Back to Journals » OncoTargets and Therapy » Volume 14
Remarkable Response to Olaparib in a Patient with Combined Hepatocellular-Cholangiocarcinoma Harboring a Biallelic BRCA2 Mutation
Authors Su YL, Ng CT, Jan YH, Hsieh YL, Wu CL, Tan KT
Received 4 May 2021
Accepted for publication 17 June 2021
Published 29 June 2021 Volume 2021:14 Pages 3895—3901
DOI https://doi.org/10.2147/OTT.S317514
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Yu-Li Su,1,2 Ca Tung Ng,3 Yi-Hua Jan,3 Yi-Lin Hsieh,3 Chia-Ling Wu,3 Kien Thiam Tan3
1Division of Hematology Oncology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University, College of Medicine, Kaohsiung, Taiwan; 2Clinical Trial Center, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan; 3ACT Genomics Co., Ltd, Taipei City, Taiwan
Correspondence: Yu-Li Su
Division of Hematology-Oncology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University, College of Medicine, Taiwan No. 123, Dapi Road, Niaosong Dist., Kaohsiung City, 833, Taiwan
Tel +886-7-7317123 ext. 8303
Fax +886-7-7322402
Email [email protected]
Abstract: Combined hepatocellular cholangiocarcinoma (cHCC-CC) is a rare subtype of primary liver malignancy characterized by aggressive behavior and poor prognosis. Radial surgical resection is the standard curative treatment. However, effective therapeutic options for recurrent or metastatic cHCC-CC are still lacking, mainly because of an insufficient understanding of the molecular and genomic alterations of cHCC-CC, preventing the discovery of specialized targeting therapy. Here, we present the case of a patient with metastatic cHCC-CC on first-line treatment of gemcitabine, cisplatin, and nab-paclitaxel. A comprehensive genomic profile revealed four clinically relevant single nucleotide variants (BRCA2, PIK3C2G, RET, and TP53), two amplified genomic regions (CRKL and MAPK1), and 11 heterozygous genomic deletions (BAP1, CDKN2A, PTCH1, TSC1, BRCA2, RB1, RAD51, PALB2, TSC2, SMAD4, and STK11). The patient underwent olaparib treatment and achieved a remarkable and sustained tumor response. Our experience indicates that BRCA2 mutations could be a potential therapeutic target for patients with cHCC-CC.
Keywords: combined hepatocellular cholangiocarcinoma, BRCA2 mutation, olaparib, comprehensive genomic panel, precision oncology
Introduction
Combined hepatocellular cholangiocarcinoma (cHCC-CC) is an extremely rare and aggressive liver cancer that shares histological features with both cholangiocarcinoma (CC) and hepatocellular carcinoma (HCC).1 The incidence of cHCC-CC is 0.05 per 10,000 per year, increases with age, and is higher among Asian populations.2 Most cases of cHCC-CC are diagnosed at a late stage, meaning such patients are unsuitable for radical surgery, leading to an unsatisfactory 5-year overall survival rate of 17.7%.2 The length of overall survival of patients with cHCC-CC is substantially shorter than that of patients with pure HCC or CC.3,4 Current primary treatment modalities include hepatic tumor resection, liver transplantation, transarterial chemoembolization, radiofrequency ablation, and percutaneous ethanol injection.5 Systemic chemotherapy is frequently used for recurrent or metastatic diseases, albeit with a lack of clear evidence for guiding clinical practice and choosing the appropriate chemotherapy regimen.6–8 Because of the arduous long-term prognosis, accurate preoperative diagnoses and effective targeted molecular therapies are essential for the efficient management of cHCC-CC.
