Back to Journals » Neuropsychiatric Disease and Treatment » Volume 14
Removal of blood amyloid-β with hemodialysis reduced brain amyloid-β, confirmed by brain imaging: a case report
Authors Kitaguchi N , Kato T , Matsunaga S, Hirano K, Iwata K , Kawaguchi K, Fujita K, Takechi H , Hasegawa M , Yuzawa Y, Ito K
Received 1 September 2018
Accepted for publication 3 October 2018
Published 1 November 2018 Volume 2018:14 Pages 2931—2937
DOI https://doi.org/10.2147/NDT.S186118
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Nobuya Kitaguchi,1 Takashi Kato,2 Shinji Matsunaga,3 Kyoko Hirano,4 Kaori Iwata,2 Kazunori Kawaguchi,1 Kiyoshi Fujita,5 Hajime Takechi,3 Midori Hasegawa,4 Yukio Yuzawa,4 Kengo Ito2
1School of Health Sciences, Fujita Health University, Toyoake, Aichi, Japan; 2Department of Brain Science and Molecular Imaging, Research Institute, National Center for Geriatrics and Gerontology, Obu, Aichi, Japan; 3Department of Geriatrics and Cognitive Disorders, School of Medicine, Fujita Health University, Toyoake, Aichi, Japan; 4Department of Nephrology, School of Medicine, Fujita Health University, Toyoake, Aichi, Japan; 5Okehazama Hospital, Fujita Kokoro Center, Seisinkai, Toyoake, Aichi, Japan
Abstract: The accumulation of amyloid-β protein (Aβ) in the brain signifies a major pathological change of Alzheimer’s disease (AD). Extracorporeal blood Aβ removal system (E-BARS) has been under development as a tool for enhancing the clearance of Aβ from the brain. Previously, we revealed that dialyzers remove blood Aβs effectively, evoking substantial Aβ influx into the blood during hemodialysis sessions as one form of blood Aβ removal by E-BARS, and that postmortem brains of hemodialysis patients exhibited lower Aβ accumulation. Here, we present a case report of a 77-year-old male patient with end-stage renal failure whose Aβ accumulation in the brain declined by initiating and continuing hemodialysis for 6 months. This report suggests that blood Aβ removal by E-BARS could be an effective therapeutic method for AD.
Keywords: amyloid-β, Aβ, PiB/PET, blood purification, blood Aβ removal, Alzheimer’s disease, E-BARS, extracorporeal blood Aβ removal system
Introduction
The deposition of amyloid-β protein (Aβ) is one of the major hallmarks in the brain of Alzheimer’s disease (AD).1 Of several Aβ species present in the brain and plasma, 40-amino acid Aβ1–40 and 42-amino acid Aβ1–42 are the most prevalent. Aβ1–42 aggregates more readily to form neurotoxic Aβ oligomers. Recent findings suggested that the decreased clearance of Aβ from the brain could be a mechanism for the increase of brain Aβ, especially in sporadic AD cases.1,2
As a tool for enhancing the clearance of Aβ from the brain, we proposed extracorporeal blood Aβ removal system (E-BARS) based on our hypothesis that the rapid removal of blood Aβ could facilitate peripheral Aβ drainage from the brain.3 As shown in Figure 1, blood Aβs are extracorporeally removed using Aβ removal devices, decreasing the concentrations of blood Aβs, which might accelerate Aβ transport from the brain into the blood. There are several effective Aβ removal devices. We found that the most efficient materials are hexadecyl-alkylated cellulose beads4 and hollow fibers in dialyzers made of hydrophobic materials such as polysulfone and polymethylmethacrylate.5,6 These materials exhibit Aβ removal efficiencies as high as ~100% for both Aβ1–40 and Aβ1–42 in vitro, with enough contacting time with Aβs,4–6 and 50% or more in extracorporeal circulation with a blood flow of 200 mL/min, such as in hemodialysis.7,8 Adsorption is the primary Aβ removal mechanism, even in dialyzers used for hemodialysis. An investigation of patients with end-stage renal failure revealed that a massive influx of Aβ from certain tissues into the blood occurred during hemodialysis sessions, which removed blood Aβs as one form of blood Aβ removal by E-BARSs.7–9 One of the origins of this large Aβ influx could be the brain, based on our histopathological studies reporting that the Aβ accumulation in the brains of patients undergoing hemodialysis was markedly lower than that in age-matched controls without hemodialysis.10 Furthermore, we revealed that cognitive functions of hemodialysis patients were maintained or marginally improved in a prospective study of 30 hemodialysis patients,9 and that a longer hemodialysis duration correlated with a lower dementia risk based on an analysis of over 200,000 hemodialysis patients in Japan.11
