induced anorexia model in rats by promoting the secretion of ghrelin, which immediately attracted the attention and research of a large number of scholars.19,20 Clinical study found that LJZD and modified LJZD improved tumor-associated anorexia effectively.18,21–33 A number of animal experiments have also proved that this prescription has the efficacy of preventing and treating tumor-associated anorexia.19,34–41 In view of the exact effect of LJZD in improving tumor-associated anorexia, this paper summarizes the literature of LJZD in the treatment of tumor-associated anorexia from CA and CIA, in order to provide ideas for further application of LJZD.
CA and LJZD
CA is anorexia caused by advanced tumor. In advanced tumor often caused a complex systemic disease “cachexia”, leading to anorexia, weakness, muscle loss and anemia clinical features, cause to further reduce the quality of life.7 CA does not have a specific name in traditional Chinese medical science (TCMS). According to symptoms, it can be classified into the categories of “fullness”, “asthenia and fatigue”, “anorexia” or “insufficient food” in TCMS.42 Its etiology is mostly due to weakness of spleen and stomach, indigestion of diet, and emotional disorders, resulting in adverse middle energizer and abnormal ascending and descending.42 At the same time, it is also the cause of death in most advanced cancer patients, which directly affects the therapeutic effect of tumor, increases the incidence of complications, reduces the quality of life of cancer patients, and shortens the survival time.7,43 The main pathological mechanism of CA is the disorder of food intake center and related peripheral signaling pathways.
Pathogenesis of CA: Central Nervous System, Peripheral Signals and Other Factors
In the arcuate nucleus (ARC) located at the bottom of the medial hypothalamus, there are two types of neurons regulating metabolism: one inhibits appetite, such as the neurons secreting proopiomelanocortin (POMC), the other promotes appetite, such as neurons secreting neuropeptide Y (NPY) and Agouti-related protein (AgRP).44 According to the literature, the studies on inhibiting appetite neurons POMC mainly through influence are as follows. Under cachexia conditions, radiolabelled recombinant human lipocalin-2 (LCN2) was injected into macaques and found LCN2 can cross the blood–brain barrier and reach to the hypothalamus, regulating food intake by affecting AgRP/POMC neurons and leading to anorexia.45,46 Under cancer cachectic conditions, POMC neurons can be activated by 5-hydroxytryptamine (5-HT) and produce satiety by releasing the shearing product α-melanin stimulating hormone (α-MSH) of POMC.47,48 Hypothalamus has the function of mediating energy balance in the body, so hypothalamus dysfunction caused by inflammation can lead to the occurrence of CA, such as the activation of hypothalamic-pituitary-adrenal axis induced by interleukin-1β (IL-1β), which promotes the development of cancer anorexia-cachexia syndrome (CACS).49,50 At the same time, it also up-regulates 5-HT levels to enhance the activity of hypothalamic anorexia neurons to some extent, stimulate POMC neurons, induce appetite decline and reduce food intake.51,52 In addition, pro-inflammatory factors such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6) and interferon-γ (IFN-γ) may directly enter the central nervous system through the blood–brain barrier and bind to the corresponding receptors in the hypothalamus to promote the development of anorexia.53,54 To appetite-promoting neurons NPY, in vitro hypothalamic cell experiment showed that 5-HT could interfere with the synthesis, transportation and secretion of NPY, which could enhance food intake, that is, the anorexia caused by 5-HT under the condition of cancer cachexia may be related to the NPY system.48 On the other hand, there are other factors that do not directly affect appetite-regulated neurons that can cause CA. Studies have shown that in the early stage of cancer development, the parasympathetic nervous system can perceive tumor signals and stimulate hypothalamic histaminergic neurons, prompting them to emit abnormal histamine signals.55 The abnormal histamine signal acts on the histamine H1 receptor (HRH1) in the arcuate nucleus, medial nucleus and paraventricular nucleus of the hypothalamus. The activation of HRH1 receptor can mediate the activation of AMP-activated protein kinase (AMPK) in the hypothalamus, further inhibit the histamine H3 receptor (HRH3), thereby inhibiting the hypothalamic starvation center and inducing CA.56,57 In one word, the main pathway of tumor leading to CA by affecting the central nervous system is to promote the secretion of related proteins and inflammatory factors, thereby abnormally regulating appetite-related neurons in the hypothalamus and activating appetite-related receptors.
Peripheral signals mainly involved in regulating appetite include leptin and ghrelin.54 Leptin regulates appetite mainly by interacting with hypothalamic neuroendocrine pathways, inhibiting appetite regulation-related peptides such as NPY and orexin-A (OX-A) and stimulating hormones such as POMC.58 Ghrelin can improve appetite, prevent weight loss, and promote the production of synthetic metabolic factors such as insulin and insulin-like growth factor 1 by stimulating growth hormone secretagogue receptor-1a (GHS-R1a) to promote the increase of synthetic metabolic hormones.59–61 Glucagon-like peptide-1 (GLP-1) secreted mainly by colon L cells is expressed in nucleus tractus solitarius (NTS), which is related to nausea and anorexia. Studies have found that the use of GLP-1 receptor antagonist exendin-9 and the knockout of GLP-1 expression genes in NTS can effectively improve food intake and the body weight of CACS model rats.62 Leukemia inhibitory factor (LIF) secreted by tumor induces adipocyte lipolysis and increases serum IL-6 and leptin levels by activating the janus kinase/signal transducer and activator of tran-ions (JAK/STAT) signaling pathway and affected cachexia-related fat consumption and anorexia.63,64 In summary, tumor mainly causes CA by affecting peripheral leptin, ghrelin, GLP-1 and LIF secretion and activating their central receptors.