Case Presentation
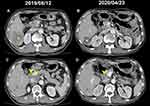
A 71-year-old man with a history of prostate adenocarcinoma (Gleason score 9), underwent robot-assisted radical prostatectomy in September 2018 and received a diagnosis of pathologic stage T3aN0M0 (stage IIIc). After adjuvant radiotherapy (6,400 cGY in 32 fractions) from April to May 2019, he remained prostate cancer-free, with a persistently low level of serum prostate-specific antigen PSA (<0.04 ng/mL). Four months later, he began to experience right upper quadrant pain, decreased appetite, and unintentional body weight loss. A CT scan of the abdomen revealed a large hypovascular tumor over S6 and enlarged hepatic hilar and retroperitoneal lymph nodes in August 2019 (Figure 1A and C). The patient underwent laparoscopic lymph nodes dissection and a biopsy of the liver tumor that both revealed poorly differentiated carcinoma. Microscopically tissue composed of neoplastic epithelial cells, bearing pleomorphic and hyperchromatic nuclei, and forming solid, trabecular and focal cribriform-like patterns with focal intervening sinusoidal-like spaces (Figure 2). The immunohistochemical (IHC) study revealed tumor cells in either liver or lymph node were positive for LMW-CK, CK7, CK19, glypican-3 and focal positive for arginase-1, and negative for PSA, hepatocyte-specific antigen (HSA) and HMW-CK. With these results taken together, the patient was diagnosed as having metastatic cHCC-CC.
The patient received first-line systemic chemotherapy with gemcitabine, cisplatin, and nab-paclitaxel for three cycles from September to December 2019 and achieved a partial radiologic and alpha-fetoprotein (AFP) response (887 to 165 ng/mL). However, the patient discontinued chemotherapy because of intolerance to chemotherapy-related adverse effects (grade 3 neutropenia, grade 2 peripheral neuropathy, nausea, vomiting, and fatigue).
To identify actionable molecular targets, the liver tumor biopsy specimen (tumor purity: 52%) taken on August 2019 was sequenced using a comprehensive genomic panel (CGP) of 440 genes (ACTOnco®; sequencing coverage: 1.8mb; NGS sequencing mean depth 2031X). Genomic profiling identified 19 nonsynonymous mutations, including 17 single nucleotides and two small insertion and deletions (InDel) variants, among which four mutations (BRCA2, PIK3C2G, RET, and TP53) were considered clinically relevant variants (Table 1). As evident from the sanger sequencing, the BRCA2 mutation (c.97G>T) is not germline mutation as it is not present in the patient’s peripheral blood mononuclear cell (PBMC) sample (Figure 3). Furthermore, an amplified genomic region encoding the CRKL and MAPK1 at chromosome 22 was identified, with a copy number of 6. Eleven genes with heterozygous deletions (BAP1, CDKN2A, PTCH1, TSC1, BRCA2, RB1, RAD51, PALB2, TSC2, SMAD4, and STK11) were also identified (Figure 4). Loss of heterozygosity of BRCA2 E33* mutations can result in the biallelic inactivation of BRCA2, which would indicate sensitivity to poly ADP-ribose polymerase (PARP) inhibitors.
 |
Table 1 Clinically Relevant Single Nucleotide Variants Detected in Patient’s Liver Tumor Biopsy |
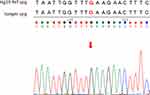 |
Figure 3 Sanger sequencing result of buffy coat on SeqStudio Genetic Analyzer. Target region: BRCA2 (Chr 13:32893243). |
 |
Figure 4 Copy number profile of the liver biopsy sample. Amplified regions are indicated by red dots; deleterious regions are indicated by blue dots. |
The patient received 300 mg of olaparib twice per day beginning in January 2020 (Figure 5), and a partial response was achieved in April 2020, with a CT of the abdomen revealing remarkable regression of the liver tumor (asterisk) and the hepatic hilar lymph nodes (arrowhead; Figure 1B and D). In addition, AFP levels significantly decreased from 675.9 to 8.0 ng/mL. The patient had grade 3 thrombocytopenia, grade 2 neutropenia, anemia, and fatigue. Because of the high-grade hematologic toxicity, the dosage of olaparib was reduced to 150 mg twice per day in May 2020. The patient is still on the olaparib treatment with a sustained response >7 months since beginning therapy.
