Back to Journals » Drug Design, Development and Therapy » Volume 17
Renzhu Ointment Regulates L-Type Voltage-Dependent Calcium Channel in Mice Model of Senna-Induced Diarrhea by Transdermal Administration
Authors Zhong L, Cao X, Li L, He Y, Liu Y, Chen W, Yang F, Xiao N, Zhang J, He H
Received 4 May 2023
Accepted for publication 21 July 2023
Published 11 August 2023 Volume 2023:17 Pages 2355—2368
DOI https://doi.org/10.2147/DDDT.S419626
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Lian Zhong,1,* Xiaoyu Cao,2,* Li Li,1 Yuanyuan He,1 Yanxia Liu,1 Weiwei Chen,1 Fuzhen Yang,1 Ni Xiao,1 Jun Zhang,1 Huifen He3
1School of Pharmaceutical Sciences, Guangzhou University of Chinese Medicine, Guangzhou, 510006, People’s Republic of China; 2School of Chinese Materia Medica, Guangdong Pharmaceutical University, Guangzhou, 510006, People’s Republic of China; 3The First Affiliated Hospital, Guangzhou University of Chinese Medicine, Guangzhou, 510405, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huifen He, The First Affiliated Hospital, Guangzhou University of Chinese Medicine, Guangzhou, 510006, People’s Republic of China, Tel +86-15920174486, Email [email protected] Jun Zhang, School of Pharmaceutical Sciences, Guangzhou University of Chinese Medicine, Guangzhou, 510006, People’s Republic of China, Tel +86-020-39356997, Email [email protected]
Purpose: In China, herbal preparation is commonly administered transdermally for treating pediatric diarrhea. However, few studies have probed into their antidiarrheal mechanisms. This study was designed to investigate the antidiarrheal effect of Renzhu ointment (Renzhuqigao, RZQG) and its underlying mechanisms via transdermal administration.
Methods: The main components of RZQG were confirmed by gas chromatography-mass spectrometry (GC-MS). The effect of RZQG on L-type voltage-dependent calcium channel (L-VDCC) was evaluated by CaCl2- and ACh-induced contraction in isolated colon. The antidiarrheal efficacy of RZQG was further investigated by the senna-induced diarrhea mice based on the frequency of loose stools, diarrhea rate and index, fecal moisture content, and the basal tension of the colon. Additionally, the protein expression of CACNA1C, CACNA1D, cAMP, and PKA were detected with Western blot and immunohistochemistry (IHC).
Results: GC-MS analysis determined 14 components in RZQG. In vitro, RZQG relaxed the CaCl2- and ACh-induced tension, while nifedipine (a L-VDCC inhibitor) and H-89 (a PKA inhibitor) decreased the relaxation. In vivo, animal model showed that transdermal administration of RZQG exhibited a significant reduction in the frequency of loose stools, diarrhea rate and index, fecal moisture content and the basal tension. Compared to the model group, the colon of mice treated with RZQG showed lower expression of CACNA1C, CACNA1D, cAMP, and PKA. IHC results showed that cAMP was downregulated in colonic smooth muscle after RZQG treatment.
Conclusion: RZQG improved diarrhea symptoms and down-regulated the expression of CACNA1C and CACNA1D via transdermal administration, which is closely associated with the cAMP/PKA signaling pathway in colonic smooth muscle.
Keywords: Renzhu ointment, transdermal administration, antidiarrheal effect, antidiarrheal mechanisms
Introduction
Pediatric diarrheal disease is caused by multiple pathogens. The Global Burden of Disease 2019 Study (GBD 2019) has reported that diarrhea is the fourth leading cause of mortality among children.1 About 480,000 children under the age of five died from diarrhea in 2019.1 The disease with high morbidity between 6 months to 2 years of age is the major cause of malnutrition, developmental disabilities and death.2
Currently, oral or intravenous rehydration is mostly used in the treatment for pediatric diarrhea, it reduces child mortality but cannot relieve diarrheal symptoms.3 In China, traditional transdermal administration to the navel has accumulated abundant clinical experience of treating pediatric diarrhea with high efficacy, few side effects, and good compliance.4 This external transdermal therapy usually involves pasting the herbal powders containing essential oil onto the patient’s navel. Navel has a thin layer of the stratum corneum, and thus drugs are readily absorbed bypassing the first-pass effect.5
There are a large variety of Chinese patent medicines made of herbal powder on the market, such as Ding Gui Infantile Navel Paste (Dingguierqitie, DGQT),4 Xiaoer Fuxie Waifu powder.6 The effect of transdermal preparations is determined by effective substance content and release rate.6 The essential oil is an effective antidiarrheal part of herbs. The herbal powders with low essential oil content are used to prepare ointment that reduces the transdermal absorption and limits favorable therapeutic effect. Renzhu ointment (Renzhuqigao, RZQG) is composed of essential oil extracted by supercritical CO2 extraction technology from Fructus Amomi (FA, fructus of Amomum villosum Lour.), Rhizoma Atractylodis (RA, rhizoma of Atractylodin lancea (Thunb.) DC), Cortex Cinnamomi (CC, cortex of Cinnamomum cassia Presl.), and Flos Caryophylli (FC, alabastrum of Ewgewia caryophyllata Thunb.). Through formulation optimization and extraction process, RZQG, with high essential oil content, enhances the transdermal absorption and shows a good application prospect.
