Back to Journals » Journal of Pain Research » Volume 15
Reported Outcomes in Interdisciplinary Pain Treatment: An Overview of Systematic Reviews and Meta-Analyses of Randomised Controlled Trials
Authors Dong HJ , Gerdle B , Dragioti E
Received 17 February 2022
Accepted for publication 10 June 2022
Published 30 August 2022 Volume 2022:15 Pages 2557—2576
DOI https://doi.org/10.2147/JPR.S362913
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Dawood Sayed
Huan-Ji Dong, Björn Gerdle, Elena Dragioti
Pain and Rehabilitation Centre, Department of Health, Medicine and Caring Sciences, Linköping University, Linköping, Sweden
Correspondence: Huan-Ji Dong, Pain and Rehabilitation Centre, Department of Health, Medicine and Caring Sciences, Linköping University, Linköping, Sweden, Email [email protected]
Background: There is considerable diversity of outcome selections and methodologies for handling the multiple outcomes across all systematic reviews (SRs) of Interdisciplinary Pain Treatment (IPT) due to the complexity. This diversity presents difficulties for healthcare decision makers. Better recommendations about how to select outcomes in SRs (with or without meta-analysis) are needed to explicitly demonstrate the effectiveness of IPT.
Objective: This overview systematically collates the reported outcomes and measurements of IPT across published SRs and identifies the methodological characteristics. Additionally, we provide some suggestions on framing the selection of outcomes and on conducting SRs of IPT.
Methods: Three electronic databases (PubMed, Cochrane Library, and Epistemonikos) and the PROSPERO registry for ongoing SR were supplemented with hand-searching ending on 30 September 2021.
Results: We included 18 SRs with data on 49007 people from 356 primary randomised controlled trials (RCTs); eight were followed by meta-analysis and ten used narrative syntheses of data. For all the SRs, pain was the most common reported outcome (72%), followed by disability/functional status (61%) and working status (61%). Psychological well-being and quality of life were also reported in half of the included SR (50%). The core outcome domains according to VAPAIN, IMMPACT, and PROMIS were seldom met. The methodological quality varied from critically low to moderate according to AMSTAR2. The AMSTAR2 rating was negatively correlated to the number of outcome domains in PROMIS, and VAPAIN was positively correlated with IMMPACT and PROMIS, indicating the intercorrelations between the reported outcomes.
Conclusion: This systematic overview showed wide-ranging disparity in reported outcomes and applied outcome domains in SRs evaluating IPT interventions for chronic pain conditions. The intercorrelations between the reported outcomes should be appropriately handled in future research. Some approaches are discussed as well.
Keywords: interdisciplinary pain treatment, interdisciplinary pain rehabilitation program, multimodal rehabilitation, multidisciplinary rehabilitation, biopsychosocial pain rehabilitation, pain management program, chronic pain, outcome domains, intercorrelation
Introduction
Description of the Patient Populations
According to the International Association for the Study of Pain (IASP), chronic pain is pain that persists or recurs for more than 3 months.1 Significant emotional distress and/or functional disability are well-known characteristics of chronic pain. The prevalence of chronic non-cancer pain is estimated to be between 8.7% and 64.4% across diverse populations around the world, depending on the assessments method used.2 In Europe, about 19% adults suffer from chronic pain conditions with moderate-severe pain intensity.3 In the US, about 8% of adults have chronic pain that cause limitations in normal life activities such as work, social, recreational, and self-care activities.4 When assessing the patient with chronic pain, it is important to determine the activated pain mechanisms, ie, if the pain is nociceptive (injury to non-neural tissues producing noxious stimulus), neuropathic (injury to the somatosensory nervous system), nociplastic (altered nociception despite no clear evidence of tissue damage) or combinations of these.5 Such characterisations are important steps towards mechanism-based management and treatments of chronic pain conditions.
Description of Interdisciplinary Pain Treatment (IPT)
Interdisciplinary pain treatment (IPT), based on the IASP, is a multimodal treatment provided by a multidisciplinary team collaborating in assessment and treatment using a shared bio-psycho-social (BPS) model and goals.6 This model suggests that chronic pain is based on a foundation of neurobiology (partially unknown) influenced by and interacting with biological, psychological, and social/contextual factors.7–11 The BPS framework is applied in modern clinical management of pain to better evaluate pain and its negative consequences for patients,12,13 including physical functioning,11 psychological well-being,10 and quality of life.14 Apart from medical treatment, IPT usually contains two or more well-synchronised treatment components such as physical, occupational, psychological, social, and educational components over a specified period (several weeks). Unlike pharmacological treatments or unimodal interventions, IPT is a complex intervention targeting the whole patient, including behaviours.15
Besides IPT, other common names used in the literature are Interdisciplinary pain rehabilitation program (IPRP), multimodal rehabilitation, multidisciplinary rehabilitation, biopsychosocial pain rehabilitation, and pain management program.
How IPT Works
IPT is generally offered when unimodal interventions (eg, pharmacological treatment, surgery, and rehabilitation with a unimodal approach) have not been associated with important improvements or when the patient with chronic pain has a complex condition, for example, with respect to comorbidities (eg, depressive and anxiety symptoms) and/or prolonged sick leave and difficulties with return to work. The BPS framework captures the total complexity of the pain condition and provides clues for reasonable interventions, including IPT. Hence, IPT targets the complexity in several components simultaneously and is well-coordinated (pain intensity, depressive symptoms, perceived health, and return to work) over time. For years, several systematic reviews (SRs) have reported that IPT is more effective compared with single-treatment or usual care.16–18 However, how this is achieved in detail and the importance of duration or other dosage aspects are not known.19
Why It is Important to Do This Review
True measures of healthcare quality are the outcomes that matter to patients.20 By using patient‐reported outcomes (PROMs), we can measure the quality of IPT and identify possible ways to improve IPT.21 Pain is a personal experience and patients with chronic pain conditions often describe wide consequences: significant pain intensity, psychological distress, insomnia, reduced work ability and sick-leave, ill-health, worse physical functioning and low quality of life.22–27 The large variation of the study procedures and published outcomes makes comparing RCTs difficult.28 Without standardised outcome measures, interpretation and implementation of evidence are challenging. To improve the choice of outcome measures to assess the effectiveness of IPT, several core outcomes sets (COSs) have been developed. In this paper, we used three relatively established guidelines for reporting outcomes: 1) Validation and application of a core set of patient-relevant outcome domains (VAPAIN);29 2) Initiative on methods, measurements, and pain assessments in clinical trials (IMMPACT);30 and 3) Patient-reported outcomes measurement information system (PROMIS).31
IPT is a complex intervention. The published systematic reviews (SRs) that explicitly select IPT outcomes have not been well studied and documented.32 Therefore, the evaluation of complex interventions such as IPT are ambiguous and different definitions of a positive outcome of an IPT trial have been presented.33 On the other hand, meta-research studies (ie, research on research) have increased rapidly to evaluate and improve research methods and reporting practices.34 To date, only one SR of randomised controlled trials (RCTs) has been used to investigate reported outcomes of an IPT,28 and no study has determined which reported outcomes are used in SRs of IPT. To fill this knowledge gap, we aim to provide a meticulous overview of the IPT outcomes reported in SRs with or without meta-analysis to evaluate the quality of IPT reported outcomes. In addition, we provide an overview of methodological aspects related to SRs of IPT to initiate a discussion of how to develop new methods of conducting and reporting SRs with complex interventions.