In addition, central histamine neurons are closely related to the basic functions of the body, such as regulation behavior, biological rhythm, body temperature and food intake. For example, taste and olfactory functions are controlled by the brain discrete structures with different histaminergic neurons as targets. Abnormal histamine signals can lead to taste and olfactory disorders, thus triggering CA.7 On the other hand, the mechanical obstruction of gastrointestinal tract, delayed gastric emptying, digestive and absorption disorders and abnormal fluid loss caused by tumor growth and oppression of surrounding organs can lead to reduced food intake.43 Figure 1 summarizes the main pathological mechanisms of CA.
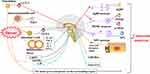 |
Figure 1 The main pathological mechanisms of CA. |
Clinical Application and Mechanism of LJZD in Prevention and Treatment of CA
Table 1 summarizes the main indexes of LJZD in the study of CA. Korean scholar Kang et al21 in a pilot, randomized, controlled study program, the selected subjects were randomly divided into two groups: control group and Yukgunja-Tang (LJZD in Korean herbal medicine) group. Through nutritional inquiry and leptin, TNF-α, ghrelin and IL-6 levels of detection, they found that LJZD in the treatment of tumor-associated anorexia has exerted efficacy and safety. Xie et al22 in the use of thalidomide, LJZD combined with transcatheter arterial chemoembolization in the treatment of advanced liver cancer clinical research found that LJZD can improve the appetite of patients, enhance their physique, and reduce the incidence of severe liver function damage. Zhan et al23 in a clinical trial, the patients in the study group were treated with Xiangsha Liujunzi Decoction combined with auricular acupressure beans and megestrol acetate, while the patients in the control group were treated with megestrol acetate; Wang et al24 divided the patients diagnosed as stage IV advanced malignant tumor patients with cachexia syndrome into the medroxyprogesterone group, the modified Xiangsha Guishao Liujunzi Decoction group and the modified Xiangsha Guishao Liujunzi Decoction combined with medroxyprogesterone group; Sun et al25 randomly divided tumor patients who met the experimental criteria into the comprehensive group, the TCM group and the control group and treated them with Xiangsha Liujunzi Decoction combined with auricular point pressing beans and medroxyprogesterone; Cheng et al26 divided 60 patients with advanced cancer into treatment group with megestrol acetate and treatment group with modified LJZD combined with megestrol acetate. In the above clinical studies, the analysis results showed that the appetite, weight and Karnofsky performance scale (KPS) scores of the patients treated with LJZD were improved, and the clinical symptoms of TCM changed well. There were no other side effects, and the patient’s anorexia was abated and the quality of life was improved.
 |
Table 1 The Effect of LJZD on the Main Evaluation in CA Study |
LJZD is effective in alleviating CA in clinical practice, but its mechanism still needs further exploration. Ohbuchi et al34 performed plasma metabolomics analysis on AH-130 ascites-induced cachexia rat model. A total of 110 metabolites were detected in the plasma. LJZD treatment significantly changed the levels of 23 metabolites. It was found that LJZD can delay weight loss, improve muscle atrophy, reduce ascites content, and alleviate inflammation and anorexia by increasing glucarate in the plasma. Fujitsuka et al35 established the AH-130 tumor-bearing anorexia rat model and found that 5-HT in hypothalamus reduced the ghrelin signal caused by the excessive interaction between 5-HT2C receptor and corticotropin-releasing factor (CRF), resulting in anorexia. 5-HT2C receptor antagonist could reduce the CRF level in hypothalamus, thereby increasing the plasma acylated ghrelin level to improve appetite. The experimental results show that LJZD not only has the efficacy of improving anorexia in tumor-bearing rats but also has the efficacy of improving gastrointestinal motility disorder, alleviating muscle atrophy, relieving anxiety and prolonging the survival time of tumor-bearing rats. It is an effective drug for the treatment of CASC. Terawaki et al36 established a rat model of cachexia by inoculating rats with human gastric cancer 85As2 cells. They found that ghrelin resistance in tumor-bearing rats was one of the causes of anorexia and weight loss. Rikkunshito may improve CA by enhancing the ghrelin signaling pathway to reduce its resistance.
CIA and LJZD
Chemotherapy is effective for cancer, but with various side effects, including anorexia, nausea, vomiting, diarrhea and neurotoxicity, it makes it difficult for patients to continue treatment, resulting in poor prognosis and poor quality of life, further limiting the clinical application of chemotherapy.65,66 Chemotherapy is one of the most effective methods for cancer treatment, and CIA is one of the serious adverse reactions caused by chemotherapy. CIA occurs in 50% of newly diagnosed cancer patients and up to 70% of patients with advanced diseases.67 Although various countermeasures have been developed to prevent or treat side effects such as anorexia, the effect is unsatisfactory.68,69 The pathological mechanism of CIA is related to many factors, especially the physiological mechanism disorder of appetite regulation center.