 |
Figure 5 Treatment timeline. Treatment course of combined hepatocellular cholangiocarcinoma from August 2019 to April 2020. |
Discussion
Because curative surgery is not suitable for advanced cHCC-CC and lacks of randomized studies of systemic treatment for metastatic cHCC-CC, the molecular characterization of cHCC-CC is urgently needed to facilitate the accurate diagnosis and development of novel targeted therapies. A genomic profiling study comparing mixed and combined hepatocellular and intrahepatic cholangiocarcinoma (ICC) demonstrated similar genomic landscapes to both ICC and HCC subtypes, with combined cHCC-CC exhibiting strong ICC-like features, and mixed cHCC-CC expressing strong HCC-like features, implying the potential utility of ICC or HCC treatments to patients with cHCC-CC.1 Unfortunately, management of both advanced ICC and HCC remains limited, with resections being excluded and systemic gemcitabine- and fluoropyrimidine-based chemotherapy being associated with poor responses.9–11 Although sorafenib, an oral multikinase inhibitor targeting vascular endothelial growth factor (VEGF), Raf, and the platelet-derived growth factor (PDGF) signaling pathway, has demonstrated a capacity to considerably improve progression-free survival (PFS) and overall survival in patients with HCC, several case-based retrospective studies have shown that its efficacy in treating cHCC-CC is limited.12,13 Because of the rarity of cHCC-CC, evidence on molecular targeted therapies is still lacking but remains urgently required.
Here, we present our experience of treating a patient with cHCC-CC harboring the biallelic inactivation of BRCA2. A genomic analysis revealed the presence of genome-wide LOH (gLOH), contributing to the “BRCAness” phenotype and indicating a potential benefit from PARP inhibitor. Germline mutations of BRCA1/2 have been associated with a higher risk of biliary tract cancer (BTC).14 In a recent comprehensive genomic study, the overall incidence of BRCA mutations in patients with BTC was 3.6%.15 Patients with BRCA mutations had a notably higher rate of mismatch repair deficiency and high tumor mutational burden. PARP inhibitors, such as olaparib, rucaparib, and niraparib, have demonstrated modest antitumor activities in cases of ovarian, breast, prostate, and pancreatic cancer with BRCA1/2 mutations.16–20 Although relatively few studies have focused on the efficacy of PARP inhibitors in treating patients with BTC with BRCA1/2 mutations, several case reports have documented a responsiveness to olaparib in cases of BTC with BRCA2 mutations.21,22 Lin et al investigated the distribution of DNA damage repair alteration by sequencing of 357 cases of primary liver cancer (PLC), including HCC, ICC, and cHCC-CC, and showed that 4.8% of PLCs had BRCA1/2 mutations.22 Out of the eight patients with PLC (7 ICC, 1 cHCC-CC) and BRCA germline or somatic mutations, three achieved partial responses on the olaparib treatment, indicating the potential therapeutic application of PARP inhibitors in cases of BTC or cHCC-CC harboring BRCA1/2 mutations. This case report was the first to reveal a BRCA2 mutation in cHCC-CC sustaining a response to olaparib, setting a valuable precedent for further clinical research and validation. Further large case series are warranted to confirm this hypothesis.
The absence of BRCA1 or BRCA2 function is characterized by mutational signature 3. Distinct mutational signature 3 is highly associated with the biallelic inactivation of BRCA, compared with single functional copies of BRCA.23 In contrast with monoallelic aberrations in BRCA1/2, which can have haploinsufficiency but no association with elevated gLOH levels, biallelic BRCA1/2 alterations show observable gLOH signatures for homologous recombination deficiency (HRD) across every type of cancer and may constitute a better therapeutic target for PARP inhibitors.24,25 Preclinical studies of human cell lines and mouse models with BRCA1/2 biallelic mutations or BRCA1/2 knockout have revealed a greater responsiveness to platinum and PARP inhibitors, compared with those retaining some level of BRCA1/2 function.23–25 For this reason, understanding BRCA1/2 alterations as a predictive biomarker for PARP inhibitors by differentiating between monoallelic and biallelic conditions can yield beneficial results.26
Conclusion
Given the lack of targeted therapies, olaparib could be a promising therapeutic agent for patients with cHCC-CC harboring BRCA1/2 mutations. