Back to Journals » Drug Design, Development and Therapy » Volume 17
Remimazolam vs Etomidate: Haemodynamic Effects in Hypertensive Elderly Patients Undergoing Non-Cardiac Surgery
Authors Chen J, Zou X, Hu B , Yang Y, Wang F, Zhou Q, Shen M
Received 17 July 2023
Accepted for publication 19 September 2023
Published 27 September 2023 Volume 2023:17 Pages 2943—2953
DOI https://doi.org/10.2147/DDDT.S425590
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Jiejuan Chen,1 Xiaohua Zou,2 Bailong Hu,2 Yang Yang,2 Feng Wang,2 Qian Zhou,2 Minhuan Shen2
1School of Anesthesiology, Guizhou Medical University, Guiyang City, Guizhou Province, People’s Republic of China; 2Department of Anesthesiology, Affiliated Hospital of Guizhou Medical University, Guiyang City, Guizhou Province, People’s Republic of China
Correspondence: Xiaohua Zou, Email [email protected]
Background: Remimazolam tosilate (RT) is a novel ultrashort-acting γ-aminobutyric acid subtype A (GABAA) agonist, with several advantages including rapid induction and recovery, stable haemodynamics, and mild respiratory inhibition. However, studies have not been conducted to explore the haemodynamic effects of RT in elderly hypertensive subjects undergoing non-cardiac surgery. Therefore, we sought to compare the effects of anaesthesia induction using different doses of RT and etomidate on the haemodynamics of this group of patients.
Methods: Patients were recruited into this single-center, prospective, randomized, double-blind trial from October 2022 to June 2023. A total of 150 hypertensive elderly undergoing non-cardiac surgery were randomly assigned into 0.2 mg/kg RT group (Group RL), 0.3 mg/kg RT group (Group RH) and 0.3 mg/kg etomidate group (Group E). The primary outcome of the study was haemodynamic changes (mean arterial pressure fluctuation value -∆MAP and heart rate fluctuation value -∆HR) observed during anaesthesia induction. Secondary outcomes included incidence of adverse cardiovascular events and adverse drug reactions (injection pain and myoclonus), cumulative doses of vasoactive drugs and vital signs at different time points.
Results: Patients in Group E and Group RL had significantly lower haemodynamic fluctuations (∆MAP), lower incidence of hypotension and cumulative dose of ephedrine than subjects in Group RH. Patients in groups RL and RH had significantly lower incidence of injection pain and myoclonus compared with patients in group E. The results showed no statistically significant differences in ∆HR, hypertension, bradycardia, tachycardia, and time to loss of eye-opening reflex and start of intubation, and vital signs at different time points among the three groups.
Conclusion: Use of low-dose RT (0.2 mg/kg) for induction of non-cardiac surgical anaesthesia in elderly hypertensive patients is more effective in maintaining haemodynamic stability and has fewer adverse effects compared with etomidate.
Keywords: remimazolam tosylate, etomidate, haemodynamics, elderly patients, hypertension, non-cardiac surgery
Introduction
Elderly patients with hypertension have a high risk of hypotension and arrhythmia, especially during anaesthesia induction. Therefore, anesthesiologists should use anesthetic drugs that do not affect the haemodynamic stability in this group of patients.
Etomidate is a non-barbiturate intravenous sedative. This drug is a hydroxylated imidazole salt that exerts sedative and anesthetic effects mainly by binding to γ-aminobutyric acid subtype a (GABAA) receptors in the central nervous system.1 Etomidate is the preferred sedative drug for induction of general anaesthesia in critically ill patients because it maintains haemodynamic stability2–6 However, etomidate has several adverse effects, such as adrenal inhibition,7,8 inducing muscle spasm,9 injection pain,10 nausea and vomiting, which limit its clinical application.
Remimazolam tosylate (RT) is a recently reported benzodiazepine that acts on GABAA receptors and has similar structure and binding activity to remimazolam. In addition, RT has a short half-life resulting in rapid acting onset and recovery than short-acting sedatives currently used clinically.11,12 Several studies report that RT has several advantages, such as rapid onset, short maintenance and recovery times, and is not accumulated in the system. In addition, RT does not induce cardiorespiratory depression or affect liver and kidney function.13–15 A previous multicentre clinical study reported the safety of RT used during upper gastrointestinal endoscopy compared with propofol, and the results showed that RT was associated with faster patient recovery.14 Moreover, a multicentre clinical study was conducted to explore the efficacy and safety of remimazolam in vulnerable patients (ASA Class III) undergoing elective general surgery, and the findings showed that the two induction regimens (6 and 12 mgkg−1 h−1) had similar efficacy and safety in ASA Class III patients undergoing surgery.16 A significantly shorter time to loss of consciousness was observed when as higher remimazolam dosage was used.16 Liu et al reported that remimazolam is safe and effective for anaesthesia induction and can be used as an alternative to propofol during anaesthesia induction in patients undergoing valve replacement surgery.17 Several studies have demonstrated that RT is a safe and effective option for anaesthesia induction during digestive endoscopy, bronchoscopy, induction and maintenance of general anesthesia, and induction of anaesthesia in high-risk patients and cardiac surgery patients.14,16,17
However, there are no clinical studies that have compared the effect of RT and etomidate on the haemodynamics of elderly patients with hypertension during anaesthesia induction. Therefore, the aim of the present study was to compare the effect of RT and etomidate on haemodynamics of elderly patients with hypertension undergoing non-cardiac surgery. The findings of the present study will provide a safer alternative for anaesthesia induction during non-cardiac surgery in elderly patients with hypertension.
Methods
Study Design
The present study was a prospective, randomized, controlled, double-blind, single-center trial. The aim of the trial was to evaluate the effect of different doses of RT and etomidate on haemodynamics change during anaesthesia induction on elderly patients with hypertension scheduled to undergo non-cardiac surgery. The study was approved by the Medical Ethics Committee of the Affiliated Hospital of Guizhou Medical University (Ref: 2022100K) and was registered in the Chinese Clinical Trial Registry (ChiCTR2200064997). The study was conducted according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients before the enrollment into the study. A flowchart showing the study design is presented in Figure 1.
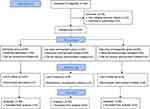 |
Figure 1 Consort flow diagram of the trial design. |
Patients aged > 65 years, with an American Society of Anesthesiologists (ASA) grade I–III and scheduled for elective non-cardiac surgery from October 2022 to June 2023 were recruited into this study. The exclusion criteria were as follows: patients diagnosed with (1) severe cardiovascular or pulmonary diseases; (2) uncontrolled or poorly controlled hypertension; (3) a history of renal or hepatic dysfunction; (4) neurocognitive or psychiatric disorders; (5) known allergic or contraindication to benzodiazepines, opioids and etomidate; (6) a history of alcohol abuse or benzodiazepines or opioids addiction; and (7) possible blocked airway.
The patients were randomly assigned to three groups at a 1:1:1 allocation ratio using a computer-generated algorithm. The numbers representing the patients were initially placed in separate opaque envelopes. The envelopes were then opened by a nurse who was not involved in the study just before performing anaesthesia induction. The study groups were as follows: 0.2 mg/kg RT group (Group RL), 0.3 mg/kg RT group (Group RH) and 0.3 mg/kg etomidate group (Group E). All the syringes and intravenous lines used to administer the experimental drugs were sealed with a masking tape to conceal the identity of the drugs. Data were collected and analysed by two researchers who were blinded to the randomization to minimise bias and achieve allocation concealment. Experienced trial supervisors supervised the trial to ensure integrity of the blinding process and regularly reviewed the experimental data for accuracy and completeness. Patients, surgeons, nurses, anesthesiologists, and outcome observers were blinded to the randomization and drugs administered to the various groups throughout the study period.
Study Interventions
All patients were requested to fast for 8h before the surgery. Standard monitoring procedures, including electrocardiogram (ECG) test, noninvasive blood pressure (NIBP) evaluation, and determination of peripheral oxygen saturation (SpO2) and bispectral index (BIS) of the patient were conducted in the surgery room. A 18-gauge intravenous cannula was used for Ringer lactate (10 mL/kg) infusion. All subjects were subjected to preoxygenation (breathing spontaneous using a closed mask with 100% oxygen), then patients in Group E received etomidate (0.3 mg/kg) (Jiang Su Heng Rui Medicine Co. Ltd, Jiangsu Province, China) for about 30s, patients in Group RL received RT (0.2 mg/kg) (Jiang Su Heng Rui Medicine Co. Ltd, Jiangsu Province, China) for approximately 30s whereas patients in Group RH received RT (0.3 mg/kg) for approximately 30s. Subsequently, patients in the three groups received an intravenous injection of sufentanil (0.4 ug/kg) for approximately 30s. The period taken for eyelash reflex disappearance was determined every 5s after drug administration by gently tapping the eyelashes with a sterile cotton swab. An additional dose of etomidate (0.05 mg/kg) or RT (0.05 mg/kg) was administered to patients if the eyelash reflex did not not disappear within a minute after intravenous administration of sufentanil. All patients received phenylsulfonyl cisatracurium (0.15 mg/kg) for muscle relaxation after loss of consciousness was achieved (eyelash reflex disappearance). Endotracheal intubation was performed 2 min after administration of phenylsulfonyl cisatracurium to maintain PetCO2 within a range of 35–45 mm Hg. The BIS index was maintained within a range of 40–60.18 Anaesthesia was maintained with sevoflurane in 50% oxygen and/or other intravenous anesthetics were administered 2 min after intubation depending on the requirements for surgery.
Systolic blood pressure (SBP), diastolic blood pressure (DBP), MAP, HR were examined and recorded at baseline and at a 10-min interval following drug administration (once per minute). Endotracheal intubation and anaesthesia administration were performed by an anesthesiologist who was blinded to the study groups. The duration between the time of initiation of drug administration and time of eyelash reflex disappearance and endotracheal intubation were recorded for each patient.
Systolic arterial pressure below 80 mmHg or a more than 20% decrease in systolic arterial pressure during induction relative to the baseline level lasting at least 1 min indicated hypotension. In this study, 6 mg ephedrine or more was administered intravenously until the MAP was restored to normal range once hypotension occurred. HR decrease below 45 beats per min (bpm) indicated severe bradycardia, and 0.5 mg atropine was administrated by intravenous injection to alleviate it. MAP increase above 120 mmHg was managed by appropriate nitroglycerin dose. HR increase above 120 bpm was managed using appropriate esmolol dose. Incidence of adverse cardiovascular events (hypotension, severe bradycardia, hypertension, tachycardia and arrhythmia) and the cumulative doses of administered vasoactive drugs were recorded.
Assessment of Primary and Secondary Outcomes
The primary outcome of the present study was the change in haemodynamic parameters (∆MAP and ∆HR). ∆MAP was defined as the difference between maximum or minimum MAP and the baseline MAP. ∆HR was defined as the difference between the maximum or minimum HR and the baseline HR. Secondary outcomes included the incidence of adverse cardiovascular events, incidence of adverse drug reactions (injection pain and myoclonus), cumulative doses of vasoactive drugs administered per patient, vital signs at different time points, the duration between the time of initiation of drug administration and time of eyelash reflex disappearance and endotracheal intubation.
Statistical Analysis
The sample size was calculated based on the primary outcome, which was the change in haemodynamic parameters (∆MAP). A review of literature showed that there were no similar previous studies that reported a method for determination of the sample size the study protocol was designed. Therefore, a pilot study comprising 10 patients in each group was conducted prior to the trial. The results of the pilot study were not included in the full-scale study. The results of the pilot study showed that the ∆MAP (mean ± SD) in group E, Group RL and Group RH were 15.68 ± 6.54 mmHg, 13.86 ± 8.02 mmHg and 20.37 ± 10.53 mmHg, respectively. Assuming α = 0.05 and power = 0.90, 38 patients were sufficient for each group according to findings from the pilot study. A total of 150 patients were enrolled in this study (n = 50 in each group) to account for a potential withdrawal rate of 20%. The sample size was calculated using the PASS software (version 15, NCSS, LCC, Kaysville, UT, United States).
Statistical data analysis was performed using SPSS 25.0 software (SPSS Inc, Chicago, IL, USA). Data were presented as means ± standard deviations, medians and interquartile ranges, or numbers and percentages, based on the type and distribution of the data. Baseline balance was assessed using absolute standardised difference (ASD), calculated as the absolute difference in means, medians or proportions divided by the combined standard deviation. Baseline variables with an absolute standardized difference of 0.877 or greater (ie, 1.96 × sqrt ((n1 + n2)/(n1 × n2))) were considered imbalanced, where n1 and n2 were the number of patients in each randomized group.19 Differences in continuous variables between the groups were evaluated by one-way analysis of variance (ANOVA). Differences in vital signs at various time points were evaluated by repeated-measures ANOVA. Data that were not normally distributed (ephedrine use) were presented as median (25th and 75th percentile) and compared using Kruskal–Wallis test. Post hoc tests were performed using the Bonferroni method. Chi-square test was performed to compare differences in independent qualitative data. Fischer’s test was conducted for data that did not meet chi-square test conditions. Data on gender, ASA grade, hypertension grade, surgical procedure, incidence of adverse reactions were presented as numbers and percentages and χ2 test was conducted to compared differences between groups. All tests were two-sided and P-value < 0.05 was considered statistically significant.