Pathogenesis of CIA: Central Nervous System, Peripheral Signals and Inflammatory Factors
NPY and ghrelin, which play roles in hypothalamic arcuate nucleus, are effective appetite inducers. Cisplatin, a chemotherapeutic drug, can reduce the concentration of Ca2+ in cells in arcuate nucleus and reduce the activity of NPY and ghrelin-responsive neurons to produce CIA.70 At the same time, chemotherapy drugs cisplatin and 5-fluorouracil can reduce the level of ghrelin and inhibit the activity of GHS-R1a receptor in hypothalamus, thereby causing anorexia.9 In the delayed phase of chemotherapy, cisplatin activates GLP-1 neurons in NTS and leads to CIA. Experiments show that the injection of GLP-1 receptor antagonist in the fourth ventricle of the rat model of chemotherapy-induced anorexia can reduce cisplatin-induced anorexia, weight loss and kaolin intake.71 In the CIA rat model, it was found that cisplatin could induce the expression of fos-like immune response in the supraoptic nucleus and paraventricular nucleus, while oxytocin (OT) receptor antagonist could inhibit the expression of fos and improve anorexia.72 Studies have shown that cisplatin can affect neuronal γ oscillation, induce abnormal coupling of γ phase amplitude in ARC neurons, and activate the nitrosation stress caused by caspase-1 in neurons, resulting in CIA.73 Overall, chemotherapy drugs such as cisplatin can reduce the activity of feeding-promoting neurons in the hypothalamus or induce abnormal neuronal responses leading to CIA.
It was found that the growth differentiation factor-15 (GDF-15) level in the cycle of cancer patients receiving platinum-based chemotherapy was high. Through the experiments of GDF-15 gene knockout mice and GDF-15 neutralizer treatment, the results showed that anorexia and weight loss caused by platinum-based treatment could be alleviated.10 Japanese scholars have found through experiments that cutting off the vagus nerve of rats can reduce the level of acylated ghrelin in plasma, and cisplatin can further reduce the level of acylated ghrelin and cause anorexia.19,20 Subsequently, the administration of ghrelin in rats can improve the reduction of food intake caused by cisplatin. Peripheral factor ghrelin signaling pathway is the most studied in CIA, and GDF-15 related pathway has gradually entered scholars’ vision.
The use of chemotherapy drugs can promote the production of inflammatory cytokines and lead to CIA. Cisplatin activates histamine H4 receptor (HRH4) to release TNF-α and IL-1β in cells, which promotes the degradation of prepro-orexin (PPO) mRNA and leads to CIA.74,75 At the same time, TNF-α can also induce the ubiquitination of orexin receptor 2 (OXR2), and the expression of OXR2 protein is reduced to reduce food intake.76 Figure 2 summarizes the main pathological mechanisms of CIA.
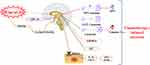 |
Figure 2 The main pathological mechanisms of CIA. |
Clinical Application and Mechanism of LJZD in Prevention and Treatment of CIA
Table 2 summarizes the main indexes of LJZD in the study of CIA. With the wide application of 5-HT3 receptor antagonist and NK-1 receptor antagonist, the adverse reactions such as nausea and vomiting caused by chemotherapy have been effectively controlled. However, the anorexia caused by chemotherapy has not been well controlled. Therefore, it is necessary to find and explore new therapeutic drugs and schemes. Nishida et al27 observed the changes of the ratio of total ghrelin and acylated ghrelin in the study of gastric emptying disorder after gastric cancer surgery. The results showed that LJZD could inhibit the inactivation of acylated ghrelin, improve its plasma concentration and promote the appetite of patients. Huang et al28 randomly divided malignant tumor patients with moderate-to-severe anorexia after systemic chemotherapy into the treatment group and the control group. The treatment group was given Xiangsha Liujunzi Decoction, and the control group was given medroxyprogesterone tablets; Sun et al29 will be advanced non-small cell lung cancer (NSCLC) patients were divided into concurrent chemotherapy with cisplatin-based combined with modified LJZD treatment group and only received cisplatin-based chemotherapy in patients with control group; Yoshiya et al30 randomly divided lung cancer patients into two groups, the treatment group took Rikkunshito, and the control group did not. All patients were given cisplatin on the first day of the experiment and combined with 5HT3, NK1 receptor antagonists and steroids for treatment; Ohnishi et al31 will accept cisplatin and paclitaxel treatment of cervical cancer or cervical cancer patients were randomly divided into LJZD group and control group (simple antiemetic drugs); Ohno et al32 randomly divided patients with gastric cancer into two groups. Group A (n = 5) took LJZD orally from the first course of treatment, and the second course of treatment did not take Rikkunshito tablets. Group B (n = 5) received reverse sequence therapy, and all patients received S-1 combined with cisplatin chemotherapy. In the above clinical studies, after evaluating the oral intake, the grade of anorexia, nausea and vomiting, and the concentration of acylated ghrelin in plasma, it was found that LJZD did not reduce the effective rate of chemotherapy and did not increase the incidence of hematological, hepatorenal toxicity and other side effects, which could effectively prevent cisplatin-induced anorexia, and its efficacy was equivalent to that of medroxyprogesterone acetate tablets. In a prospective, randomized, crossover trial of a lung cancer patient receiving cisplatin chemotherapy and an esophageal cancer patient receiving cisplatin chemotherapy, Rikkunshito increased plasma ghrelin levels and effectively alleviated CIA.18,33