As a different method from our study to remove blood Aβs, plasma exchange therapy (discarding plasma containing Aβs, followed by the administration of albumin that is an Aβ-binding substance) was also effective in improving cognitive functions in patients with AD.12 In addition, peritoneal dialysis, which uses patients’ peritonea as dialysis membranes, reduced plasma Aβ in humans and brain Aβ in mouse AD models.13 Thus, the therapeutic strategy of removing blood Aβ has gathered attention as the peripheral Aβ clearance for AD.14
However, seemingly, no direct evidence indicates that Aβ in the brain could be reduced by hemodialysis, which removes blood Aβ. The present study prospectively investigates the change in the brain Aβ caused by hemodialysis in a patient with renal failure whose Aβ accumulation in the brain was confirmed at the hemodialysis initiation by positron emission tomography (PET) imaging with [C-11]-(2-[4-methyl-amino phenyl]-1,3-benzothiazol-6-ol) or Pittsburgh compound B (PiB) as a probe (PiB/PET). This prospective study was approved by the Institutional Review Board at Fujita Health University (latest approval number: HM16-266); the approval included permission to publish a case report. In addition, the patient provided written informed consent to participate in this study and to have the case details and any accompanying images published.
Case report
A 77-year-old male patient with end-stage renal failure was admitted to our hospital for hemodialysis initiation. He was nondiabetic and had a 60-year smoking history from age 16 to 76 years, and had no ApoE4 (ε4 allele). His preexisting diseases were hypertension and hyperuricemia. On admission, initial investigation revealed high serum creatinine (Cr; 8.63 mg/dL) and blood urea nitrogen (87.8 g/dL) concentrations in the patient’s blood. Other results were as follows: white blood cells, 5,900/μL; hemoglobin, 9.4 g/dL; platelets, 23.7×104/μL; total protein, 5.7 g/dL; and albumin, 2.8 g/dL. Urinalysis revealed urinary protein (3+) and occult blood (1+). Ultrasonography showed atrophy of the kidneys. Although the reason for renal failure was unclear, we considered it to be nephrosclerosis. On the basis of these data, the patient was started on hemodialysis.
At the hemodialysis initiation, the patient was diagnosed with mild cognitive impairment per the criteria of Petersen et al.15 Although he exhibited mild memory impairment, he experienced no trouble in his routine life. His Mini-Mental State Examination (MMSE) score was 23 points (disorientation to time, recall, and calculation). In addition, the score of Wechsler Memory Scale-Revised was 14/50 for Logical Memory I and 13/50 for Logical Memory II (the period of his education was within 9–12 years), suggesting a score near the low border of the normal range. His score on the short Japanese version of the Geriatric Depression Scale (GDS-S-J; published by Sugishita and Asada with the permission of the copyright holder16) was 6, indicating mild depressives. He exhibited no abnormality in the thyroid function, and vitamin B12 and serum calcium concentration levels. Furthermore, he displayed no signs and symptoms of any other psychological disorder.
The cranial magnetic resonance imaging (MRI; Figure 2) was performed 3 days before the hemodialysis initiation. The MRI revealed no apparent atrophy, and the patient’s hippocampus seemed to be normal. Regarding white matter lesions, the periventricular hyperintensity was grade 2, and deep white matter hyperintensity was grade 1, as evaluated using the scale of Fazekas et al.17 Furthermore, the Hachinski et al’s ischemic score18 of the patient was 3, suggesting a low possibility of vascular dementia. Hence, his cerebrovascular impairment was concluded as very mild and seemed not to affect his clinical symptoms.