Results
A total of 168 patients were recruited into this study. Five participants declined to participate, eight patients exhibited potential difficulty with intubation and five patients presented with haemodynamic instability, so the procedure was suspended. Therefore, 150 patients were included in the final analysis (Figure 1). The baseline characteristics and demographic data of the patients in the three groups are presented in Table 1. The results showed that the demographic and baseline characteristics of patients between three groups were balanced (ASD < 0.877).
 |
Table 1 Demographic and Baseline Characteristics of Patients |
Patients in the E and RL groups exhibited significantly lower haemodynamic fluctuations (∆MAP) than subjects in the RH group (17.02 ± 5.16, 15.94 ± 5.48 and 19.72 ± 4.65, respectively, F = 7.267, P < 0.05). The difference in ∆MAP between the RL and RH groups was not statistically significant. The three groups did not show statistically significant differences in ∆HR (Table 2).
 |
Table 2 Haemodynamic Fluctuations Between the Three Groups |
The incidence of hypotension and the cumulative dose of ephedrine were significantly lower in patients in group E and Group RL than in Group RH (P < 0.05), but the differences in the two variables between groups E and RL were not statistically significant. Patients in groups RL and RH had a significantly lower incidence of injection pain and myoclonus compared with subjects in group E (P < 0.05), but the difference between groups RL and RH was not statistically significant. The incidence of hypertension, bradycardia and tachycardia were not significantly different among the three groups (Table 3).
 |
Table 3 Comparison of Adverse Reactions Between the Three Groups |
The results showed no statistically significant differences in duration between initiation of drug administration to the disappearance of the eye-opening reflex and the period of intubation, and number of vital signs at different time points among the three groups (Table 4 and Figure 2).
 |
Table 4 Comparison of the Time from the Initiation of Drug Administration to Eyelash Reflex Disappearance and Endotracheal Intubation Between the Three Groups |
Discussion
In the present prospective, randomized, double-blind controlled clinical trial, participants were randomly assigned to the etomidate group, the low-dose RT group and the high-dose RT group. In this study, we compared the efficacy of RT and etomidate in anaesthesia induction and the effect on haemodynamics in elderly patients with hypertension undergoing non-cardiac surgery. The findings showed that induction of anaesthesia in elderly patients with hypertension using RT (0.2 mg/kg) was characterized by stable haemodynamic and fewer adverse reactions compared with use of etomidate and 0.3 mg/kg RT.
Elderly patients with hypertension have a high risk of hypotension during anaesthesia induction due to reduced organ function and low cardiac reserve function.20 This group of patients, especially elderly patients with higher hypertension classification, presents with various cardiovascular conditions during induction of anaesthesia. Therefore, it is imperative to prevent the occurrence of hypotension, myocardial ischaemia and hypoxia, and to maintain a stable circulatory function during induction of anaesthesia in this group of patients. Etomidate is a common clinical drug for anaesthesia induction in elderly patients presenting with haemodynamic instability because it is associated with low hypotension incidence.21–23 However, etomidate is associated with adverse effects, such as adrenocortical suppression and myoclonus, which limit its clinical application.1,24
RT is a recently developed ultra-short-acting GABAA agonist characterized by rapid onset of action, short half-life, rapid metabolism and non-hepatic and renal dependence, and it ensures haemodynamic stability.25,26 The results showed that patients in the etomidate and low-dose RT groups had significantly lower haemodynamic fluctuations (∆MAP) than patients in the high-dose RT group (17.02 ± 5.16, 15.94 ± 5.48 and 19.72 ± 4.65 mmHg respectively). Notably, no significant differences in ∆HR were observed among the three groups. Although the difference in ∆MAP was only subtle a 3–5 mmHg change in blood pressure can cause imbalance in myocardial oxygen supply and demand.27,28 The effect is significant in elderly hypertensive patients with reduced cardiac reserve function and subjects at with high risk of myocardial ischaemia. Therefore, reducing haemodynamic fluctuations during induction of anaesthesia and use of safer drugs that maintain and haemodynamic stability during induction of anaesthesia in this group of patients is key.