 |
Table 2 The Effect of LJZD on the Main Evaluation in CIA Study |
It has been reported that some TCM have no effect on the pharmacokinetics or anti-tumor effect of chemotherapeutic drugs in animal experiments, indicating that these TCM can be used in combination with chemotherapeutic drugs without reducing the efficacy of chemotherapy. Therefore, in the future, TCM may be more and more used as treatment for side effects of chemotherapy.77 With the clinical application of LJZD more and more widely, the mechanism of its prevention and treatment of CIA is also being studied. Takeda et al19 compared with cisplatin model group, 5-HT2B/2C receptor agonist group and LJZD group found that LJZD can inhibit cisplatin-induced plasma levels of ghrelin and alleviate the loss of appetite in rats. At the same time, the author speculated that flavonoids contained in LJZD may inhibit the activity of 5-HT2B/2C receptor and promote the release of ghrelin, thereby improving cisplatin-induced gastrointestinal dysfunction. Yakabi et al37 found that 5-HT2C receptor antagonist and Rikkunshito can enhance GHS-R1a signal transduction in hypothalamus to inhibit cisplatin-induced CIA by exploring the effect of ghrelin receptor expression in hypothalamus. Sadakane et al38 explored whether Rikkunshito was involved in peripheral ghrelin degradation in CIA rat model, and found that multiple components (such as 10-gingerol) could inhibit ghrelin deacetylase activity, thereby increasing the level of acyl-ghrelin in plasma to alleviate CIA. Yoshimura et al39 established anorexia rat model by cisplatin and treated with LJZD. The changes of regulatory peptides in hypothalamus were measured by in situ hybridization histochemical method. It was found that the treatment group could reduce the levels of POMC, cocaine and amphetamine-regulated transcript (CART) in arcuate nucleus and increase NPY. The results showed that LJZD had a therapeutic effect on cisplatin-induced anorexia. Zenitani et al40 found that Rikkunshito could prevent cisplatin-induced intestinal mucosal injury by increasing intestinal epithelial cell proliferation and significantly reducing ileal cell apoptosis, thereby improving appetite and weight loss in CIA rats. Through metabolomics analysis, Dai et al41 found that inhibiting JAK-STAT signaling pathway, regulating the expression of anorexigenic and orexigenic peptides, and mediating multiple metabolic pathways may be the potential mechanism of LJZD in the treatment of CIA.
Discussion
In summary, TCM has played a favorable role in the treatment of various cancers as alternative therapy. TCM can not only alleviate the symptoms of tumor patients and improve their quality of life but also alleviate the adverse reactions and complications caused by chemotherapy, radiotherapy or targeted therapy.78 Evidence from preclinical studies continues to support the view that TCM is effective in preventing or mitigating side effects caused by chemotherapy. More importantly, some reported TCM have no effect on pharmacokinetics or antitumor activity of chemotherapy agents in animal experiments, suggesting that they can be used in combination with chemotherapy without reducing their efficacy.77 In one word, exploring effective TCM is conducive to the adjuvant treatment of cancer and provides useful information for the development of more effective anti-cancer treatment methods.
LJZD is a famous tonifying formula in TCM, which has been paid more and more attention by researchers because of its alleviation of adverse reactions (such as anorexia) in tumor treatment. LJZD and its modified prescription can treat anorexia by increasing appetite, protecting gastric mucosa and promoting secretion of digestive juice. By sorting out all the literature on the treatment of CA and CIA by LJZD in recent years, it is found that the main therapeutic mechanisms are to activate ghrelin signaling pathway, produce 5-HT2B/2C receptor-like antagonism and protect gastrointestinal mucosal barrier. Figure 3 summarizes the main mechanisms of LJZD in the treatment of CA and CIA. In addition, our laboratory conducted a multivariate chemometrics analysis of LJZD, combined with network pharmacology and molecular docking to construct drug and disease networks, and found that the main bioactive compounds in LJZD (especially ephedrine hydrochloride, hesperidin, ginsenoside rg1 and jujuboside A) may exert anti-inflammatory and anti-oxidative stress effects by interacting with sarcoma (SRC), phosphatidylinositol 3-kinase regulatory subunit alpha (PIK3R1), mitogen-activated protein kinase 1 (MAPK1), protein kinase b (AKT1) and other targets to treat CIA.79
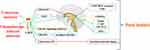 |
Figure 3 The main pathological mechanisms of LJZD in the treatment of CA and CIA. |
Conclusion
However, there are still some imperfections in the study of LJZD. The composition of LJZD is complex, the target is numerous, the mechanism is unclear, and the sample size reported in clinical practice is small so that the persuasion is not strong. In recent years, many scholars have established CA34,35,64,80 and CIA19,39 animal models through various methods and explored their pathogenesis. It has been reported that GDF-15, GLP-1, NPY, HRH1, HRH4, central OT and microbe-gut-brain axis are related to tumor-associated anorexia, which may become a new research direction to explore LJZD in improving anorexia. Taken together, multidisciplinary research is needed to explore the pharmacodynamics and pharmacokinetics of Chinese herbal compound by using the joint analysis of transcriptome, metabolomics, genome and other multi-omics, so as to improve the credibility and repeatability of the research, which is of great significance for the clinical application and mechanism exploration of LJZD.
Abbreviations
AgRP, agouti-related protein; AKT1, protein kinase b; AMPK, adenosine 5‘-monophosphate-activated protein kinase; ARC, arcuate nucleus; CACS, cancer anorexia-cachexia syndrome; CA, cancerous anorexia; CIA, chemotherapy-induced anorexia; CRF, corticotropin-releasing factor; CART, cocaine and amphetamine-regulated transcript; GLP-1, glucagon-like peptide-1; GHS-R1a, growth hormone secretagogue receptor-1a; GDF-15, growth differentiation factor-15; HRH1, histamine H1 receptor; HRH3, histamine H3 receptor; HRH4, histamine H4 receptor; IL-1β, interleukin-1β; IL-1, interleukin-1; IL-6, interleukin-6; IFN-γ, interferon-γ; JAK/STAT, janus kinase/signal transducer and activator of tran-ions; KPS, Karnofsky performance scale; LIF, leukemia inhibitory factor; LJZD, Liujunzi decoction; LCN2, lipocalin-2; MAPK1, mitogen-activated protein kinase 1; NPY, neuropeptide Y; NTS, nucleus tractus solitarius; NSCLC, non-small cell lung cancer; OX-A, orexin-A; OXR2, orexin receptor 2; OT, oxytocin; PIK3R1, phosphatidylinositol 3-kinase regulatory subunit alpha; POMC, proopiomelanocortin; PPO, prepro-orexin; SRC, sarcoma; TCM, traditional Chinese medicine; TCMS, traditional Chinese medical science; TNF-α, tumor necrosis factor-α; 5-HT, 5-hydroxytryptamine; α-MSH, α-melanin stimulating hormone.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China NO.82174143.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Zhang F, Shen A, Jin Y, Qiang W. The management strategies of cancer-associated anorexia: a critical appraisal of systematic reviews. BMC Complement Altern Med. 2018;18(1):236. doi:10.1186/s12906-018-2304-8
3. Olson B, Marks DL, Grossberg AJ. Diverging metabolic programmes and behaviours during states of starvation, protein malnutrition, and cachexia. J Cachexia Sarcopenia Muscle. 2020;11(6):1429–1446. doi:10.1002/jcsm.12630
4. Herremans KM, Riner AN, Cameron ME, et al. The Microbiota and Cancer Cachexia. Int J Mol Sci. 2019;20(24):6267. doi:10.3390/ijms20246267
5. Blauwhoff-Buskermolen S, Ruijgrok C, Ostelo RW, et al. The assessment of anorexia in patients with cancer: cut-off values for the FAACT-A/CS and the VAS for appetite. Support Care Cancer. 2016;24(2):661–666. doi:10.1007/s00520-015-2826-2
6. Ishikawa A, Ohara G, Nakazawa K, et al. Chemotherapy-induced complications in patients with lung cancer: an evaluation by pharmacists. Mol Clin Oncol. 2013;1(1):65–68. doi:10.3892/mco.2012.33
7. Peixoto da Silva S, Santos JMO, Costa E Silva MP, et al. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle. 2020;11(3):619–635. doi:10.1002/jcsm.12528
8. Sang Y, Zhang Y, Liu Y, et al. Study Progress in Pathogenesis of Cancer Cachexia and Prevention and Treatment with Traditional Chinese MedicineJ. Chin J Exp Traditional Med Formulae. 2021;27(01):203–213. doi:10.13422/j.cnki.syfjx.20202425
9. Shiomi Y, Ohira Y, Yoshimura M, et al. Z-505 hydrochloride ameliorates chemotherapy-induced anorexia in rodents via activation of the ghrelin receptor, GHSR1a. Eur J Pharmacol. 2018;818:148–157. doi:10.1016/j.ejphar.2017.10.047
10. Breen DM, Kim H, Bennett D, et al. GDF-15 Neutralization Alleviates Platinum-Based Chemotherapy-Induced Emesis, Anorexia, and Weight Loss in Mice and Nonhuman Primates. Cell Metab. 2020;32(6):938–950.e6. doi:10.1016/j.cmet.2020.10.023
11. Siddiqui JA, Pothuraju R, Jain M, et al. Advances in cancer cachexia: intersection between affected organs, mediators, and pharmacological interventions. Biochim Biophys Acta Rev Cancer. 2020;1873(2):188359. doi:10.1016/j.bbcan.2020.188359
12. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10(2):90–99. doi:10.1038/nrclinonc.2012.209
13. Currow DC, Glare P, Louw S, et al. A randomised, double blind, placebo-controlled trial of megestrol acetate or dexamethasone in treating symptomatic anorexia in people with advanced cancer. Sci Rep. 2021;11(1):2421. doi:10.1038/s41598-021-82120-8
14. Zhou J, Fu L, Luo L, et al. Research Progress in Diagnosis and Treatment of Cancer Cachexia by Chinese Medicine and Western MedicineJ. J Oncol Chin Med. 2019;1(5):
15. Ruiz Garcia V, López-Briz E, Carbonell Sanchis R, et al. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;2013(3):CD004310. doi:10.1002/14651858.CD004310.pub3
16. Sidaway P. Palliative care: anamorelin provides benefit to patients with cachexia. Nat Rev Clin Oncol. 2018;15(2):68. doi:10.1038/nrclinonc.2017.204
17. King L, Tsilioni I, Theoharides TC. Time to look past TNF and thalidomide for cachexia - could mast cells and flavonoids be the answer? J Biol Regul Homeost Agents. 2018;32(3):443–447.