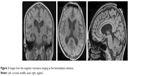  | Figure 2 Images from the magnetic resonance imaging at the hemodialysis initiation. |
Regarding the Aβ accumulation in the brain, three-dimensional static PET imaging for 50–70 minute after the intravenous injection of PiB was conducted using a PET–computed tomography camera, Biograph True V (Siemens Healthineers, Erlangen, Germany), as reported previously.9 PiB images were visually rated as PiB-positive or PiB-negative, as described previously.19 The first PiB/PET was performed 14 days after the hemodialysis initiation (Figure 3, top), which showed a PiB-positive result. A quantitative analysis using the method of Australian Imaging, Biomarkers and Lifestyle (AIBL)20 showed 1.91 as the global standard uptake value ratio with whole cerebellum as reference (SUVRWCb). After the patient received maintenance hemodialysis (4 h/day, 3 days/week) for 6 months (visit 2), PiB/PET was performed again. After 6-month hemodialysis, the Aβ accumulation in the brain was decreased (Figure 3, middle). The global SUVRWCb was 1.72, which was lower by “0.19” than that of visit 1. On visit 3, PiB/PET was performed again after maintenance hemodialysis of 12 months (Figure 3, bottom). The global SUVRWCb was 1.78, which was lower by “0.13” than that of visit 1. The change in SUVRWCb is summarized in Table 1.
Regarding cognitive functions, MMSE scores were the lowest at 23 points at the hemodialysis initiation but gradually increased during 3 months of maintenance hemodialysis (Figure 4). Then, the scores marginally increased (improved) and were maintained at 26 points after 194 and 285 days of the hemodialysis initiation, and at 27 points after 355, 453, and 719 days (the last data are not shown in Figure 4).
We measured the concentrations of Aβ monomers and oligomers in the plasma, as described previously (Figure 4).9 At the hemodialysis initiation, both plasma Aβ1–40 and Aβ1–42 concentrations, measured with High Sensitive Human β Amyloid (1–40) and (1–42) ELISA Kit Wako II (WAKO Pure Chemical, Osaka, Japan), were as high as 971 and 73.7 pg/mL, respectively, because of renal failure. After the hemodialysis initiation, both plasma Aβ1–40 and Aβ1–42 concentrations decreased for around 6 months, but then increased and were maintained as 709–1,033 and 77.2–99.2 pg/mL, respectively. In addition, the ratios of Aβ1–40 to Aβ1–42 were 0.077 (Day 3; Figure 4) and 0.076 (Day 0) just before the hemodialysis initiation. However, the ratios increased up to 0.117 for 6 months after hemodialysis initiation, but then were maintained between 0.08 and 0.10 (Figure 4).
Contrary to Aβ monomers, the concentrations of Aβ oligomers in the plasma, measured with Human Amyloid β Oligomers (82E1-specific) Assay Kit (IBL, Fujioka, Japan), were below the detection limit (0; Figure 4) at the hemodialysis initiation and were maintained at 0 for 194 days after hemodialysis initiation. Then, plasma Aβ oligomer concentrations increased to 13.6–33.8 pmol/L after 285 days of the hemodialysis initiation (Figure 4).
Serum Cr and β2-microglobulin (β2MG) concentrations just before hemodialysis sessions are shown in Figure 4 (measured by clinical test companies such as SRL, Nagoya, Japan). The change in both Cr and β2MG concentrations was small compared to Aβ monomers and oligomers.
Discussion
Although this study reports only one case, it is worth mentioning that 6-month hemodialysis decreased Aβ in the brain by “−0.19 SUVR” as measured by PiB/PET (Figure 3; Table 1). The reduction of “−0.19 SUVR” is comparable with that observed in a clinical study of aducanumab, where a decrease in SUVR of around 0.14 for 6 mg/kg and 0.21 for 10 mg/kg was noted after 26 weeks of administration.21
Our previous histochemical study reported that the Aβ accumulation in the postmortem brains of hemodialysis patients was markedly lower than those of age-matched controls.10 Although this histochemical study was cross-sectional, even 2-year hemodialysis seemed to decrease the Aβ accumulation in the brain;10 this finding is consistent with the finding of the present case report. Furthermore, the concentrations of both Aβ1–40 and Aβ1–42 in the cerebrospinal fluid decreased during the removal of blood Aβs by extracorporeal circuits with an Aβ adsorbent, hexadecyl-alkylated cellulose beads, in a study on rats;22 this finding also supports the decrease of the Aβ accumulation in the brain measured by PiB/PET in this study.