The transdermal administration for treating diarrhea has achieved remarkable efficacy in the clinic, which can significantly reduce the frequency of liquid stools, relieve abdominal pain, improve appetite, and shorten disease course.4 However, few have probed into the mechanism of adequate antidiarrhea effect of transdermal administration.
Diarrhea is often accompanied by accelerated gastrointestinal motility. In our studies, the effect of RZQG on L-VDCC and PKA was assayed by isolated colonic contractility first. Then young mice were selected to further explore the antidiarrheal effect and underlying mechanism of RZQG at different doses on senna-induced diarrhea.
Materials and Methods
Plant Materials, Reagents, and Their Preparations
Folium Sennae (Voucher No. 210801) was purchased from Zisun Medicine Co., Ltd. (Guangzhou, China). Type 40 mixed fatty acid glyceride (Voucher No. 160312) was produced by Hubei Tungshun Medicine Co., Ltd. (Hubei, China). Acetylcholine chloride (Voucher No. Z30S11H126592) was manufactured by the Shanghai yuanye Bio-Technology Co., Ltd. (Shanghai, China). DMSO was purchased from Guangzhou Risu Biotechnology Co., Ltd. (Guangzhou, China). Nifedipine (Voucher No. RH305187) was purchased from Xi’an Yizhichen Biological Technology Co., Ltd. (Xi’an, China). NaCl, KCl, MgSO4, NaHCO3, and CaCl2 were all manufactured and marketed by Tianjin Damao Chemical Reagent Factory (Tianjin, China). Glucose and KH2PO4 were produced by Tianjin Zhiyuan Chemical Reagent Co. Ltd. (Tianjin, China).
RZQG (Voucher No. 20170802) was produced by Guangzhou University of Chinese Medicine (Guangdong, China). It was composed of essential oils of FA, RA, CC, FC, as well as mixed fatty acid glycerides. Renzhu essential oils (RZEO) were made by supercritical CO2 extraction from FA, RA, CC, and FC in a ratio of 4:4:3:5. Mixed fatty acid glycerides were used as excipients for RZQG. The ratio of RZEO and mixed fatty acid glycerides was 1:4.
Folium sennae powder was taken, added 10-fold distilled water, and soaked at 90°C for 30 minutes. After filtration, the water extracts were concentrated to 0.4 g/mL under reduced pressure. Then folium sennae extract (senna) was obtained.
The Krebs solution consisted of 117 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L MgCl2, 24.8 mmol/L NaHCO3, 1.2 mmol/L KH2PO4, 2.56 mmol/L CaCl2, and 11.1 mmol/L glucose.
GC-MS Analysis of RZQG
RZQG (0.5 g) in 25 mL of ethanol was weighed precisely and ultrasonicated (280 W, 40 kHz) for 30 min. After standing for 30 min at 4°C, the extract was left at room temperature and the loss of weight was replenished with ethanol. Then the sample was filtrated. The essential oil of FA, RA, CC, and FC were obtained by supercritical CO2 extraction respectively. RZQG, RZEO, FA, RA, CC, and FC were analyzed by Agilent 7890B-5977A GC-MS. HP-5MS capillary column (30 m × 0.25 mm i.d., 0.25 μm film, Agilent J&W Scientific, USA) was utilized to separate the compounds. The temperature program started at 86°C for 15 minutes, then raised it to 140°C at 2°C/min, kept it for 0 minutes, then elevated to 250°C at 5 °C/min. The split injection was used with a ratio of 10:1, the flow rate of helium was set at 1.0 mL/min, and the injection volume was 1 μL. The mass spectrometer was operated in electron impact ionization at 70 eV, scanning from 35 to 550 amu. The injection inlet and ion source temperatures were set to 300°C and 230°C, respectively.
Animals
Male KM mice (15–20 g) were obtained from Guangdong Medical Laboratory Animal Center, China (License No. SCXK (Yue) 2018–0002). All experimental animals were given free access to water and food under controlled temperature, humidity, and photoperiod environment. The experimental scheme protocol was approved by the Guangzhou University of Chinese Medicine Ethics Committee (approval number 20210311001) and conformed to the Regulations of Guangzhou University of Chinese Medicine on Ethical Review of Animal Experiments.
CaCl2- and Acetylcholine (ACh)-Induced Tonic Contraction in vitro
Mice proximal colon (1–2 cm away from the cecum, 0.8 cm × 0.2 cm) was taken and divided into Control, DMSO, and RZQG groups. The muscle strips were hung diagonally on the tension transducers (PL3508, Harvard Apparatus, USA). Colon was allowed to equilibrate under zero force for 30 min in Ca2+-free Krebs with 95% oxygen and 5% carbon dioxide. Then, the tissue was equilibrated under a resting force of 0.5 g. After resting for 30 min, experiments were performed. Then CaCl2 was added to a final concentration of 3 × 10−3 mol/L. After recording tension for 1 min, tension was recorded with Ca2+-free Krebs, DMSO and RZQG (1 × 10−2 mg/mL, 1 × 10−3 mg/mL, 1 × 10−4 mg/mL, 1 × 10−5 mg/mL, 1 × 10−6 mg/mL with 0.2% DMSO).