Methods
Study Design
We conducted a systematic overview of reviews35 following a standardised methodological approach in line with a published protocol (International Registered Report Identifier: PRR1-10.2196/17795).32 We also reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) statement, including the PRISMA 2020 for Abstracts checklist36 (see PRISMA Tables S1 and S2 in the Supplementary materials).
Search Strategy
We undertook a comprehensive literature search using PubMed, Cochrane Database of Systematic Reviews, and Epistemonikos from the earliest record through 30 September 2021. A search strategy was created using a combination of the following terms adjusted per database if needed: chronic pain, neuropathic pain, chronic persistent pain, pain rehabilitation, pain therapy, multidisciplinary, interdisciplinary, multimodal, multidisciplinary biopsychosocial rehabilitation, combined modality therapy, and patient care team. Hence, with respect to selection of pain conditions, we included nociceptive, neuropathic and nociplastic chronic pain conditions. We also used a sensitive filter created to retrieve only SRs citations in PubMed.37 The full search strategy is outlined in Box S1 in the Supplementary material. The search strategy was limited by study design, but no restrictions were made on setting or context of the included articles or publication date. We also screened the reference lists of relevant records for additional articles and searched the International Prospective Register of Systematic Reviews (PROSPERO) for relevant ongoing reviews (Table S3 in the Supplementary material). Two independent reviewers (ED and H-JD) searched the databases, evaluated the titles and abstracts of each identified record, examined the full text eligibility, and assessed whether a potentially eligible article fulfilled our inclusion criteria. Any disagreement was solved by discussion between the two reviewers or, if required, a third reviewer (BG) was consulted until agreement was reached.
Study Selection
We included SRs with or without meta-analysis assessing the effectiveness of IPT strategies for chronic pain. SRs that assessed IPT as a part of other interventions were included if separate results and outcomes for IPT were given. We also included other types of evidence synthesis that have been published and fulfilled our following inclusion criteria – ie, network meta-analyses or living SRs, individual patient data meta-analysis, and health agency reviews.
To be eligible, SRs needed to accomplish the following: 1) present a definition of IPT throughout the full text along with description of components involved in the intervention; 2) include adult male or female participants or both (at least 75% of the participants must be ≥18 years of age) with chronic/persistent nociceptive, neuropathic and/or nociplastic pain condition (ie, pain lasting at least 3 months) as described in our protocol;32 3) include only RCTs published in English or Swedish; and 4) compare IPT with treatment as usual, wait list, no treatment, or an alternative active intervention including comparisons between two different types of IPT.
Although there is some overlap, SRs that assessed “back school interventions” or work-hardening programmes were excluded because they are not considered identical treatment to IPT.38 We further excluded SRs if they had any of the following characteristics: 1) included patients with a diagnosis of chronic pain due to cancer, infection, inflammatory arthropathy, osteoporosis, fracture, pregnancy, rheumatoid arthritis, or other rheumatic pain;32 2) included patients with subacute or acute pain (ie, pain less than 3 months); 3) included study designs other than RCTs (eg, clinical trials without randomization, single study designs, and cohort studies); 4) included secondary research studies as the unit of analysis (ie, overview of reviews, umbrella reviews, scoping reviews, summary of systematic reviews, or mixed methods review); and 5) published in languages other than English or Swedish. In cases of duplicate publications (ie, the same SR from the same research group in a different journal) (see38,39), we kept the most comprehensive and detailed version of the article. However, updated versions of the same SRs were considered for inclusion (see38,40).
Data Extraction
Endnote software (X9 version) was used to collect the results of the searches and to remove duplicates. Two independent reviewers (ED and H-JD) performed data extraction using pre-defined excel forms. The list of variables of interest included the following: PMID/DOI of the included SR, first author, publication year, chronic pain conditions, control/comparison arms, number of RCTs of IPTs included in the SR, outcomes investigated (primary and secondary if such categorization exists), and total number of participants. Furthermore, we abstracted data in terms of the duration of the treatment (weeks and hours), treatment components, setting, and follow-up length. Finally, we recorded any method, strategy, considerations, or discussion regarding how the authors chose which outcomes to study and which methods to use to evaluate the evidence (eg, the GRADE approach) as well as methodological aspects such as reporting of funding, methods for risk of bias appraisal, synthesis of results, and details of meta-analytic processes if meta-analysis were conducted.
Any discrepancy was solved by discussion between the two reviewers. Data extraction on characteristics of included studies and reported outcomes of ITP was double checked by a third reviewer (BG).