Clinical studies of anti-Aβ antibody therapy for AD revealed amyloid-related imaging abnormality (ARIA), such as microedema/microeffusion or hemosiderin deposition, as one of the adverse events to be considered.23 Because E-BARS removes blood Aβs, it may remove Aβs deposited in cerebral blood vessels24 as well as in brain parenchyma.10 Therefore, ARIA could occur in hemodialysis patients. Although we did not perform follow-up MRI for this patient, we have checked for the presence or absence of ARIA-like changes in a cross-sectional study of Aβ deposition in the brain slices of 17 hemodialysis patients. We did not find any ARIA-like changes, even in their brains, after 6–24 months of hemodialysis periods. Therefore, hemodialysis is less likely to cause ARIA-like changes.
Regarding the concentration change of Aβs in the blood, a study showed that the concentrations of Aβ1–40 and Aβ1–42 in the blood of patients with renal failure increased along with a decline of renal functions.8 Thus, the blood concentrations of Aβ1–40 and Aβ1–42 of our patient were very high at the hemodialysis initiation (Figure 4). After the hemodialysis initiation, which removes blood Aβ effectively, both Aβ1–40 and Aβ1–42 decreased for about 6 months, but then increased and maintained (Figure 4); this tendency is consistent with the findings of a previous prospective study of 30 hemodialysis patients.9 The ratio of Aβ1–42 to Aβ1–40 increased just after the hemodialysis initiation from 0.077 to 0.108 and was sustained at around 0.10.
The MMSE scores of our patient increased and were almost maintained at 26–28 points after 1 year of hemodialysis initiation (Figure 4); this tendency of improvement or maintenance of cognitive functions by hemodialysis, which removes blood Aβs, was also observed in our previous prospective study.9 As reported recently, higher concentrations of Aβ1–42 in the blood correlate with higher cognitive functions and larger hippocampal volume.25 Furthermore, a larger ratio of Aβ1–42 to Aβ1–40 in the blood predicts less risk of development of AD and dementia.26 Based on these reports, our finding of high concentrations of blood Aβ1–42 and a higher Aβ1–42:Aβ1–40 ratio after 6 months of hemodialysis might correlate with high MMSE scores in the same period.
Uremic toxins also cause cognitive impairment.27 Hemodialysis can remove uremic toxins from the blood and improve cognitive functions. Cognitive impairment caused by uremia is generally worst at initiation of hemodialysis and improves in a few weeks’ time. In our previous prospective study, MMSE scores were improved within just a few weeks after hemodialysis initiation.9 This short-term improvement may be attributed to removal of uremic toxins. In contrast, extracorporeal blood Aβ removal might contribute to the long-term effects of hemodialysis on cognitive improvement or maintenance, as shown in this study (Figure 4), in our prospective study of 30 hemodialysis patients during 18–36 months,9 and in our cross-sectional study of over 200,000 hemodialysis patients.11
It is known that β2MG deposition is observed in some hemodialysis patients resulting in dialysis-related amyloidosis, which causes carpal tunnel syndrome.28 β2MG is amyloidogenic but has a totally different amino acid sequence compared to Aβs. Recently, hemodialyzers have been improved to enhance the efficiency of removal of blood β2MG, resulting in fewer patients suffering from carpal tunnel syndrome. In our independent study, we found the removal efficiency of dialyzers (pre-/post-dialyzers) for β2MG was ~50%, similar to that for Aβ1–42 during hemodialysis sessions.29 The blood β2MG concentrations in our patient showed a small change after hemodialysis initiation compared to Aβs (Figure 4). This difference may be attributed to molecular weights (11.8 kDa for β2MG vs ~4 kDa for Aβ monomers), origins (almost all nucleated cells vs neurons), influx pathway (absence vs presence of the blood–brain barrier), absolute concentrations (mg/L vs pg/mL), and other factors such as the amounts of insoluble deposition and production rates.