The experiment of ACh-induced tension was divided into control, DMSO, RZQG, and nifedipine + RZQG groups. Strips of nifedipine + RZQG group were incubated with nifedipine (1 × 10−5 mol/L) for 20 minutes or H-89 (1 × 10−7 mol/L) for 5 minutes. After the resting tension had stabilized, ACh (1 × 10−5 mol/L) was added to induce a rapid increase in colon tone. Then, tension was recorded with Krebs, DMSO and RZQG.
The relaxant response to RZQG was calculated as follows:
Relaxation (%) = (the maximal contraction by CaCl2 (or ACh) - tension at the corresponding time after incubation with RZQG)/(the maximal contraction by CaCl2 (or ACh) - basal tension) × 100%.
Ointments and Their Preparations
1 g RZQG, containing mixed fatty acid glycerides and 200 mg RZEO, was applied to the navel once daily for 5 days in the clinic. The dose of RZQG was designed according to Guidelines for the Pharmacodynamics of Chinese Medicine New Drugs issued by the Ministry of Health, China. The guideline recorded that a dose should be equivalent to 2–5 times the human equivalent dose (10–15 times in mice). Dosages are equivalent to 3, 6 and 12 times the clinical dosage for a 20 kg child.
The area-based dose was used to achieve low, medium and high doses. 0.1 g RZQG (with a diameter of 1.0 cm) containing 20 mg of RZEO, was marked as 1S. The corresponding area of the ointment was then cut out in the ratio of 1/2 and 1/4, marked as 1/2S and 1/4S. 1/4S, 1/2S and 1S RZQG were respectively applied to RZQG low, medium and high dose groups (equivalent to 3, 6, and 12 times of the human equivalent dose in 20 g mice).
0.1 g Blank ointment (BN, diameter was 1.0 cm) contained mixed fatty acid glycerides. BN was applied to the control group and the model group.
DGQT (Voucher No. 150743) was purchased from Yabao Pharmaceutical Group Co., Ltd. (Shanxi, China). 1.6 g DGQT was applied to the navel once daily in the clinic. 0.16 g DGQT (diameter was 1.0 cm) was used as the positive control drug in the efficacy test for the positive control group (equivalent to 12 times the human equivalent dose).
Animal Experiments
Male KM mice were randomly divided into 6 groups. There were control group (Control), model group (Model), RZQG low dose group (Low, 1.25 mg/g), RZQG medium dose group (Medium, 2.5 mg/g), and RZQG high dose group (High, 5 mg/g), positive control group (Ding, 8 mg/g), with 10 mice in each group. Subsequently, abdomen hair was depilated using 8% Na2S solution. Mice were fasted for 4 h before the experiment and then weighed.
Evaluation of Antidiarrheal Test
In this test, a mouse model of diarrhea was induced by senna. Mice in the different groups were treated with the corresponding ointment by transdermal administration. The specific transdermal administration methodology was as follows: after being wiped and disinfected with alcohol swabs, RZQG or mixed fatty acid glycerides was directly contacted with the abdominal skin of mice. The abdomen was then covered with a layer of sulphate paper, a layer of gauze, and fixed with non-irritating tape. One hour later, except for the control, the groups were administered senna by gavage (0.25 mL/10g), and feces were collected under 7 h observation. The frequency of dry stools and loose stools, and diarrhea grade were recorded. The diarrhea rate, diarrhea index, and fecal moisture content were calculated by the following formula (1–4). The diarrhea grade was classified into four grades, which were < 1.0 cm (grade 1), 1.0 ~ 1.9 cm (grade 2), 2.0 ~ 3.0 cm (grade 3), and > 3.0 cm (grade 4).
Effects of RZQG on the Basal Tension
Mice were euthanized 5 h after gavage. The proximal colon (1–2 cm proximally to the cecum) was removed and the intestinal segments were cleaned by ice-cold saline. The muscle strips were hung diagonally on the tension transducers (PL3508, Harvard Apparatus, USA). After the resting tension stabilized, the strips of 6 groups were equilibrated for 60 min under a resting force of 0.5 g in Krebs with 95% oxygen and 5% carbon dioxide. Basal tension was recorded for 5 min.
Western Blot
Total protein was extracted from the proximal colon tissues with RIPA buffer containing PMSF and determined by a BCA kit (Keygen Biotech, KGP902, China). The protein samples (60 μg/lane) were loaded on SDS-PAGE gels (Beyotim, P0012A, China) and transferred onto PVDF membranes (Merck, IPVH00010, United States) after electrophoresis. Membranes were blocked with 5% skimmed milk for 2 h and then incubated overnight at 4°C with CACNA1C (1:500, Affinity, DF2267), CACNA1D (1:500, Affinity, DF2267), cyclic adenosine monophosphate (cAMP, 1:10000, Abcam, ab76238), protein kinase A (PKA, 1:500, Affinity, AF7746), and β-Tubulin (1:5000, Affinity, AF7011) antibodies. Afterward, an HRP-conjugated antibody (1:5000, Affinity, S0001, China) were incubated with the membrane. The chemiluminescence (ECL) kit was employed for the membrane visualization by a Tanon 5200 system (Shanghai, China). ImageJ 7.0 was used for quantification.