18. Yoshiya T, Mimae T, Ito M, et al. Prospective, randomized, cross-over pilot study of the effects of Rikkunshito, a Japanese traditional herbal medicine, on anorexia and plasma-acylated ghrelin levels in lung cancer patients undergoing cisplatin-based chemotherapy. Invest New Drugs. 2020;38(2):485–492. doi:10.1007/s10637-019-00836-x
19. Takeda H, Sadakane C, Hattori T, et al. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology. 2008;134(7):2004–2013. doi:10.1053/j.gastro.2008.02.078
20. Yamada C, Hattori T, Ohnishi S, et al. Ghrelin Enhancer, the Latest Evidence of Rikkunshito. Front Nutr. 2021;8:761631. doi:10.3389/fnut.2021.761631
21. Kang HJ, Jeong MK, Park SJ, Jun HJ, Yoo HS. Efficacy and safety of Yukgunja-Tang for treating anorexia in patients with cancer: the protocol for a pilot, randomized, controlled trial. Medicine. 2019;98(40):e16950. doi:10.1097/MD.0000000000016950
22. Xie J, Hou E. Effect of thalidomide and Liujunzi decoction combined with TACE in the treatment of advanced liver cancerJ. Contemporary Med Symposium. 2018;16(07):130–131.
23. Zhan Y. Xiangsha Liujunzi Decoction Combined with Ear Points Pressuring Beans and Megestrol Acetate in Treatment of Tumor Patients with Anorexia. Chin Arch Traditional Chin Med. 2016;34(11):2703–2705. doi:10.13193/j.issn.1673-7717.2016.11.041
24. Wang L. Flavored Xiangsha Guishao Liujun Decoction Treatment of Cancer Loss of Appetite Analysis of Clinical Research D [Dissertation]. Nanjing university of traditional chinese medicine; 2017.
25. Sun J. Xiang Sha Liu Jun Zi-Based Clinical Observation of Integrated Treatment of Cancer Patients with Anorexia [Dissertation]. Shandong university of traditional chinese medicine; 2012.
26. Chen L, Zhai C, Xu J, et al. Clinical study of Xiangsha Liujunzi Decoction combined with hawthorn and Jiaoliuqu in the treatment of anorexia in patients with advanced cancer. Heilongjiang J Traditional Chin Med. 2020;49(06):426–427.
27. Xi Y, Gao Q Current situation and research progress of perioperative Chinese application in Japanese digestive tract surgery.
28. Huang J, Lin Q, Chen Y, et al. Clinical study on the curative effect of additive Xiangsha Liujunzi decoction on anorexia from chemotherapy in patients with malignant tumor. Clin J Chine Med. 2015;1(8):64–66.
29. Sun L, Lai H, Chen Z, et al. Modified Liujunzi Decoction () Alleviates Chemotherapy-Induced Anorexia in Advanced Non-Small Cell Lung Cancer: a Propensity Score Matched Case-Control Study. Chin J Integr Med. 2020;26(4):256–262. doi:10.1007/s11655-020-3185-5
30. Yoshiya T, Ito M, Misumi K, et al. The effect of rikkunshito, a traditional Japanese herbal medicine, on food intake and plasma acylated ghrelin levels in lung cancer patients treated with platinum-based chemotherapy. Ann Oncol. 2016;27(06):497–521. doi:10.1093/annonc/mdw390.41
31. Ohnishi S, Watari H, Kanno M, et al. Additive effect of rikkunshito, an herbal medicine, on chemotherapy-induced nausea, vomiting, and anorexia in uterine cervical or corpus cancer patients treated with cisplatin and paclitaxel: results of a randomized Phase II study (JORTC KMP-02). J Gynecol Oncol. 2017;28(5):e44. doi:10.3802/jgo.2017.28.e44
32. Ohno T, Yanai M, Ando H, et al. Rikkunshito, a traditional Japanese medicine, suppresses cisplatin-induced anorexia in humans. Clin Exp Gastroenterol. 2011;4:291–296. doi:10.2147/CEG.S26297
33. Hamai Y, Yoshiya T, Hihara J, et al. Traditional Japanese herbal medicine rikkunshito increases food intake and plasma acylated ghrelin levels in patients with esophageal cancer treated by cisplatin-based chemotherapy. J Thorac Dis. 2019;11(6):2470–2478. doi:10.21037/jtd.2019.05.67
34. Ohbuchi K, Nishiumi S, Fujitsuka N, et al. Rikkunshito Ameliorates Cancer Cachexia Partly through Elevation of Glucarate in Plasma. Evid Based Complement Alternat Med. 2015;2015:871832. doi:10.1155/2015/871832
35. Fujitsuka N, Asakawa A, Uezono Y, et al. Potentiation of ghrelin signaling attenuates cancer anorexia-cachexia and prolongs survival. Transl Psychiatry. 2011;1(7):e23. doi:10.1038/tp.2011.25
36. Terawaki K, Kashiwase Y, Sawada Y, et al. Development of ghrelin resistance in a cancer cachexia rat model using human gastric cancer-derived 85As2 cells and the palliative effects of the Kampo medicine rikkunshito on the model. PLoS One. 2017;12(3):e0173113. doi:10.1371/journal.pone.0173113
37. Yakabi K, Kurosawa S, Tamai M, et al. Rikkunshito and 5-HT2C receptor antagonist improve cisplatin-induced anorexia via hypothalamic ghrelin interaction. Regul Pept. 2010;161(1–3):97–105. doi:10.1016/j.regpep.2010.02.003
38. Sadakane C, Muto S, Nakagawa K, et al. 10-Gingerol, a component of rikkunshito, improves cisplatin-induced anorexia by inhibiting acylated ghrelin degradation. Biochem Biophys Res Commun. 2011;412(3):506–511. doi:10.1016/j.bbrc.2011.08.002
39. Yoshimura M, Matsuura T, Ohkubo J, et al. The gene expression of the hypothalamic feeding-regulating peptides in cisplatin-induced anorexic rats. Peptides. 2013;46:13–19. doi:10.1016/j.peptides.2013.04.019
40. Zenitani M, Sasaki T, Oue T. Kampo medicines Rikkunshito and Hangeshashinto prevent cisplatin-induced intestinal mucosal injury in rats. J Pediatr Surg. 2021;56(7):1211–1218. doi:10.1016/j.jpedsurg.2021.03.033
41. Dai Y, Chen S, Li Y, et al. Liujunzi Decoction ameliorated cisplatin-induced anorexia by inhibiting the JAK-STAT signaling pathway and coordinating anorexigenic and orexigenic neuropeptides in rats. J Ethnopharmacol. 2022;285:114840. doi:10.1016/j.jep.2021.114840
42. Li P, Xin B, Qian W. Advances in TCM Treatment of Cancer Anorexia in Recent 6 Years. Modern Traditional Chin Med. 2017;37(04):107–109. doi:10.13424/j.cnki.mtcm.2017.04.038