In this study, the incidence of hypotension and the cumulative dose of ephedrine in patients in the etomidate and low-dose RT groups were significantly lower than subjects in the high-dose RT group. Hypotension is a common side effect of clinical drugs used for induction of anaesthesia. Approximately one third of perioperative hypotension incidence occurs between induction of anaesthesia and surgical incision.29,30 Previous studies report that post-induction hypotension can be attributed to the reduced myocardial contractility and venodilatation, which cause reduced venous return. In addition, post-induction hypotension is potentially caused by arterial dilatation accompanied by reduced systemic vascular resistance following induction of anaesthesia.31–34 Incidence of hypotension in elderly patients with hypertension is significantly associated with increased occurrence of organ damage, cardiovascular conditions and postoperative cognitive impairment.35 Hypotension and the associated conditions may prolong hospital stay of patients and result in higher healthcare costs for this group of patients. In the present study, the results showed that reducing MAP fluctuations during induction of anaesthesia significantly reduced the incidence of hypotension. Therefore, a highly stable MAP during induction of anaesthesia can reduce the incidence of hypotension in elderly patients with hypertension. The present findings showed no statistically significant difference in incidence of hypertension, tachycardia and bradycardia during induction of anaesthesia among the three groups of patients, which is consistent with findings reported by Hu et al.36 The incidence of tachycardia and bradycardia was higher in patients receiving high doses of remimazolam than in patients administered with low doses of remimazolam and etomidate, but the difference was not statistically significant. The appropriate doses of remimazolam and etomidate have less impact on the cardiovascular system,17,36 but a larger sample size may yield different results.
Previous findings indicate that RT reduces the incidence of hypotension, probably through the subtle effect of rimazolam on cardiac output and systemic vascular resistance.37 Moreover, RT can maintain haemodynamic stability by reducing haemodynamic fluctuations, which decreasing the stress response and enhancing myocardial contractility.38 In the current study, the findings show that induction of anaesthesia in elderly hypertensive patients using a low-dose of RT (0.2 mg/kg) is results in similar haemodynamic stability and but fewer adverse effects compared with use of etomidate. Liu et al reported that patients that received RT (0.3 mg/kg) exhibited higher haemodynamic stability than subjects administered with propofol during cardiac surgery.17 Hu et al used RT for induction of anaesthesia in patients undergoing cardiac surgery and reported that 0.2 mg/kg RT induced similar haemodynamic stability as etomidate, but RT was associated with fewer complications.36 Zhang et al observed that 0.2–0.4 mg/kg RT induced higher haemodynamic stability compared with propofol in elderly patients undergoing hip surgery.39 In the present study, two doses of 0.2 mg/kg and 0.3 mg/kg were used based on the hypertension status and the poor cardiac reserve function of elderly patients. The present findings indicate that 0.2 mg/kg RT is safe for induction of anaesthesia in elderly hypertensive patients. However, further studies should be conducted to determine the optimal dose of RT that reduces occurrence of hypotension and other complications during non-cardiac surgery in elderly hypertensive patients.
Myoclonus is a common adverse effect of etomidate. Occurrence of myoclonus in patients with poor cardiovascular reserve can lead to serious adverse consequences, such as myalgia, muscle fibre damage, increased cerebral metabolic rate and increased energy consumption.40,41 In this study, the incidence of myoclonus in patients in the etomidate group was 26%, which is similar to the incidence of myoclonus reported in a study conducted by Liu et al.42 On the contrary, myoclonus occurred in one and two patients in the low-dose RT group and the high-dose RT group, respectively. The severity of myoclonus was significantly lower in the RT group than that of the etomidate group. These results indicate that RT is safe and effective in reducing myoclonic adverse reactions. The underlying mechanism of induction of myoclonus by etomidate is unclear. Previous findings indicate that etomidate-induced myoclonus is dependent on the dose of the drug and is mainly caused by neocortical glutamate accumulation and is medicated through the N-methyl-D-aspartate receptor (NMDAR). Myoclonus induced by etomidate is associated with downregulation of the expression of KCC2 protein mediated by NMDR.43 Moreover, induction of myoclonus by etomidate may be through inhibition of GABAA neurons, which activates the pathways associated with skeletal muscle.44,45 Although RT also exerts its effect through GABAA receptors, the incidence of myoclonus caused by RT is lower, and the specific mechanism of RT should be explored further.
Injection pain is also a common adverse effect of etomidate. Previous studies report varying degrees of injection pain associated with etomidate.36,46,47 The incidence of etomidate injection pain among the subjects in this study was 14%. The incidence of injection pain was significantly lower in the low-dose RT group and the high-dose RT group compared with etomidate group. The chemical structure of RT is different from that of isoproterenol because RT lacks a phenol group. As a result, RT causes less vascular irritation and less injection pain. Notably, there was no statistically significant difference in the duration between the start of drug administration to the disappearance of the eye-opening reflex and the time taken to reach tracheal intubation among the three groups, indicating rapid onset of drug action.