Immunohistochemistry (IHC)
The colon tissue was analyzed using IHC. Endogenous peroxidases activity were blocked using 0.3% H2O2, and sections were repaired with a high-pressure method. The sections were blocked for 30 min and then incubated with cAMP antibody (1:800, Abcam, ab76238) overnight at 4°C. The HRP-conjugated antibody (1:200, Servicebio, GB23303, China) was then added and incubated at 37°C for 50 min. DAB (Servicebio, G1211) was used for chromogenic detection. Brown staining was considered positive. ImageJ 7.0 was used to analyze the staining intensity and expressed as the mean optical density (MOD).
Statistical Analysis
All data analyses were conducted using SPSS 26.0, expressed as the mean ± standard deviation (SD), and performed using GraphPad Prism version 8.0. Normality tests were performed before statistical analysis of the data. One-way ANOVA was used to compare the multiple group comparisons. The LSD and Dunnett’s T3 test were used when homogeneous or non-homogeneous variance was found in the data respectively.
Result
GC-MS Analysis of RZQG
RZQG and RZEO were identified to contain 14 components (Figure 1 and Table 1). (+)-2-Bornanone and Bornyl acetate were assignable to the FA essential oil. Humulene, Agarospirol, β-Eudesmol, Atractylodin, and 2-Methoxybiphenyl were attributed to RA essential oil. (E)-Cinnamaldehyde, Copaene, α-Muurolene, and δ-Cadinene were assigned to CC essential oil. Moreover, Eugenol, β-Caryophyllene, and Eugenol acetate were attributed to FC essential oil.
 |
Table 1 Characterization of Compounds of RZQG, RZEO, FA, RA, CC, FC by GC-MS |
Effects of RZQG on CaCl2 and ACh Induced Tension
The strips were subjected to 0.5 g load tension. The results showed RZQG concentration-dependently relaxed the contraction induced by CaCl2 and ACh with a maximum relaxation of 99.12 ± 15.14% and 94.33 ± 7.01%, respectively (Figure 2, P < 0.05). In the presence or absence of nifedipine (10−5 mol/L), the maximal relaxation effects were 94.33 ± 7.01% and 62.31 ± 5.81%, respectively. Blocking of L-VDCC with nifedipine significantly decreased the relaxation of RZQG (Figure 2, P < 0.05). Moreover, in the presence or absence of H-89 (10−7 mol/L), the maximal relaxation effects were 94.33 ± 7.01% and 64.16 ± 8.10%, respectively. Blocking of PKA with H-89 significantly decreased the relaxation of RZQG (Figure 2, P < 0.05). The above method was in order to confirm the role of L-VDCC and PKA in soothing effects of RZQG on the colon.
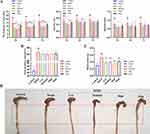
The Antidiarrheal Efficacy of RZQG
Senna is the most commonly used herb to induce diarrhea.7 The frequency of loose stools, diarrhea rate, diarrhea index, fecal moisture content, and basal tension were significantly increased in the model group (Figure 3, compared with the control group, P < 0.01). These results suggested that the diarrhea model was established. After transdermal administration of drugs, the frequency of loose stools, diarrhea rate, diarrhea index, and basal tension were decreased (Figure 3, compared with the model group, P < 0.05), especially in the RZQG high-dose group (Figure 3, P < 0.01). There was a trend of reduced fecal moisture content in each dose group (Figure 3B). Macroscopic observation in the model group at experimental termination showed that mice exhibited apparent swelling on the proximal colon when compared to the control group (Figure 3D). The symptoms were significantly improved in the administration group (Figure 3D, compared with the model group). Moreover, RZQG was preferred over the marketed transdermal preparations DGQT in the above indicators.
For the frequency of loose stools, compared to the model group (7.60 ± 2.12, 8.30 ± 2.11, 8.70 ± 2.31), the low (5.10 ± 1.60, 5.70 ± 1.42, 5.80 ± 1.62), medium (4.50 ± 2.88, 5.50 ± 1.96, 5.80 ± 2.04), and high dose groups (5.00 ± 1.15, 5.70 ± 1.34, 6.20 ± 1.23) of RZQG significantly inhibited senna-induced diarrhea in mice within 5 h, 6 h and 7 h (Figure 3A, P < 0.01). Within 6 h, the Ding group (6.50 ± 2.17) had significant antidiarrheal effect (Figure 3A, P < 0.05). For the diarrhea rate, compared to the model group (0.53 ± 0.10, 0.55 ± 0.09), the RZQG low dose group (0.44 ± 0.10) within 5 h and RZQG medium dose group (0.40 ± 0.24, 0.48 ± 0.14) within 5 h and 6 h had significant antidiarrheal effect (Figure 3A, P < 0.05). For the diarrhea index, compared to the model group (0.83 ± 0.20), the RZQG high dose group (0.61 ± 0.14) and Ding group (0.65 ± 0.18) had significant antidiarrheal effect within 6 h (Figure 3A, P < 0.05). For the basic tension, compared to the model group (0.53 ± 0.06), the RZQG low dose group (0.38 ± 0.08), RZQG medium dose group (0.40 ± 0.06), RZQG high dose group (0.34 ± 0.03) and Ding group (0.42 ± 0.06) significantly reduced the colonic basic tension of mice with diarrhea (Figure 3C, P < 0.01).