Quality Assessment of Included Reviews
The quality of the included SRs was rated by means of the AMSTAR 2 tool (A Measurement Tool to Assess Systematic Reviews version 2)41 and by two independent reviewers (ED and H-JD). Any discrepancy between two reviewers for individual AMSTAR-2 questions was discussed until agreement was reached. AMSTAR-2 comprises 16 items and four options are provided to answer the questions of items: “Yes”, “Partial Yes”, “No” or “No meta-analysis conducted”. Seven critical items involve protocol content and registration, comprehensive literature search, providing reasons for excluding studies, assessment of RoB for individual studies, appropriate synthesis of results (meta-analytic methods), consideration of RoB in results and impact of publication bias. AMSTAR 2 categorises the quality of systematic reviews into four domains – high quality, moderate quality, low quality, or critically low quality – based on 16 items and sets a very high threshold for a high overall rating.42
Data Synthesis
We performed descriptive statistics to present our results using median and interquartile range for quantitative variables and absolute and relative frequencies for qualitative variables. We also used a narrative synthesis approach employing tabular presentations stratified by the AMSTAR2 assessment per SR. For conceptual classification, outcome domains were organised using three established guidelines of reporting outcomes: 1) VAPAIN statement for IPTs including eight core outcome domains;29 2) IMMPACT statement including six core outcome domains;30 and 3) PROMIS recommendations including three core outcome domains.31 Each domain per statement was scored as positive (Y) or negative (N). The total score was computed by counting the number of outcome domains scored as positive. For AMSTAR2, we rated critically low quality as one, low quality as two, moderate quality as three, and high quality as four. Lastly, we used the spearman rho correlation coefficient (0.00–0.19: very weak; 0.20–0.39: weak; 0.40–0.59: moderate; 0.60–0.79: strong; ≥0.80: very strong) to determine the relationship between the AMSTAR2 rating and the other variables such as number of outcome domains in VAPAIN, IMMPACT, or PROMIS, total number of outcomes, total sample size, number of included studies, and year of publication. All analyses were conducted in STATA, version 17.0 (StataCorp LLC) and R software, version 4.1.2.
Differences Between Protocol and Review
We made small changes in terms of the inclusion criteria between this version and the published protocol.32 This involved clarifying that other types of SRs as described in study selection were considered for inclusion and excluding the criterion of the detailed description of professionals involved in the intervention as only a trivial number of the included SRs provided this information in detail. We also updated our search up to 30 September 2021, including PROSPERO database.
Results
Search Results
Figure 1 illustrates the PRISMA flow of the selection process. After deduplication, the initial electronic database search identified 3253 potentially eligible systematic reviews. After screening records by title and abstract, we considered 145 potentially eligible reviews for inclusion and retrieved full-text articles. Grounds for exclusion after full-text assessment are illustrated in Table S3 in the Supplementary material. We also identified six ongoing reviews on PROSPERO; three were completed and published,43–45 but only one45 met our inclusion criteria and therefore was included in the final set (see Table S2 in the Supplementary material for details). Finally, 18 published articles met the inclusion criteria and were included in this review.16–18,38,40,45–57
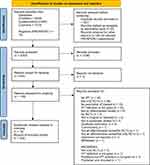 |
Figure 1 PRISMA flow chart. Notes: Adapted from: Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71.36 Creative Commons Attribution (CC BY 4.0) license (https://creativecommons.org/licenses/by/4.0/legalcode). |
Characteristics of Included IPT Systematic Reviews
The 18 selected SRs included a total of 356 primary RCTs and a total of 49006 participants. Eight of the included SRs were quantitative syntheses followed by meta-analysis and ten used narrative syntheses of data. Most of the SRs focused largely or completely on chronic low back pain and three on fibromyalgia. The remaining focused on chronic non-cancer pain in general. No SR eligible for our overview focused specifically on neuropathic pain. The proportion of female participants ranged between 21% and 100%. The median number of the total sample size per SR was 2212 (inter-quartile range [IQR] = 1334 to 4925, range 600 to 6858) and the median number of included primary RCTs was 15.5 (IQR = 9 to 27, range 2 to 46). A comprehensive description of each SR is provided in Tables 1 and 2. The methodological quality varied from critically low to moderate according to AMSTAR2 (see Table S4 in the Supplementary material for details).
 |
Table 1 Characteristics of Systematic Reviews with Meta-Analysis Stratified by AMSTAR2 Evaluation |
 |  |  |
Table 2 Characteristics of Systematic Reviews with Narrative Synthesis Stratified by AMSTAR2 Evaluation |
The duration of the IPT treatment of the RCTs evaluated varied considerably across SRs (ranged from 1 week to 24 weeks) and only two SRs38,40 clearly reported the hours of treatment. All but three SRs45,47,57 included RCTs that compared an IPT with both active and passive comparison arms (eg, treatment as usual (TAU), waiting list control (WLC), no intervention, No-IPT interventions, and different types of IPTs). Details of treatment components and professionals involved were rarely reported in SRs. Only six16,38,40,49,55,58 gave details about population settings for the primary RCTs (eg, outpatients or inpatients, and primary care or specialist care).
Characteristics of Reported Outcomes in IPT Systematic Reviews
In all included SRs, the reported outcomes were assessed by validated self-report instruments with few exceptions for work status outcomes, which used data from labour markets, social security systems, or unemployment rates. Only four SRs (three SRs with meta-analysis and one without)16,18,38,48 clearly stated primary and secondary outcomes. In one SR,38 the authors specified only measures collected at long-term follow-up (12 months or more) were considered the primary outcomes (Tables 1 and 2). The median number of reported outcomes per SR was 5 (IQR = 3 to 7, range 1 to 14). The most common reported outcomes were pain (72%), disability or functional status (61%), work status (61%), psychological or emotional strain and cognitive function (50%), and quality of life (50%). However, only seven SRs provided a specific strategy for selecting outcomes (39%).18,38,45–47,51,57 Notably, only two SRs18,47 (11%) assessed outcome domains using established guidelines (OMERACT-10 and IMMACT) of reporting outcomes (Tables 1 and 2).
Reported Outcomes Domains Measured by VAPAIN, IMMPACT, and PROMIS Guidelines in IPT Systematic Reviews
According to VAPAIN, no SR assessed a combination of all eight core outcome domains (Table 3). The median number of reported outcome domains per SR was 3.5 (IQR = 1 to 5, range 1 to 7). Physical activity (14/18 SRs, 78%), pain intensity (12/18 SRs, 67%), productivity (11/18 SRs, 61%), emotional well-being (10/18 SRs, 55%), and health-related quality of life (9/18 SRs, 50%) were most frequently reported. The less commonly reported outcome domains were satisfaction with social roles and the patient’s activities and perceptions of treatment goal achievement (both 4/18 SRs, 22%).
 |
Table 3 Outcome Domains Assessed by VAPAIN Statement in Included Systematic Reviews |
Only two SRs18,48 assessed a combination of all six core outcome domains according to IMMPACT (Table 4). The median number of reported outcome domains per SR was 2.5 (IQR = 1 to 3, range 1 to 6). Physical functioning (15/18 SRs, 83%), pain (12/18 SRs, 67%), and emotional functioning (11/18 SRs, 61%) were most frequently reported. The less commonly reported outcome domains were symptoms and adverse events (5/18 SRs, 28%) and participant disposition (2/18 SRs, 11%).
 |
Table 4 Outcome Domains Assessed by IMMPACT and PROMIS Statements in Included Systematic Reviews |
According to PROMIS, six SRs16–18,48,56,57 assessed a combination of all three core outcome domains (Table 4). The median number of reported outcome domains per SR was 2 (IQR = 1 to 3, range 1 to 3). Physical health was reported in 15/18 SRs (83%), social health in 11/18 SRs (61%), and mental health in 10/18 SRs (55%).
Univariate Spearman rho analysis (Figure 2) showed a negative correlation between AMSTAR2 rating and PROMIS score (weak) as well as AMSTAR2 rating and total number of outcomes (moderate). VAPAIN score was positively correlated with IMMPACT score and PROMIS score (very strong). The scores for IMMPACT, VAPAIN, and PROMIS had negative correlations with year of publication (strong and moderate). Total sample size was positively correlated with number of included studies (very strong).
Methodological Characteristics of Included IPT Systematic Reviews
None of the SRs assessed IPT as a complex intervention. However, 11 SRs reported the quality of the evidence with an appropriate guidance and comprehensive methods.16–18,38,40,45,46,48,49,51–53 Likewise, two other SRs16,55 used simple methods (ie, “the higher effectiveness had to be demonstrated in at least two out of the five primary outcomes, or at least in one of the primary and two of the secondary outcomes” and “effectiveness was judged to be robust if more than 50% of the high-quality studies showed effectiveness on the outcome studied” (Tables 1 and 2).
Authors in three SRs50,53,56 did not report any potential sources of conflict of interest, including any funding they received for conducting their review. Fifteen SRs16–18,38,40,45–52,54,55 (83%) assessed the methodological quality of included studies, but less than half clearly stated the tool used and none used the risk of bias of included RCTs as an inclusion criterion. The most common checklists were Cochrane Risk of Bias Tool and van Tulder-11 criteria. The random effect models with Der Simonian and Laird variance estimator were mainly used as statistical synthesis of data in the eight SRs38,45–50,58 with meta-analysis. All the models used the I2 Index as an indicator of heterogeneity. Subgroup or sensitivity analysis was done by six meta-analytic SRs,38,45–47,49,50 but meta-regression analysis was done using only one meta-analytic SR.46
Discussion
Summary of Main Results
This systematic overview showed wide-ranging disparity in reported outcomes and applied outcome domains in SRs evaluating IPT interventions for chronic non-cancer pain conditions. This finding was based on the 18 published SRs with data on more than 49000 people in 356 primary RCTs. Most RCTs reported nociplastic pain (eg, fibromyalgia, low back pain), and no SR focused specifically on neuropathic pain. The duration of IPT varied across the SRs. Primary or secondary outcomes and the rationale of selecting outcome domains were not always stated. Compared to IMMPACT or VAPAIN guidelines, more SRs followed PROMIS to assess outcome domains. However, by counting the number of reported outcome domains according to the three guidelines, we found a positive correlation of VAPAIN with IMMPACT and PROMIS, indicating an intercorrelation between the outcome domains included per standardised statement/guideline. A variety of methodological characteristics of SRs are also notable, which affects the quality of the reporting evidence.
Common Reported Outcomes in SRs Included IPT
Apart from pain as the most common reported outcome (72%) in all included SRs, disability or functional status (61%) and working status (61%) were also frequently evaluated as outcomes after IPT. Only half of the SRs reported psychological well-being and quality of life. The results reflect the complexity of chronic pain in a biopsychosocial context, and the outcomes after IPT are supposed to include a variety of physical, mental, and social aspects. However, it is uncertain which outcomes are prioritised. For example, whether pain intensity should be included as a major outcome of IPT is often debatable.17,59–62 In addition, SRs did not consider outcomes of adverse events or harms. Lack of specificity on such outcome domains may also bias SR conclusions.63
Reporting many outcomes in SRs did not increase the quality of the SRs as a negative correlation was found between AMSTAR2 rating and total number of outcomes. Based on these results, we believe the guidelines for complex intervention for conducting a SR on pain should be re-evaluated. Recently, a new PRISMA extension, known as PRISMA-CI statement and checklist, has been developed to address the important reporting gaps relative to complex interventions in healthcare.64
Why is It Difficult to Report Standardised Outcomes of IPT?