According to the findings of this case report, a patient’s response to the hemodialysis initiation could be divided into two phases as follows. During the first phase – from 0 to 6 months during which a decrease in SUVR can be measured by PiB/PET – decrease in blood Aβs and improvement in cognitive functions are observed; during this phase, not so tightly aggregated/deposited Aβs might migrate into the blood. The influx of Aβs into the blood may not be so large, resulting in marginally lower concentrations of blood Aβs. The second phase – from 6 to 15 months during which no obvious change in SUVR can be shown by PiB/PET – increase in blood Aβs, especially Aβ1–42, and maintenance of cognitive functions can be observed. Aβ oligomers in the blood increase in this phase, may be because the influx of Aβs from the brain into the blood is gradually increased. The influx of Aβ monomers into the blood may increase, because the concentrations of blood Aβ monomers are high in the second phase despite high Aβ removal efficiencies of dialyzers compared to the first phase. The production and clearance of Aβs in the brain almost become balanced; however, this speculation should be evaluated with a larger sample size.
In conclusion, this case report suggests that E-BARS could be an effective therapeutic tool for AD.
Acknowledgments
The authors thank Dr Yoshiki Inui for discussing the ischemic status, and Clinical Psychologist Ms Tomoko Kitayama, Clinical Engineer Mr Shojiro Kitagawa, and Ms Miwa Sakata for their assistance. The authors also thank Dr Kazuyoshi Sakai and Mr Hiroshi Kawachi for discussion about ARIA and β2MG, respectively. This work was partly supported by KAKENHI (20509008, 23500531, 26282126), and the Smoking Research Foundation.
Author contributions
Nobuya Kitaguchi contributed to study design, steering the study, and measurements of blood Aβ monomers and oligomers. Takashi Kato, Kaori Iwata, and Kengo Ito contributed to performing PiB/PET, analyzing the data of PiB/PET and MRI, and single-photon emission computed tomography. Shinji Matsunaga and Hajime Takechi contributed to neurological diagnosis. Kyoko Hirano, Kiyoshi Fujita, Midori Hasegawa, and Yukio Yuzawa contributed to recruitment of the patient, diagnosis and treatment of renal failure, and initiation and maintenance of his hemodialysis. Kazunori Kawaguchi contributed to measurements of blood Aβ monomers and oligomers. All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Dr Kitaguchi has patents issued to this work (JP5843345 and US 10,112,000). The authors report no other conflicts of interest in this work.
References
Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8(6):595–608. | ||
Mawuenyega KG, Sigurdson W, Ovod V. Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science. 2010;330:1774. | ||
Kawaguchi K, Kitaguchi N, Nakai S, et al. Novel therapeutic approach for Alzheimer’s disease by removing amyloid beta protein from the brain with an extracorporeal removal system. J Artif Organs. 2010;13(1):31–37. | ||
Kawaguchi K, Takeuchi M, Yamagawa H, et al. A potential therapeutic system for Alzheimer’s disease using adsorbents with alkyl ligands for removal of blood amyloid β. J Artif Organs. 2013;16(2):211–217. | ||
Kawaguchi K, Saigusa A, Yamada S, et al. Toward the treatment for Alzheimer’s disease: adsorption is primary mechanism of removing amyloid β protein with hollow-fiber dialyzers of the suitable materials, polysulfone and polymethyl methacrylate. J Artif Organs. 2016;19(2):149–158. | ||
Kitaguchi N, Kawaguchi K, Yamazaki K, et al. Adsorptive filtration systems for effective removal of blood amyloid β: a potential therapy for Alzheimer’s disease. J Artif Organs. 2018;21(2):220–229. | ||
Kitaguchi N, Kawaguchi K, Nakai S, et al. Reduction of Alzheimer’s disease amyloid-β in plasma by hemodialysis and its relation to cognitive functions. Blood Purif. 2011;32(1):57–62. | ||