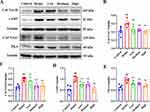
CACNA1C, CACNA1D, cAMP, and PKA Protein Expression in the Colon
CACNA1C, CACNA1D, cAMP and PKA protein expression were dramatically up-regulated in the model group (Figure 4, compared with the control group, P < 0.01). After RZQG administration, CACNA1C and CACNA1D, cAMP and PKA expression were significantly reduced (Figure 4, compared with the model group, P < 0.01).
IHC of cAMP Proteins in Colonic Tissue
In the model group, the colonic smooth muscle displayed very strong staining of cAMP (Figure 5, compared with the control group, P < 0.01), while the protein expression levels of cAMP decreased significantly in mice treated with RZQG (Figure 5, compared with the model group, P < 0.01).
Discussion
The main results of the present study supported that RZQG could effectively treat senna-induced diarrhea. In vitro, RZQG inhibited the CaCl2- and ACh-induced excessive contraction of the colon, associated with L-VDCC and PKA. Colon motility participated in the mechanisms of RZQG treatment. RZQG down-regulated the expression of CACNA1C and CACNA1D in the colon, associated with the cAMP/PKA signaling pathway.
The GC-MS analysis showed that fourteen components in RZQG were identified (Figure 1 and Table 1). Published literature has reported the antidiarrheal effects of cinnamaldehyde, bornyl acetate, eugenol, β-Caryophyllene, humulene, and atractylodin by oral gavage.8–12 And multiple components could effectively alleviate intestinal motility such as humulene, eugenol, and β-Eudesmol.13–15 In addition, some components have spasmolytic effects, such as eugenol, copaene, β-Caryophyllene.16–18 The main mechanisms of spasmolysis are inhibition of voltage- dependent calcium channels and regulation of intracellular cAMP, etc.19 L-type voltage-dependent calcium channel is a crucial component in regulating smooth muscle contraction in the gastrointestinal tract.20 By regulating the activity of this channel, it is possible to regulate the contraction of smooth muscle and alleviate symptoms related to diarrhea. cAMP, the major intracellular second messenger, is directly involved in the relaxation of smooth muscle.19 cAMP can activate PKA, which in turn may relax smooth muscle by increasing intracellular calcium excretion.19 Due to the presence of components in RZQG, such as cinnamaldehyde, eugenol, β-Caryophyllene, Copaene, which possess antidiarrheal and spasmolytic effects, it is possible that RZQG inhibits L-type voltage-dependent calcium channels, leading to a decrease in intracellular calcium levels and subsequent relaxation of smooth muscle. This effect may help alleviate the symptoms related to diarrhea. Moreover, it may also regulate the intracellular cAMP level. By regulating intracellular cAMP levels, it activates the cAMP/PKA signaling pathway, which in turn relaxes smooth muscle and may alleviate diarrhea symptoms. In summary, the potential spasmolytic and antidiarrheal effects of the active ingredients in RZQG may be achieved through the regulation of L-type voltage-dependent calcium channel and the cAMP/PKA signaling pathway in colonic smooth muscle.
Previous studies have shown that senna-induced models resulted in severe diarrhea.21 This agreed with our model. RZQG transdermal treatment led to a significant reduction in the frequency of loose stools, diarrhea rate, diarrhea index, and basal tension (Figure 3). DGQT, a marketed transdermal preparation in China for pediatric diarrhea,4 is used by approximately 60% of patients.22 DGQT treatment significantly reduced the frequency of loose stools induced by senna and castor oil.23 Our study showed that RZQG preferred over the DGQT. Moreover, RZQG administration has displayed effect on castor oil-induced diarrhea, it is also proved effective against gastrointestinal motility through the charcoal meal test, indicating the antidiarrheal efficacy of RQZG. A similar preparation, Xiaoer Fuxie Waifu powder, made of Fructus Piperis and recorded in Chinese Pharmacopoeia,6 inhibits intestinal motility and exerts an antidiarrheal effect.24 Therefore, we focused on calcium channel to investigate colonic smooth muscle, which was more closely related to the antidiarrheal effect.