43. Wu G. Cancer cachexia: pathogenic mechanisms and therapeutic perspectives. Chin J Practical Surg. 2015;35(01):36–39.
44. Aklan I, Sayar Atasoy N, Yavuz Y, et al. NTS Catecholamine Neurons Mediate Hypoglycemic Hunger via Medial Hypothalamic Feeding Pathways. Cell Metab. 2020;31(2):313–326.e5. doi:10.1016/j.cmet.2019.11.016
45. Petropoulou PI, Mosialou I, Shikhel S, et al. Lipocalin-2 is an anorexigenic signal in primates. Elife. 2020;9:e58949. doi:10.7554/eLife.58949
46. Huisman C, Norgard MA, Levasseur PR, et al. Critical changes in hypothalamic gene networks in response to pancreatic cancer as found by single-cell RNA sequencing. Mol Metab. 2022;58:101441. doi:10.1016/j.molmet.2022.101441
47. Harno E, Gali Ramamoorthy T, Coll AP, et al. POMC: the Physiological Power of Hormone Processing. Physiol Rev. 2018;98(4):2381–2430. doi:10.1152/physrev.00024.2017
48. Dwarkasing JT, Boekschoten MV, Argilès JM, et al. Differences in food intake of tumour-bearing cachectic mice are associated with hypothalamic serotonin signalling. J Cachexia Sarcopenia Muscle. 2015;6(1):84–94. doi:10.1002/jcsm.12008
49. van Norren K, Dwarkasing JT, Witkamp RF. The role of hypothalamic inflammation, the hypothalamic-pituitary-adrenal axis and serotonin in the cancer anorexia-cachexia syndrome. Curr Opin Clin Nutr Metab Care. 2017;20(5):396–401. doi:10.1097/MCO.0000000000000401
50. Molfino A, Iannace A, Colaiacomo MC, et al. Cancer anorexia: hypothalamic activity and its association with inflammation and appetite-regulating peptides in lung cancer. J Cachexia Sarcopenia Muscle. 2017;8(1):40–47. doi:10.1002/jcsm.12156
51. D’Agostino G, Lyons D, Cristiano C, et al. Nucleus of the Solitary Tract Serotonin 5-HT2C Receptors Modulate Food Intake. Cell Metab. 2018;28(4):619–630.e5. doi:10.1016/j.cmet.2018.07.017
52. Grossberg AJ, Scarlett JM, Marks DL. Hypothalamic mechanisms in cachexia. Physiol Behav. 2010;100(5):478–489. doi:10.1016/j.physbeh.2010.03.011
53. Argilés JM, Busquets S, Toledo M, et al. The role of cytokines in cancer cachexia. Curr Opin Support Palliat Care. 2009;3(4):263–268. doi:10.1097/SPC.0b013e3283311d09
54. Paval DR, Patton R, McDonald J, et al. A systematic review examining the relationship between cytokines and cachexia in incurable cancer. J Cachexia Sarcopenia Muscle. 2022. doi:10.1002/jcsm.12912
55. Zwickl H, Zwickl-Traxler E, Pecherstorfer M. Is Neuronal Histamine Signaling Involved in Cancer Cachexia? Implications and Perspectives. Front Oncol. 2019;9:1409. doi:10.3389/fonc.2019.01409
56. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic Drugs: from Receptor-binding Profiles to Metabolic Side Effects. Curr Neuropharmacol. 2018;16(8):1210–1223. doi:10.2174/1570159X15666170630163616
57. Singh R, Bansal Y, Medhi B, et al. Antipsychotics-induced metabolic alterations: recounting the mechanistic insights, therapeutic targets and pharmacological alternatives. Eur J Pharmacol. 2019;844:231–240. doi:10.1016/j.ejphar.2018.12.003
58. Trayhurn P, Bing C. Appetite and energy balance signals from adipocytes. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1237–1249. doi:10.1098/rstb.2006.1859
59. Khatib MN, Gaidhane A, Gaidhane S, et al. Ghrelin as a Promising Therapeutic Option for Cancer Cachexia. Cell Physiol Biochem. 2018;48(5):2172–2188. doi:10.1159/000492559
60. Nakanishi Y, Higuchi J, Honda N, et al. Pharmacological profile and clinical efficacy of anamorelin HCl (ADLUMIZ® Tablets), the first orally available drug for cancer cachexia with ghrelin-like action in Japan. Nihon Yakurigaku Zasshi. 2021;156(6):370–381. doi:10.1254/fpj.21046
61. Yoshimura M, Shiomi Y, Ohira Y, et al. Z-505 hydrochloride, an orally active ghrelin agonist, attenuates the progression of cancer cachexia via anabolic hormones in Colon 26 tumor-bearing mice. Eur J Pharmacol. 2017;811:30–37. doi:10.1016/j.ejphar.2017.05.036