Kato M, Kawaguchi K, Nakai S, et al. Potential therapeutic system for Alzheimer’s disease: removal of blood Aβs by hemodialzyers and its effect on the cognitive functions of renal-failure patients. J Neural Transm. 2012;119(12):1533–1544. | ||
Kitaguchi N, Hasegawa M, Ito S, et al. A prospective study on blood Aβ levels and the cognitive function of patients with hemodialysis: a potential therapeutic strategy for Alzheimer’s disease. J Neural Transm. 2015;122(11):1593–1607. | ||
Sakai K, Senda T, Hata R, et al. Patients that have Undergone Hemodialysis Exhibit Lower Amyloid Deposition in the Brain: Evidence Supporting a Therapeutic Strategy for Alzheimer’s Disease by Removal of Blood Amyloid. J Alzheimers Dis. 2016;51(4):997–1002. | ||
Nakai S, Wakai K, Kanda E, Kawaguchi K, Sakai K, Kitaguchi N. Is hemodialysis itself a risk factor for dementia? An analysis of nationwide registry data of patients on maintenance hemodialysis in Japan. Renal Replacement Therapy. 2018;4(1):12. | ||
Boada M, Ortiz P, Anaya F, et al. Amyloid-targeted therapeutics in Alzheimer’s disease: use of human albumin in plasma exchange as a novel approach for Abeta mobilization. Drug News Perspect. 2009;22(6):325–239. | ||
Jin WS, Shen LL, Bu XL, et al. Peritoneal dialysis reduces amyloid-beta plasma levels in humans and attenuates Alzheimer-associated phenotypes in an APP/PS1 mouse model. Acta Neuropathol. 2017;134(2):207–220. | ||
Wood H. Alzheimer disease: Peripheral Aβ clearance – a therapeutic strategy for AD? Nat Rev Neurol. 2017;13(7):386. | ||
Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. | ||
Sugishita M, Asada T. Creating Geriatric Depression Scale-Short Version-Japanese, GDS-S-J. Jpn J Cognit Neurosci. 2009;11:87–90. | ||
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–356. | ||
Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol. 1975;32(9):632–637. | ||
Kaneko N, Nakamura A, Washimi Y, et al. Novel plasma biomarker surrogating cerebral amyloid deposition. Proc Jpn Acad Ser B Phys Biol Sci. 2014;90(9):353–364. | ||
Zhou L, Salvado O, Dore V, et al. MR-less surface-based amyloid assessment based on 11C PiB PET. PLoS One. 2014;9(1):e84777. | ||
Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–56. | ||
Kitaguchi N, Kawaguchi K, Kinomura J, et al. Extracorporeal Blood Aβ Removal System (EBARS) reduced soluble Aβ in the brain by triggering influx into the blood: rat studies. Poster presented at: Alzheimer’s Association International Conference AAIC; July 17, 2017; London. | ||
Salloway S, Marshall GA, Lu M, Brashear HR. Long-Term Safety and Efficacy of Bapineuzumab in Patients with Mild-To-Moderate Alzheimer’s Disease: A Phase 2, Open Label Extension Study. Curr Alzheimer Res. Epub 2018 Aug 20. | ||
Sakai K, Senda T, Hata R, et al. Evidence supporting a therapeutic strategy for Alzheimer’s disease by removal of blood amyloid; Patients that have undergone hemodialysis exhibit lower Amyloid deposition in the brain. Poster presented at: Alzheimer’s Association International Conference AAIC; July 25, 2016; Toronto. | ||
Hilal S, Wolters FJ, Verbeek MM, et al. Plasma amyloid-β levels, cerebral atrophy and risk of dementia: a population-based study. Alzheimers Res Ther. 2018;10(1):63. | ||
Koyama A, Okereke OI, Yang T, Blacker D, Selkoe DJ, Grodstein F. Plasma amyloid-β as a predictor of dementia and cognitive decline: A systematic review and meta-analysis. Arch Neurol. 2012;69:824–831. | ||
Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol. 2013;24(3):353–363. | ||
Labriola L, Jadoul M. Dialysis-related Amyloidosis: Is It Gone or Should It Be? Semin Dial. 2017;30(3):193–196. | ||
Morikawa H, Ohashi N, Kawaguchi K, et al. Potential therapeutic system for Alzheimer’s disease by removal of blood Aβ; Efficient Aβ removal system by enhancing adsorption on hollow fibers with hemodiafiltration. Poster presented at: Alzheimer’s Association International Conference AAIC; July 25, 2016; Toronto. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.