The present study has several limitations. Firstly, this was a single-centre study with a relatively small sample size. Therefore, study trials with a larger sample size should be conducted to determine the haemodynamic effects of RT in elderly hypertensive patients undergoing non-cardiac surgery. Secondly, the effective dose of etomidate and RT was not explored in this study and therefore etomidate and RT doses used in the study were determined based on a pilot study. Thirdly, we did not monitor other indicators of cardiac function such as cardiac output, volume per beat and vascular resistance. Therefore, we could not effectively interpret the effect of RT on haemodynamics and the mechanism underlying this effect. Further clinical studies should be conducted to address these limitations and further validate the results reported in this study.
Conclusion
The present findings show that use of low-dose RT (0.2 mg/kg) for induction of anaesthesia during non-cardiac surgery in elderly patients with hypertension promotes haemodynamic stability and has fewer adverse effects compared with etomidate. RT has good efficacy and high safety profile and is a potential alternative sedative drug for induction of general anaesthesia in elderly patients with hypertension.
Abbreviations
GABAA, γ-aminobutyric acid subtype A; MAP, Mean Arterial Pressure; HR, Heart rate; RT, Remimazolam tosylate; ASA, American Society of Anesthesiologists; ECG, Electrocardiogram; NIBP, Noninvasive Blood Pressure; SpO2, Peripheral Oxygen Saturation; BIS, Bispectral Index; PetCO2, Partial pressure of end-expiratory carbon dioxide; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; BMI, Body mass index; NMDAR, N-methyl-D-aspartate receptor; SD, Standard deviation; P25, Lower quartile; P75, Upper quartile.
Data Sharing Statement
The datasets generated during and/or analyzed in the current study are not publicly available due to the privacy policy when using human subjects but are available from the corresponding authors upon reasonable requests.
Ethical Approval and Consent to Participate in the Study
This study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol was approved by the institution review board (IRB) of the Affiliated Hospital of Guizhou Medical University (Ref. No.2022100K/IRB). The study was registered on chiCTR.org website (ChiCTR2200064997; date of registration: 25 October 2022). Written informed consent was acquired from all patients.
Acknowledgments
The authors thank all the participants enrolled into in this study and the researchers involved in the study design, recruitment of patients, treatment, and data collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study did not receive external funding.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Valk BI, Struys MMRF. Etomidate and its analogs: a review of pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2021;60(10):1253–1269. doi:10.1007/s40262-021-01038-6
2. Song JC, Lu ZJ, Jiao YF, et al. Etomidate anesthesia during ERCP caused more stable haemodynamic responses compared with propofol: a randomized clinical trial. Int J Med Sci. 2015;12(7):559–565. doi:10.7150/ijms.11521
3. Yao YT, He LX, Fang NX, et al. Anesthetic induction with etomidate in cardiac surgical patients: a PRISMA-compliant systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2020;35(4):1–10. doi:10.1053/j.jvca.2020.11.068
4. Hannam JA, Mitchell SJ, Cumin D, et al. Haemodynamic profiles of etomidate vs propofol for induction of anaesthesia: a randomised controlled trial in patients undergoing cardiac surgery. Br J Anaesth. 2019;122(2):198–205. doi:10.1016/j.bja.2018.09.027
5. Kay B. A dose-response relationship for etomidate, with some observations on cumulation. Br J Anaesth. 1976;48(3):213–216. doi:10.1093/bja/48.3.213
6. Egan ED, Johnson KB. The influence of hemorrhagic shock on the disposition and effects of intravenous anesthetics: a narrative review. Anesth Analg. 2020;130(5):1320–1330. doi:10.1213/ANE.0000000000004654
7. Wagner RL, White PF, Kan PB, et al. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med. 1984;310(22):1415–1421. doi:10.1056/NEJM198405313102202