We need to be aware that the evaluation of complex interventions such as IPT is not clear cut and definitions of a positive outcome of a IPT trial vary in different publications.33 Although VAPAIN, IMMPACT, and PROMIS are established recommendations for assessing IPT outcomes, few SRs applied them to categorise their outcome domains. A main consequence might be a decreased quality of SRs since a negative correlation between AMSTAR2 and PROMIS was noted. A possible reason of not using these guidelines can be overlooking of the complexity of IPT. In this systematic overview, we found no SR clearly presented the complexity of IPT and considered the intercorrelations between the selected outcomes. For example, outcome measures evaluated separately in one SR38 may be problematic as the outcomes are most likely to be intercorrelated.15
Methodological Characteristics Affect the Quality of the Evidence
The approach GRADE for evidence ratings was used in many but not all included SRs. However, since IPT is a complex intervention with high levels of heterogeneity and indirectness assessment, using only GRADE may not adequately describe the evidence base.65,66 The latter is supported by Movsisyan and colleges;67 they found that the outcomes of complex interventions were more likely to be rated as “very low” quality of evidence compared with those of simple interventions (37.5% vs 9.1% for the primary benefit outcomes). New methods for grading the evidence such as threshold analysis in guideline development have been recently used.68 We also found diversities in methodological quality assessments of included RCTs and even more often did not state the tool they used in assessment as well as did not use risk of bias of RCTs as an inclusion criterion. With respect to included meta-analytic SRs, we found that subgroup or sensitivity analysis and meta-regression analysis were seldom conducted. Such methodological shortcomings may inflate the quality of the reported evidence.69,70
Our Suggestions on Handling the Multi-Correlated Outcomes of IPT in SRs
First, clear definitions of positive outcomes from IPT need to be presented in each SR. RCTs’ different definitions of positive outcomes from IPT should be addressed before an SR is conducted. For example, some SRs treated an outcome of a RCT as a positive outcome when a majority of outcomes were significantly better than for the control intervention.17,18 In another SR, the authors predetermined primary and secondary outcomes and what was necessary to classify an intervention as positive before reviewing the RCTs.16 The recommended guidelines may help categorise the variables into separate domains and minimise the risk of multivariate correlations. Furthermore, advanced graphical approaches such as harvest plots or bubble plots71 can be useful tools for illustrating and synthesising the matrix of the negative effects, no effects, and positive effects across RCTs in a SR with complex interventions.72
Second, intercorrelations between reported outcomes for different RCTs should be considered. One approach is to use the suggested outcome guidelines along with preliminary responder criteria based on several variables per outcome. For example, the evaluation of IPTs for patients with pain in fibromyalgia may be incorporated using both OMERACT core domains and preliminary responder criteria that best favour IPT over control.47,73,74 Another approach is to use appropriate statistical methods to handle the intercorrelations. Multivariate methods that are can handle intercorrelated outcomes in one analysis have been presented.75,76 We have recently suggested how simultaneous goals can be handled using scores from principal component analysis (PCA) in RCTs and observational studies.15 Other methods can obtain non-dependent effect sizes and include rationales for selecting effect sizes such as a random selection of effect sizes or averaging them to minimise their interrelations.76 For example, when the measurement of pain is evaluated with two or more instruments (eg, VAS and NRS), then it may be more feasible to average the effect size of both instruments into one effect size before meta-analysis of the data.
Third, rather than conducting separate univariate meta-analysis, researchers may consider applying multivariate meta-analysis (MVMA) to provide a framework to address multi-correlated outcomes from IPT.77,78 MVMA can simultaneously analyse multiple outcomes of interest and the summary result for each outcome depends on correlated results from related outcomes. However, implementing such an approach requires information about the correlations between the effect sizes, which is rarely reported in original RCTs, as well as knowledge of advance statistical programs like R software.76,79 Other meta-analytic strategies include multilevel hierarchical modelling which can show the variation in the true effect sizes across studies or multivariate meta regression which can allow intervention characteristics and mediating effects of intermediate outcomes to be examined together.70,76 Finally, mixed treatment comparison meta-analysis methods can also be useful for exploring the different components and combinations of components of IPT as such an approach can examine the effectiveness of a particular component (or combination of components).80
Strengths and Limitations
Most importantly, to our knowledge, this is the first overview that addresses disparity in reported outcomes and applies outcome domains in published SRs when evaluating IPT for chronic pain conditions. Using the latest AMSTAR2 checklist, we were able to evaluate the evidence quality of all the included SRs. Despite the complexity of IPT, we mapped, categorised, and compared the reported outcomes according to the VAPAIN statement, IMMPACT, and PROMIS recommendations to evaluate the current evidence of pain rehabilitation processes. This review was designed by experienced reviewers and researchers in this field, guided by a protocol, and carefully stated the difference between the protocol and this review.
This systematic overview has several limitations. First, selection bias cannot be avoided. SRs of RCTs may be associated with risk for bias resulting from an unrepresentative selection of patients and researcher allegiance.81 Other study designs, for example, SRs with registry cohorts and observational studies, were not included. Thus, real-world data are lacking. Second, in addition to the varied components of IPT, IPT had great variability in terms of duration of treatment and time of outcome assessment. Whether the reported outcomes from RCTs were related to these factors is not reflected in the present work. Third, the search strategy was limited to three electronic databases and SRs published in English or Swedish. There were no searches of the grey literature, so we might have missed some eligible reviews. However, we searched PROSPERO to identify any ongoing SRs on the topic. One may also argue that our investigation is limited to a small number of included SRs and therefore we may have not captured the entire field. However, we adopted very strict inclusion criteria related to the topic to assess the cutting edge evidence. Finally, the study population considered, ie, different pain conditions and the definition adopted in the SRs for IPT might have influenced the outcomes reported. Given these limitations, future research is needed to improve the framing the selection of research outcomes of IPT.
Implications for Future Research
To facilitate evidence synthesis and assessment in complex treatments for chronic pain in every day clinical practice, we need to find a proper way to frame the selection of the research on IPT outcomes. The currently established guidelines for assessing IPT outcomes (VAPAIN, IMMPACT, and PROMIS) are important for categorising outcome domains. Moreover, researchers who conduct SRs and meta-analyses are supposed to appropriately handle the intercorrelations between outcomes and the interactions between components of complex interventions.71,72
There is also a need to develop clinically accepted ways to apply the established guidelines for reporting outcome domains of IPT. A lack of consensus of evaluating reported outcomes of IPT in SRs makes it difficult to decide whether the evidence from SRs should be applied in clinical practice. A lack of consensus may also confuse health-care providers and policy makers when evaluating the need of tailoring current IPT. Clinical implications will suggest how to report outcomes that capture the information from real-world data to assess the effects of IPT.
Conclusion
Currently, there is a wide-ranging disparity in reported outcomes and applied outcome domains in SRs evaluating IPT for chronic pain conditions. According to the definition of complex interventions, IPT has two common characteristics: it has intercorrelated outcomes or mediators and moderators of effect (outcome complexity) and it has multiple components (intervention complexity).82 Given this, we present a menu of SR procedures for addressing sources of complexity when answering questions about the effects of IPT in research synthesis:
- The SRs of IPT should follow the PRISMA-CI extension.
- The inclusion criteria should clearly identify and describe the outcome domains and provide guidance for handling different outcome measurements on the same domain.
- The selection of outcomes should follow the established guidelines such as VAPAIN.
- The scope of included outcomes should address both effectiveness and harms on which strength of the evidence will be graded.
- Quantitative graphical synthesis approaches such as harvest or bubble plots should be adopted to illustrate patterns in results of multiple outcomes. They can also be used as an alternative method of vote counting in SRs when meta-analysis is not feasible.
- Subgroup analysis and meta-regression should be performed to examine how features of the interventions impact effect size. Mixed treatment comparison meta-analysis may also be useful when examining the intervention complexity and the effects of the different intervention components.