Due to the accelerated intestinal motility, the transit time for food to travel through the intestine is shortened and thus time for fluid/electrolytes to make contact with epithelial cells is reduced.25 Decreased absorption of fluid and electrolytes leads to water retention in the intestinal lumen, resulting in diarrhea.25 RZQG reduced colonic tension, and slowed colonic motility, which prolonged time for water absorption and improved fecal moisture content, thereby treating diarrhea (Figure 3). Ca2+ participates in smooth muscle cell contraction.26 Voltage-dependent calcium channel (VDCC), as major machinery for Ca2+ influx,27 has been placed into five essential groups, termed L, N, P/Q, R, and T.20 L-VDCC is highly expressed on colonic smooth muscle.28 Previous researches manifested that an increase in [Ca2+]i via the influx of Ca2+ from L-type Ca2+ channels could stimulate intestinal contraction, accelerate intestinal motility, and develop diarrhea.29 In vitro, RZQG significantly inhibited CaCl2- and ACh-induced colonic contractile responses. This relaxation was reduced by nifedipine. It was speculated that RZQG inhibited Ca2+ influx via L-type Ca2+ channels to regulate colonic motility. CACNA1C (Cav1.2) and CACNA1D (Cav1.3) are two subtypes of L-VDCC.28,30,31 Studies have shown that the accelerated colonic motility was associated with CACNA1C channels excitation-contraction.32 The expression of CACNA1C and CACNA1D were significantly increased in the colonic smooth muscle of the senna-induced diarrhea rat, which may be directly related to the enhanced colonic contraction.28 Our results of Western blot showed that the expression of CACNA1C and CACNA1D were up-regulated in the model group (Figure 4), which was in agreement with the previous studies. After RZQG treatment, the CACNA1C and CACNA1D expression level decreased (Figure 4). Therefore, RZQG may exert a relaxing effect on smooth muscle contraction by inhibiting CACNA1C and CACNA1D.
The current study has shown that inhibition of cAMP/ PKA signaling regulated Ca2+-gated channels resulting in relaxing colonic smooth muscle.33 cAMP regulates the movement of the gastrointestinal tract and participates in the regulation of intestinal sensation, secretion, and movement.34 cAMP exerts its effects mainly through the stimulation of PKA.35 Studies have indicated a pivotal role of cAMP/PKA signaling in diarrhea-related protein regulation.36 The initiation of Cav1.2 (CACNA1C) transcription is mediated through the activation of the cAMP-PKA signaling pathway.37 The C terminus Ser1928 of the Cav1.2 (CACNA1C) is the target for PKA-dependent phosphorylation.38 Also, the C-terminal region of Cav1.3 contains sites for phosphorylation by PKA, and is effectively up-regulated by the cAMP/PKA pathway.39 In vitro, blocking of PKA with H-89 significantly decreased the relaxation of RZQG (Figure 2, P < 0.05). It is speculated that the expression of CACNA1C and CACNA1D in smooth muscle may regulate intestinal motility through the cAMP/PKA signaling pathway. In vivo, 0.4 g/mL senna was given to the mice by gavage, and the expression of colonic cAMP and PKA was significantly up-regulated (Figure 4). The results of IHC showed that cAMP was highly expressed in smooth muscle (Figure 5). For patients with diarrhea, the expression of PKA was significantly up-regulated in their colon.40 There was a tendency to increase cAMP in the colon of rats after gavage with sennosides.41 After RZQG treatment, the expressions of cAMP and PKA were down-regulated, especially in the smooth muscle (Figures 4 and 5).
The sub-chronic toxicity testing in rats showed that the no observed adverse effect level (NOAEL) of RZQG was 0.3 g/kg/day (equivalent to 30 times the clinically planned dose and 4.92 times the human equivalent dose).42 According to the sub-chronic toxicity and the efficacy tests, RZQG presented promising perspectives. Electrolyte and water imbalance were also involved in the pathogenesis of diarrhea. It is worthwhile to explore whether RZQG has regulated the aquaporin and Na+/H+ exchanger.
Conclusion
In summary, the up-mentioned findings supported that RZQG may alleviate intestinal motility, and improve diarrhea symptoms by down-regulating the expression of CACNA1C and CACNA1D, which is closely related to the cAMP/PKA signaling pathway in colonic smooth muscle. The present study sheds new light on the pharmacodynamics of transdermal preparation.
Abbreviations
ACh, Acetylcholine; BN, Blank ointment; CACNA1C, L-type voltage-dependent calcium channel alpha1C subunits; CACNA1D, L-type voltage-dependent calcium channel alpha1D subunits; cAMP, Cyclic adenosine monophosphate; CC, Cortex Cinnamomi; DGQT, Dinggui Infantile Navel Paste; FA, Fructus Amomi; FC, Flos Caryophylli; GC-MS, Gas chromatography-mass spectrometry; IHC, Immunohistochemistry; IOD, Integrated optical density; L-VDCC, L-type voltage-dependent calcium channel; MOD, Mean optical density; RA, Rhizoma Atractylodis; PKA, Protein kinase A; RZEO, Renzhu essential oils; RZQG, Renzhu ointment; VDCC, Voltage-dependent calcium channel.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics Approval and Consent to Participate
The experimental scheme protocol was approved by the Guangzhou University of Chinese Medicine Ethics Committee (approval number 20210311001) and conformed to the Regulations of Guangzhou University of Chinese Medicine on Ethical Review of Animal Experiments.
Acknowledgments
We thank all authors for their substantial contributions to this trial.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the National Nature Science Foundation of China (No. 81973830) and the Science and Technology Planning Project of Guangdong Province (2016B020239005).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Perin J, Mulick A, Yeung D, et al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the sustainable development goals. Lancet Child Adolesc Health. 2022;6(2):106–115. doi:10.1016/s2352-4642(21)00311-4
2. Folwaczny C. Zinc and diarrhea in infants. J Trace Elem Med Biol. 1997;11(2):116–122. doi:10.1016/s0946-672x(97)80036-3
3. Harrell JE, Cheng SX. Inability to reduce morbidity of diarrhea by ORS: can we design a better therapy? Pediatr Res. 2018;83(3):559–563. doi:10.1038/pr.2017.295
4. Chen MR, Zhao J, Fu SF, et al. Clinical practice of Chinese medicine navel therapy for chronic diarrhea: a literature review. J Gastroenterol Hepatol. 2019;34(4):643–649. doi:10.1111/jgh.14549
5. Kluger N. Dermatoses ombilicales et péri-ombilicales [Umbilical and periumbilical dermatoses]. Ann Dermatol Venereol. 2014;141(3):224–235. French. doi:10.1016/j.annder.2013.10.039
6. Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia. Chinese Medical Science and Technology Press; 2020.
7. Li Z, Li J, Zhang F, et al. Antidiarrheal effect of sechang-zhixie-san on acute diarrhea mice and network pharmacology deciphering its characteristics and potential mechanisms. Evid Based Complement Alternat Med. 2020;2020(2020):8880298. doi:10.1155/2020/8880298
8. Li J, Wu XL, Chen Y, et al. Antidiarrheal properties of different extracts of Chinese herbal medicine formula Bao-Xie-Ning. J Integr Med. 2013;11(2):125–134. doi:10.3736/jintegrmed2013019
9. Yu C, Xiong Y, Chen D, et al. Ameliorative effects of atractylodin on intestinal inflammation and co-occurring dysmotility in both constipation and diarrhea prominent rats. Korean J Physiol Pharmacol. 2017;21(1):1–9. doi:10.4196/kjpp.2017.21.1.1
10. Zhang T, Lu SH, Bi Q, et al. Volatile oil from amomi fructus attenuates 5-fluorouracil-induced intestinal mucositis. Front Pharmacol. 2017;8:786. doi:10.3389/fphar.2017.00786
11. Nieto-Bobadilla MS, Siepmann F, Djouina M, et al. Controlled delivery of a new broad spectrum antibacterial agent against colitis: in vitro and in vivo performance. Eur J Pharm Biopharm. 2015;96:152–161. doi:10.1016/j.ejpb.2015.07.012
12. Madeddu S, Marongiu A, Sanna G, et al. Bovine Viral Diarrhea Virus (BVDV): a preliminary study on antiviral properties of some aromatic and medicinal plants. Pathogens. 2021;10(4):403. doi:10.3390/pathogens1004040
13. Kimura Y, Sumiyoshi M. Effects of an Atractylodes lancea rhizome extract and a volatile component β-eudesmol on gastrointestinal motility in mice. J Ethnopharmacol. 2012;141(1):530–536. doi:10.1016/j.jep.2012.02.031
14. Rao CV, Vijayakumar M, Sairam K, Kumar V. Antidiarrhoeal activity of the standardised extract of Cinnamomum tamala in experimental rats. J Nat Med. 2008;62(4):396–402. doi:10.1007/s11418-008-0258-8
15. Gilani AH, Shah AJ, Zubair A, et al. Chemical composition and mechanisms underlying the spasmolytic and bronchodilatory properties of the essential oil of Nepeta cataria L. J Ethnopharmacol. 2009;121(3):405–411. doi:10.1016/j.jep.2008.11.004
16. Leal-Cardoso JH, Lahlou S, Coelho-de-souza AN, et al. Inhibitory actions of eugenol on rat isolated ileum. Can J Physiol Pharmacol. 2002;80(9):901–906. doi:10.1139/y02-117
17. Kheder DA, Al-Habib OAM, Gilardoni G, Vidari G. Components of volatile fractions from eucalyptus camaldulensis leaves from Iraqi-Kurdistan and their potent spasmolytic effects. Molecules. 2020;25(4):804. doi:10.3390/molecules25040804
18. Leonhardt V, Leal-Cardoso JH, Lahlou S, et al. Antispasmodic effects of essential oil of Pterodon polygalaeflorus and its main constituent β-caryophyllene on rat isolated ileum. Fundam Clin Pharmacol. 2010;24(6):749–758. doi:10.1111/j.1472-8206.2009.00800.x
19. Heghes SC, Vostinaru O, Rus LM, et al. Antispasmodic effect of essential oils and their constituents: a review. Molecules. 2019;24(9):1675. doi:10.3390/molecules24091675
20. Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev. 2015;67(4):821–870. doi:10.1124/pr.114.009654
21. Mo L, Zeng Z, Li Y, et al. Animal study of the anti-diarrhea effect and microbial diversity of dark tea produced by the Yao population of Guangxi. Food Funct. 2019;10(4):1999–2009. doi:10.1039/c9fo00110g
22. Ren WX, Xu ZJ, Tang K, et al. Study on the treatment of diarrhea abdominal pain in children with Ding Gui Infantile Navel Paste. Nation Chinese Medi Innovation & Research Forum Academic Proceedings. 2009.
23. Yu YH, Zhang GW, Yang XN, et al. Study on antidiarrhea and analgesic effect of Dinggui Infantile Navel Paste on mice. Inter J Tradit Chinese Med. 2014;36(6):535–538.
24. Liu HM, Liu L, Liu Q. 小儿腹泻外敷散对胃肠动力学的影响 [Effect of Xiaoer Fuxie Waifu powder on gastrointestinal dynamics]. Zhongguo Zhong Yao Za Zhi. 2013;38(14):2399–2402. Chinese.