8. Albert SG, Ariyan S, Rather A. The effect of etomidate on adrenal function in critical illness: a systematic review. Intensive Care Med. 2011;37(6):901–910. doi:10.1007/s00134-011-2160-1
9. Miner JR, Danahy M, Moch A, et al. Randomized clinical trial of etomidate versus propofol for procedural sedation in the emergency department. Ann Emerg Med. 2007;49(1):15–22. doi:10.1016/j.annemergmed.2006.06.042
10. Komatsu R, You J, Mascha EJ, et al. Anesthetic induction with etomidate, rather than propofol, is associated with increased 30-day mortality and cardiovascular morbidity after noncardiac surgery. Anesth Analg. 2013;117(6):1329–1337. doi:10.1213/ANE.0b013e318299a516
11. Zhou Y, Hu P, Huang Y, et al. Population pharmacokinetic/pharmacodynamic model-guided dosing optimization of a novel sedative hr7056 in Chinese healthy subjects. Front Pharmacol. 2018;9:1316. doi:10.3389/fphar.2018.01316
12. Hu Q, Liu X, Wen C, Li D, Lei X. Remimazolam: an updated review of a new sedative and anaesthetic. Drug Des Devel Ther. 2022;16:3957–3974. doi:10.2147/DDDT.S384155
13. Rex DK, Bhandari R, Desta T, et al. A Phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88(3):427–437. doi:10.1016/j.gie.2018.04.2351
14. Chen SH, Yuan TM, Zhang J, et al. Remimazolam tosilate in upper gastrointestinal endoscopy: a multicenter, randomized, non-inferiority, phase III trial. J Gastroenterol Hepatol. 2021;36(2):474–481. doi:10.1111/jgh.15188
15. Worthington MT, Antonik LJ, Goldwater DR, et al. A phase Ib, dose-finding study of multiple doses of remimazolam (CNS 7056) in volunteers undergoing colonoscopy. Anesth Analg. 2013;117(5):1093–1100. doi:10.1213/ANE.0b013e3182a705ae
16. Doi M, Hirata N, Suzuki T, et al. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020;34(4):491–501. doi:10.1007/s00540-020-02776-w
17. Liu T, Lai T, Chen J, et al. Effect of remimazolam induction on hemodynamics in patients undergoing valve replacement surgery: a randomized, double-blind, controlled trial. Pharmacol Res Perspect. 2021;9(5):e00851. doi:10.1002/prp2.851
18. Vellinga R, Hannivoort LN, Introna M, et al. Prospective clinical validation of the Eleveld propofol pharmacokinetic-pharmacodynamic model in general anaesthesia. Br J Anaesth. 2021;126(2):386–394. doi:10.1016/j.bja.2020.10.027
19. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi:10.1002/sim.3697
20. Du X, Yu J, Mi W. The effect of dexmedetomidine on the perioperative hemodynamics and postoperative cognitive function of elderly patients with hypertension: study protocol for a randomized controlled trial. Medicine. 2018;97(43):e12851. doi:10.1097/MD.0000000000012851
21. Matchett G, Gasanova I, Riccio CA, et al. Etomidate versus ketamine for emergency endotracheal intubation: a randomized clinical trial. Intensive Care Med. 2022;48:78–91. doi:10.1007/s00134-021-06577-x
22. Zgola K, Kulakowski P, Czepiel A, et al. Haemodynamic effects of etomidate, propofol and electrical shock in patients undergoing implantable cardioverter-defibrillator testing. Kardiol Pol. 2014;72:707–715. doi:10.5603/KP.a2014.0086
23. Ko BJ, Oh JN, Lee JH, et al. Comparison of effects of fentanyl and remifentanil on hemodynamic response to endotracheal intubation and myoclonus in elderly patients with etomidate induction. Korean J Anesthesiol. 2013;64(1):12–18. doi:10.4097/kjae.2013.64.1.12
24. Nyman Y, Von HK, Ritzmo C, et al. Effect of a small priming dose on myoclonic movements after intravenous anaesthesia induction with Etomidate-Lipuro in children. Br J Anaesthesiol. 2011;107(2):225–228. doi:10.1093/bja/aer129
25. Liu M, Sun Y, Zhou L, et al. The median effective dose and bispectral index of remimazolam tosilate for anesthesia induction in elderly patients: an up-and-down sequential allocation trial. Clin Interv Aging. 2022;17:837–843. doi:10.2147/CIA.S364222
26. Chen X, Xin D, Xu G, et al. The efficacy and safety of remimazolam tosilate versus dexmedetomidine in outpatients undergoing flexible bronchoscopy: a prospective, randomized, blind, non-inferiority trial. Front Pharmacol. 2022;13:902065. doi:10.3389/fphar.2022.902065
27. FDA. Guidance for industry. Non-inferiority clinical trials; 2010. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM202140.pdf.