- More advanced meta-analytical methods are required that will encourage the exploration and handling of the intercorrelation of the outcomes.76
- There is a clear need for the development and implementation of new methods of grading evidence of IPT and, considering proper framing of the questions, judgements about directness and consistency of evidence and the need for additional contextual and qualitative evidence are needed that provide information about the circumstances when the intervention works best.66
- We believe that our work is the first step to further test our suggestions in future evidence synthesis.
Abbreviations
IPT, interdisciplinary pain treatment; SRs, systematic reviews; AMSTAR2, Assessment of systematic Reviews version 2; VAPAIN, Validation and application of a core set of patient-relevant outcome domains; IMMPACT, Initiative on methods, measurements, and pain assessments in clinical trials; PROMIS, Patient-reported outcomes measurement information system.
Acknowledgments
This study was supported by grants from County Council of Östergötland (SC-2021, to Huan-Ji Dong), Sweden. The content is solely the responsibility of the authors and does not necessarily represent the official views of County Council of Östergötland.
Disclosure
Björn Gerdle reports grants from Vetenskapsrådet (Swedish research council), outside the submitted work. The authors report no other potential conflicts of interest in relation to this work.
References
1. Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the International Classification of Diseases (ICD-11). Pain. 2019;160(1):19–27. doi:10.1097/j.pain.0000000000001384
2. Steingrimsdottir OA, Landmark T, Macfarlane GJ, Nielsen CS. Defining chronic pain in epidemiological studies: a systematic review and meta-analysis. Pain. 2017;158(11):2092–2107. doi:10.1097/j.pain.0000000000001009
3. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. doi:10.1016/j.ejpain.2005.06.009
4. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–1006. doi:10.15585/mmwr.mm6736a2
5. Kosek E, Cohen M, Baron R, et al. Do we need a third mechanistic descriptor for chronic pain states? Pain. 2016;157(7):1382–1386. doi:10.1097/j.pain.0000000000000507
6. IASP. Task force on multimodal pain treatment defines terms for chronic pain care. The International Association for the Study of Pain (IASP); 2021. Available from: https://www.iasp-pain.org/publications/iaspnews/?ItemNumber=6981.
7. Linton S, Bergbom S. Understanding the link between depression and pain. Scand J Pain. 2011;2(2):47–54. doi:10.1016/j.sjpain.2011.01.005
8. Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120(11):3779–3787. doi:10.1172/JCI43766
9. Gatchel R, Peng Y, Peters M, Fuchs P, Turk D. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133(4):581–624. doi:10.1037/0033-2909.133.4.581
10. Meints SM, Edwards RR. Evaluating psychosocial contributions to chronic pain outcomes. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt B):168–182. doi:10.1016/j.pnpbp.2018.01.017
11. Varallo G, Giusti EM, Scarpina F, Cattivelli R, Capodaglio P, Castelnuovo G. The association of Kinesiophobia and pain catastrophizing with pain-related disability and pain intensity in obesity and chronic lower-back pain. Brain Sci. 2021;11(1):684. doi:10.3390/brainsci11060684
12. WHO. International Classification of Functioning, Disability and Health (ICF). The World Health Organization (WHO); 2001.
13. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi:10.1016/j.pain.2004.09.012
14. Hadi MA, McHugh GA, Closs SJ. Impact of chronic pain on patients’ quality of life: a comparative mixed-methods study. J Patient Exp. 2018;6(2):133–141. doi:10.1177/2374373518786013
15. Ringqvist Å, Dragioti E, Björk M, Larsson B, Gerdle B. Moderate and stable pain reductions as a result of interdisciplinary pain rehabilitation-A cohort study from the Swedish Quality Registry for Pain Rehabilitation (SQRP). J Clin Med. 2019;8(6):905. doi:10.3390/jcm8060905
16. Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology. 2008;47(5):670–678. doi:10.1093/rheumatology/ken021
17. Swedish Council on Health Technology A. SBU systematic review summaries. In: Methods of Treating Chronic Pain: A Systematic Review. Swedish Council on Health Technology Assessment (SBU); 2006.
18. Swedish Council on Health Technology A. SBU systematic review summaries. In: Rehabilitation of Patients with Chronic Pain Conditions: A Systematic Review. Swedish Council on Health Technology Assessment (SBU); 2010.
19. Dragioti E, Björk M, Larsson B, Gerdle B. A meta-epidemiological appraisal of the effects of interdisciplinary multimodal pain therapy dosing for chronic low back pain. J Clin Med. 2019;8(6):871. doi:10.3390/jcm8060871
20. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–2481. doi:10.1056/NEJMp1011024
21. Raja SN, Carr DB, Cohen M, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi:10.1097/j.pain.0000000000001939
22. Turk DC, Dworkin RH, Revicki D, et al. Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. Pain. 2008;137(2):276–285. doi:10.1016/j.pain.2007.09.002
23. Casarett D, Karlawish J, Sankar P, Hirschman K, Asch DA. Designing pain research from the patient’s perspective: what trial end points are important to patients with chronic pain? Pain Med. 2001;2(4):309–316. doi:10.1046/j.1526-4637.2001.01041.x
24. Robinson ME, Brown JL, George SZ, et al. Multidimensional success criteria and expectations for treatment of chronic pain: the patient perspective. Pain Med. 2005;6(5):336–345. doi:10.1111/j.1526-4637.2005.00059.x
25. Brown JL, Edwards PS, Atchison JW, Lafayette-Lucey A, Wittmer VT, Robinson ME. Defining patient-centered, multidimensional success criteria for treatment of chronic spine pain. Pain Med. 2008;9(7):851–862. doi:10.1111/j.1526-4637.2007.00357.x