25. Adeniyi OS, Akomolafe RO, Ojabo CO, Eru EU, Olaleye SB. Effect of zinc treatment on intestinal motility in experimentally induced diarrhea in rats. Niger J Physiol Sci. 2014;29(1):11–15.
26. Ihara E, Beck PL, Chappellaz M, et al. Mitogen-activated protein kinase pathways contribute to hypercontractility and increased Ca 2+ sensitization in murine experimental colitis. Mol Pharmacol. 2009;75(5):1031–1041. doi:10.1124/mol.108.049858
27. Tang Y, Liang SW, Quan XJ, Luo HS, Liu Y. 白介素6通过阻断L型钙离子通道活性抑制急性胰腺炎大鼠结肠纵行肌条收缩 [IL-6 inhibits colonic longitudinal muscle contraction by inactivating L-type calcium channel in rats with pancreatitis]. Sheng Li Xue Bao. 2019;71(5):717–724. Chinese. doi:10.13294/j.aps.2019.0069
28. Zhu J, Luo HS, Chen L, Zhou T. 腹泻型肠易激综合征大鼠结肠L-型钙通道α1C及α1D亚基的表达变化 [Altered expression of L-type calcium channel alpha1C and alpha1D subunits in colon of rats with diarrhea-predominant irritable bowel syndrome]. Zhonghua Yi Xue Za Zhi. 2009;89(38):2713–2717. Chinese.
29. Lee CW, Sarna SK, Singaram C, Casper MA. Ca2+ channel blockade by verapamil inhibits GMCs and diarrhea during small intestinal inflammation. Am J Physiol. 1997;273(4):G785–794. doi:10.1152/ajpgi.1997.273.4.G785
30. Gonzalez-Lozano MA, Klemmer P, Gebuis T, et al. Dynamics of the mouse brain cortical synaptic proteome during postnatal brain development. Sci Rep. 2016;6:35456. doi:10.1038/srep35456
31. Howarth FC, Qureshi MA, Jayaprakash P, et al. The pattern of mRNA expression is changed in sinoatrial node from Goto-Kakizaki type 2 diabetic rat heart. J Diabetes Res. 2018;2018:8454078. doi:10.1155/2018/8454078
32. Choudhury BK, Shi XZ, Sarna SK. Gene plasticity in colonic circular smooth muscle cells underlies motility dysfunction in a model of postinfective IBS. Am J Physiol Gastrointest Liver Physiol. 2009;296(3):G632–642. doi:10.1152/ajpgi.90673.2008
33. Qiu YT. PKA/cAMP in DRG Neurons Regulates Calcium-Gated-Channels Mediating Colonic Smooth Muscle Tone and Intervention of Shugan Jianpi Recipe. Guangxi Uni Chinese Med. 2019.
34. Guerra DD, Bok R, Lorca RA, Hurt KJ. Protein kinase A facilitates relaxation of mouse ileum via phosphorylation of neuronal nitric oxide synthase. Br J Pharmacol. 2020;177(12):2765–2778. doi:10.1111/bph.15001
35. Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59(4):675–680. doi:10.1016/0092-8674(89)90013-5
36. Yun CH, Lamprecht G, Forster DV, Sidor A. NHE3 kinase A regulatory protein E3KARP binds the epithelial brush border Na+/H+ exchanger NHE3 and the cytoskeletal protein ezrin. J Biol Chem. 1998;273(40):25856–25863. doi:10.1074/jbc.273.40.25856
37. Shi XZ, Choudhury BK, Pasricha PJ, Sarna SK. A novel role of VIP in colonic motility function: induction of excitation-transcription coupling in smooth muscle cells. Gastroenterology. 2007;132(4):1388–1400. doi:10.1053/j.gastro.2007.02.016
38. De Jongh KS, Murphy BJ, Colvin AA, et al. Specific phosphorylation of a site in the full-length form of the alpha 1 subunit of the cardiac L-type calcium channel by adenosine 3’,5’-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35(32):10392–10402. doi:10.1021/bi953023c
39. Vandael DH, Mahapatra S, Calorio C, Marcantoni A, Carbone E. Cav1.3 and Cav1.2 channels of adrenal chromaffin cells: emerging views on cAMP/cGMP-mediated phosphorylation and role in pacemaking. Biochim Biophys Acta. 2013;1828(7):1608–1618. doi:10.1016/j.bbamem.2012.11.013
40. Xu HX, Yu J. Curative effective of Shenbei Guchang capsules in the treatment of diarrhea type irritable bowel syndrome and its influence on PKA/TRPV1 pathway. Chinese J Integrated Tradit Western Med Digest. 2018;26(12):1005–1008.
41. Leng-Peschlow E. Sennoside-induced secretion and its relevance for the laxative effect. Pharmacology. 1993;47(Suppl 1):14–21. doi:10.1159/000139838
42. Yang F, Cao M, Zhong L, et al. Sub-chronic and developmental toxicity of transdermal delivery of Renzhu ointment in young SD rats. Cutan Ocul Toxicol. 2022;41(3):226–237. doi:10.1080/15569527.2022.2088781
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.