28. Gosho M. Non-inferiority margins employed in clinical trials in Japan. J Clin Pharm Ther. 2015;40:289–298. doi:10.1111/jcpt.12268
29. Maheshwari K, Turan A, Mao G, et al. The association of hypotension during non cardiac surgery, before and after skin incision, with postoperative acute kidney injury: a retrospective cohort analysis. Anaesthesia. 2018;73(10):1223–1228. doi:10.1111/anae.14416
30. Südfeld S, Brechnitz S, Wagner JY, et al. Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br J Anaesth. 2017;119(1):57–64. doi:10.1093/bja/aex127
31. Goodchild CS, Serrao JM. Propofol-induced cardiovascular depression: science and art. Br J Anaesth. 2015;115(4):641–642. doi:10.1093/bja/aev320
32. Green DW. Cardiac output decrease and propofol: what is the mechanism. Br J Anaesth. 2015;114(1):163–164. doi:10.1093/bja/aeu424
33. Kakazu CZ, Lippmann M. Playing with fire: debate about propofol-induced hypotension. Br J Anaesth. 2015;114(1):164–165. doi:10.1093/bja/aeu425
34. Saugel B, Bebert EJ, Briesenick L, et al. Mechanisms contributing to hypotension after anesthetic induction with sufentanil, propofol, and rocuronium: a prospective observational study. J Clin Monit Comput. 2022;36(2):341–347. doi:10.1007/s10877-021-00653-9
35. Yang W, Luo H, Ma Y, et al. Effects of antihypertensive drugs on cognitive function in elderly patients with hypertension: a review. Aging Dis. 2021;12(3):841–851. doi:10.14336/AD.2020.1111
36. Hu B, Zhang M, Wu Z, et al. Comparison of remimazolam tosilate and etomidate on hemodynamics in cardiac surgery: a randomised controlled trial. Drug Des Devel Ther. 2023;17:381–388. doi:10.2147/DDDT.S401969
37. Qiu Y, Gu W, Zhao M, et al. The hemodynamic stability of remimazolam compared with propofol in patients undergoing endoscopic submucosal dissection: a randomized trial. Front Med. 2022;9:938940. doi:10.3389/fmed.2022.938940
38. Tang F, Yi JM, Gong HY, et al. Remimazolam benzenesulfonate anesthesia effectiveness in cardiac surgery patients under general anesthesia. World J Clin Cases. 2021;9:10595–10603. doi:10.12998/wjcc.v9.i34.10595
39. Zhang J, Wang X, Zhang Q, et al. Application effects of remimazolam and propofol on elderly patients undergoing Hip replacement. BMC Anesthesiol. 2022;22:118. doi:10.1186/s12871-022-01641-5
40. Hueter L, Schwarzkopf K, Simon M, et al. Pretreatment with sufentanil reduces myoclonus after etomidate. Acta Anaesthesiol Scand. 2003;47:482–484. doi:10.1034/j.1399-6576.2003.00081.x
41. Zhou C, Zhu Y, Liu Z, et al. Effect of pretreatment with midazolam on etomidate-induced myoclonus: a meta-analysis. J Int Med Res. 2017;45:399–406. doi:10.1177/0300060516682882
42. Liu J, Liu R, Meng C, et al. Propofol decreases etomidate-related myoclonus in gastroscopy. Medicine. 2017;96:e7212. doi:10.1097/MD.0000000000007212
43. Feng Y, Chang P, Kang Y, et al. Etomidate-induced myoclonus in Sprague-Dawley rats involves neocortical glutamate accumulation and N -Methyl- d -Aspartate receptor activity. Anesth Analg. 2023;137(1):221–233. doi:10.1213/ANE.0000000000006292
44. Gancher S, Laxer KD, Krieger W. Activation of epileptogenic activity by etomidate. Anesthesiology. 1984;61:616–618. doi:10.1097/00000542-198411000-00029
45. Wang W, Lv J, Wang Q, et al. Oxycodone for prevention of etomidate-induced myoclonus: a randomized double-blind controlled trial. J Int Med Res. 2018;46:1839–1845. doi:10.1177/0300060518761788
46. Liu X, Ding B, Shi F, et al. The efficacy and safety of remimazolam tosilate versus etomidate-propofol in elderly outpatients undergoing colonoscopy: a prospective, randomized, single-blind, non-inferiority trial. Drug Des Devel Ther. 2021;15:4675–4685. doi:10.2147/DDDT.S339535
47. Nyman Y, Von Hofsten K, Palm C, et al. Etomidate-Lipuro is associated with considerably less injection pain in children compared with propofol with added lidocaine. Br J Anaesth. 2006;97:536–539. doi:10.1093/bja/ael187
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