26. Merrick D, Sundelin G, Stålnacke BM. One-year follow-up of two different rehabilitation strategies for patients with chronic pain. J Rehabilitat Med. 2012;44(9):764–773. doi:10.2340/16501977-1022
27. Varallo G, Scarpina F, Giusti EM, et al. The role of pain catastrophizing and pain acceptance in performance-based and self-reported physical functioning in individuals with fibromyalgia and obesity. J Pers Med. 2021;11(8):810. doi:10.3390/jpm11080810
28. Deckert S, Kaiser U, Kopkow C, Trautmann F, Sabatowski R, Schmitt J. A systematic review of the outcomes reported in multimodal pain therapy for chronic pain. Eur J Pain. 2016;20(1):51–63. doi:10.1002/ejp.721
29. Kaiser U, Kopkow C, Deckert S, et al. Developing a core outcome domain set to assessing effectiveness of interdisciplinary multimodal pain therapy: the VAPAIN consensus statement on core outcome domains. Pain. 2018;159(4):673–683. doi:10.1097/j.pain.0000000000001129
30. Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337–345. doi:10.1016/j.pain.2003.08.001
31. Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3–s11. doi:10.1097/01.mlr.0000258615.42478.55
32. Dragioti E, Dong HJ, Larsson B, Gerdle B. Reported outcomes in published systematic reviews of interdisciplinary pain treatment: protocol for a systematic overview. JMIR Res Protoc. 2020;9(5):e17795. doi:10.2196/17795
33. Gerdle B, Molander P, Stenberg G, Stålnacke BM, Enthoven P. Weak outcome predictors of multimodal rehabilitation at one-year follow-up in patients with chronic pain-a practice based evidence study from two SQRP centres. BMC Musculoskelet Disord. 2016;17(1):490. doi:10.1186/s12891-016-1346-7
34. Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. doi:10.1371/journal.pmed.1000326
35. Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11(1):15. doi:10.1186/1471-2288-11-15
36. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
37. National Library of Medicine. Search strategy used to create the pubmed systematic reviews filter. Available from: https://www.nlm.nih.gov/bsd/pubmed_subsets/sysreviews_strategy.html. accessed Augue
38. Kamper SJ, Apeldoorn AT, Chiarotto A, et al.Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database Syst Rev. 2014;(9):CD000963. doi:10.1002/14651858.CD000963.pub3
39. Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: cochrane systematic review and meta-analysis. BMJ. 2015;350:h444. doi:10.1136/bmj.h444
40. Guzmán J, Esmail R, Karjalainen K, Malmivaara A, Irvin E, Bombardier C. Multidisciplinary bio-psycho-social rehabilitation for chronic low back pain. Cochrane Database Syst Rev. 2002;1:Cd000963.
41. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi:10.1136/bmj.j4008
42. Lorenz RC, Matthias K, Pieper D, et al. AMSTAR 2 overall confidence rating: lacking discriminating capacity or requirement of high methodological quality? J Clin Epidemiol. 2020;119:142–144. doi:10.1016/j.jclinepi.2019.10.006
43. Elbers S, Wittink H, Konings S, et al. Longitudinal outcome evaluations of interdisciplinary multimodal pain treatment programs for patients with chronic primary musculoskeletal pain: a systematic review and meta-analysis. Eur J Pain. 2021;26(2):310–335. doi:10.1002/ejp.1875
44. Skelly AC, Chou R, Dettori JR, et al. AHRQ Comparative Effectiveness Reviews. In: Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review Update. Agency for Healthcare Research and Quality (US); 2020.
45. Casey MB, Smart KM, Segurado R, Doody C. Multidisciplinary-based Rehabilitation (MBR) compared with active physical interventions for pain and disability in adults with chronic pain: a systematic review and meta-analysis. Clin J Pain. 2020;36(11):874–886.
46. Martinez-Calderon J, Flores-Cortes M, Morales-Asencio JM, Fernandez-Sanchez M, Luque-Suarez A. Which interventions enhance pain self-efficacy in people with chronic musculoskeletal pain? A systematic review with meta-analysis of randomized controlled trials, including over 12 000 participants. J Orthop Sports Phys Ther. 2020;50(8):418–430. doi:10.2519/jospt.2020.9319
47. Papadopoulou D, Fassoulaki A, Tsoulas C, Siafaka I, Vadalouca A. A meta-analysis to determine the effect of pharmacological and non-pharmacological treatments on fibromyalgia symptoms comprising OMERACT-10 response criteria. Clin Rheumatol. 2016;35(3):573–586. doi:10.1007/s10067-015-3144-2
48. van Middelkoop M, Rubinstein SM, Kuijpers T, et al. A systematic review on the effectiveness of physical and rehabilitation interventions for chronic non-specific low back pain. Eur Spine J. 2011;20(1):19–39. doi:10.1007/s00586-010-1518-3
49. Häuser W, Bernardy K, Arnold B, Offenbächer M, Schiltenwolf M. Efficacy of multicomponent treatment in fibromyalgia syndrome: a meta-analysis of randomized controlled clinical trials. Arthritis Rheum. 2009;61(2):216–224. doi:10.1002/art.24276
50. Norlund A, Ropponen A, Alexanderson K. Multidisciplinary interventions: review of studies of return to work after rehabilitation for low back pain. J Rehabil Med. 2009;41(3):115–121. doi:10.2340/16501977-0297
51. Martinez-Calderon J, Flores-Cortes M, Morales-Asencio JM, Luque-Suarez A. Conservative interventions reduce fear in individuals with chronic low back pain: a systematic review. Arch Phys Med Rehabil. 2020;101(2):329–358. doi:10.1016/j.apmr.2019.08.470
52. Martinez-Calderon J, Flores-Cortes M, Morales-Asencio JM, Luque-Suarez A. Intervention therapies to reduce pain-related fear in fibromyalgia syndrome: a systematic review of randomized clinical trials. Pain Med. 2021;22(2):481–498. doi:10.1093/pm/pnaa331
53. Garschagen A, Steegers MA, van Bergen AH, et al. Is there a need for including spiritual care in interdisciplinary rehabilitation of chronic pain patients? Investigating an innovative strategy. Pain Pract. 2015;15(7):671–687. doi:10.1111/papr.12234
54. Waterschoot FPC, Dijkstra PU, Hollak N, de Vries HJ, Geertzen JHB, Reneman MF. Dose or content? Effectiveness of pain rehabilitation programs for patients with chronic low back pain: a systematic review. Pain. 2014;155(1):179–189. doi:10.1016/j.pain.2013.10.006