62. Arora GK, Gupta A, Narayanan S, Guo T, Iyengar P, Infante RE. Cachexia-associated adipose loss induced by tumor-secreted leukemia inhibitory factor is counterbalanced by decreased leptin. JCI Insight. 2018;3(14):e121221. doi:10.1172/jci.insight.121221
63. Borner T, Liberini CG, Lutz TA, et al. Brainstem GLP-1 signalling contributes to cancer anorexia-cachexia syndrome in the rat. Neuropharmacology. 2018;131:282–290. doi:10.1016/j.neuropharm.2017.12.024
64. Arora G, Gupta A, Guo T, et al. JAK Inhibitors Suppress Cancer Cachexia-Associated Anorexia and Adipose Wasting in Mice. JCSM Rapid Commun. 2020;3(2):115–128. doi:10.1002/rco2.24
65. Wyatt G, Sikorskii A, Tesnjak I, et al. Chemotherapy interruptions in relation to symptom severity in advanced breast cancer. Support Care Cancer. 2015;23(11):3183–3191. doi:10.1007/s00520-015-2698-5
66. Bosnjak SM, Dimitrijevic J, Djordjevic F. Cancer and chemotherapy-induced nausea and vomiting: a focus on olanzapine. Curr Opin Support Palliat Care. 2016;10(2):180–188. doi:10.1097/SPC.0000000000000206
67. Yavuzsen T, Davis MP, Walsh D, et al. Systematic review of the treatment of cancer-associated anorexia and weight loss. J Clin Oncol. 2005;23(33):8500–8511. doi:10.1200/JCO.2005.01.8010
68. Jordan K, Gralla R, Jahn F, et al. International antiemetic guidelines on chemotherapy induced nausea and vomiting (CINV): content and implementation in daily routine practice. Eur J Pharmacol. 2014;722:197–202. doi:10.1016/j.ejphar.2013.09.073
69. Hershman DL, Lacchetti C, Loprinzi CL. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline Summary. J Oncol Pract. 2014;10(6):e421–e424. doi:10.1200/JOP.2014.001776
70. Goswami C, Dezaki K, Wang L, et al. Ninjin-yoeito activates ghrelin-responsive and unresponsive NPY neurons in the arcuate nucleus and counteracts cisplatin-induced anorexia. Neuropeptides. 2019;75:58–64. doi:10.1016/j.npep.2019.03.001
71. De Jonghe BC, Holland RA, Olivos DR, et al. Hindbrain GLP-1 receptor mediation of cisplatin-induced anorexia and nausea. Physiol Behav. 2016;153:109–114. doi:10.1016/j.physbeh.2015.10.031
72. Arase K, Hashimoto H, Sonoda S, et al. Possible involvement of central oxytocin in cisplatin-induced anorexia in rats. J Physiol Sci. 2018;68(4):471–482. doi:10.1007/s12576-017-0550-z
73. Sun M, Mao XF, Li ZM, et al. Endothelial peroxynitrite causes disturbance of neuronal oscillations by targeting caspase-1 in the arcuate nucleus. Redox Biol. 2021;47:102147. doi:10.1016/j.redox.2021.102147
74. Yamamoto K, Okui R, Yamatodani A. Effects of a histamine H4 receptor antagonist on cisplatin-induced anorexia in mice. Neurosci Lett. 2018;676:103–107. doi:10.1016/j.neulet.2018.04.019
75. Yamamoto K, Okui R, Yamatodani A. Activation of orexinergic and histaminergic pathway involved in therapeutic effect of histamine H4 receptor antagonist against cisplatin-induced anorexia in mice. Naunyn Schmiedebergs Arch Pharmacol. 2019;392(8):925–936. doi:10.1007/s00210-019-01646-x
76. Zhan S, Cai GQ, Zheng A, et al. Tumor necrosis factor-alpha regulates the Hypocretin system via mRNA degradation and ubiquitination. Biochim Biophys Acta. 2011;1812(4):565–571. doi:10.1016/j.bbadis.2010.11.003
77. Chen D, Zhao J, Cong W. Chinese Herbal Medicines Facilitate the Control of Chemotherapy-Induced Side Effects in Colorectal Cancer: progress and Perspective. Front Pharmacol. 2018;9:1442. doi:10.3389/fphar.2018.01442
78. Zhang X, Qiu H, Li C, et al. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci Trends. 2021;15(5):283–298. doi:10.5582/bst.2021.01318
79. Wu X, Dai Y, Nie K. Multievaluation Strategy for Liujunzi Decoction: fingerprint Characterization, Chemometrics Analysis, Network Pharmacology, and Molecular Docking. J Chem. 2022;11:2022. doi:10.1155/2022/9257614
80. Zhou W, Jiang Z, Jiang J, et al. Establishment of a model of cancer cachexia. Chin J Exp Surgery. 2004;1(4):105–106.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