55. van Geen JW, Edelaar MJ, Janssen M, van Eijk JT. The long-term effect of multidisciplinary back training: a systematic review. Spine. 2007;32(2):249–255. doi:10.1097/01.brs.0000251745.00674.08
56. Nielson WR, Weir R. Biopsychosocial approaches to the treatment of chronic pain. Clin J Pain. 2001;17(4 Suppl):S114–127. doi:10.1097/00002508-200112001-00020
57. Gianola S, Andreano A, Castellini G, Moja L, Valsecchi MG. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: the need to present minimal important differences units in meta-analyses. Health Qual Life Outcomes. 2018;16(1):91. doi:10.1186/s12955-018-0924-9
58. Cochrane A, Higgins NM, FitzGerald O, et al. Early interventions to promote work participation in people with regional musculoskeletal pain: a systematic review and meta-analysis. Clin Rehabil. 2017;31(11):1466–1481. doi:10.1177/0269215517699976
59. Henry SG, Bell RA, Fenton JJ, Kravitz RL. Goals of chronic pain management: do patients and primary care physicians agree and does it matter? Clin J Pain. 2017;33(11):955–961. doi:10.1097/AJP.0000000000000488
60. McCracken LM, Zhao-O’Brien J. General psychological acceptance and chronic pain: there is more to accept than the pain itself. Eur J Pain. 2010;14(2):170–175. doi:10.1016/j.ejpain.2009.03.004
61. Thompson M, McCracken LM. Acceptance and related processes in adjustment to chronic pain. Curr Pain Headache Rep. 2011;15(2):144–151. doi:10.1007/s11916-010-0170-2
62. Ballantyne JC, Sullivan MD. Intensity of chronic pain — the wrong metric? N Engl J Med. 2015;373(22):2098–2099. doi:10.1056/NEJMp1507136
63. Dwan K, Gamble C, Williamson PR, Kirkham JJ. Systematic review of the empirical evidence of study publication bias and outcome reporting bias - an updated review. PLoS One. 2013;8(7):e66844. doi:10.1371/journal.pone.0066844
64. Guise JM, Butler ME, Chang C, Viswanathan M, Pigott T, Tugwell P. AHRQ series on complex intervention systematic reviews-paper 6: PRISMA-CI extension statement and checklist. J Clin Epidemiol. 2017;90:43–50. doi:10.1016/j.jclinepi.2017.06.016
65. Movsisyan A, Melendez-Torres GJ, Montgomery P. A harmonized guidance is needed on how to “properly” frame review questions to make the best use of all available evidence in the assessment of effectiveness of complex interventions. J Clin Epidemiol. 2016;77:139–141. doi:10.1016/j.jclinepi.2016.04.003
66. Murad MH, Almasri J, Alsawas M, Farah W. Grading the quality of evidence in complex interventions: a guide for evidence-based practitioners. Evid Based Med. 2017;22(1):20–22. doi:10.1136/ebmed-2016-110577
67. Movsisyan A, Melendez-Torres GJ, Montgomery P. Outcomes in systematic reviews of complex interventions never reached “high” GRADE ratings when compared with those of simple interventions. J Clin Epidemiol. 2016;78:22–33. doi:10.1016/j.jclinepi.2016.03.014
68. Phillippo DM, Dias S, Welton NJ, Caldwell DM, Taske N, Ades AE. Threshold analysis as an alternative to GRADE for assessing confidence in guideline recommendations based on network meta-analyses. Ann Intern Med. 2019;170(8):538–546. doi:10.7326/M18-3542
69. Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323(7303):42–46. doi:10.1136/bmj.323.7303.42
70. Tanner-Smith EE, Grant S. Meta-Analysis of Complex Interventions. Annu Rev Public Health. 2018;39(1):135–151. doi:10.1146/annurev-publhealth-040617-014112
71. Ogilvie D, Fayter D, Petticrew M, et al. The harvest plot: a method for synthesising evidence about the differential effects of interventions. BMC Med Res Methodol. 2008;8(1):8. doi:10.1186/1471-2288-8-8
72. Higgins JPT, López-López JA, Becker BJ, et al. Synthesising quantitative evidence in systematic reviews of complex health interventions. BMJ Glob Health. 2019;4(Suppl 1):e000858. doi:10.1136/bmjgh-2018-000858
73. Arnold LM, Williams DA, Hudson JI, et al. Development of responder definitions for fibromyalgia clinical trials. Arthritis Rheum. 2012;64(3):885–894. doi:10.1002/art.33360
74. Vervoort VM, Vriezekolk JE, van den Ende CH. Development of responder criteria for multicomponent non-pharmacological treatment in fibromyalgia. Clin Exp Rheumatol. 2017;35 Suppl 105(3):86–92. doi:10.1136/annrheumdis-2017-eular.3629
75. Teixeira-Pinto A, Mauri L. Statistical analysis of noncommensurate multiple outcomes. Circ Cardiovasc Qual Outcomes. 2011;4(6):650–656. doi:10.1161/CIRCOUTCOMES.111.961581
76. López-López JA, Page MJ, Lipsey MW, Higgins JPT. Dealing with effect size multiplicity in systematic reviews and meta-analyses. Res Synth Methods. 2018;9(3):336–351. doi:10.1002/jrsm.1310
77. Riley RD, Jackson D, Salanti G, et al. Multivariate and network meta-analysis of multiple outcomes and multiple treatments: rationale, concepts, and examples. BMJ. 2017;358:j3932. doi:10.1136/bmj.j3932
78. Jackson D, White IR, Riley RD. Multivariate meta-analysis. In: Handbook of Meta-Analysis. Chapman and Hall/CRC; 2020:163–186.
79. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. doi:10.18637/jss.v036.i03
80. Welton NJ, Caldwell DM, Adamopoulos E, Vedhara K. Mixed treatment comparison meta-analysis of complex interventions: psychological interventions in coronary heart disease. Am J Epidemiol. 2009;169(9):1158–1165. doi:10.1093/aje/kwp014
81. Munder T, Brütsch O, Leonhart R, Gerger H, Barth J. Researcher allegiance in psychotherapy outcome research: an overview of reviews. Clin Psychol Rev. 2013;33(4):501–511. doi:10.1016/j.cpr.2013.02.002
82. Guise JM, Chang C, Butler M, Viswanathan M, Tugwell P. AHRQ series on complex intervention systematic reviews-paper 1: an introduction to a series of articles that provide guidance and tools for reviews of complex interventions. J Clin Epidemiol. 2017;90:6–10. doi:10.1016/j.jclinepi.2017.06.011
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

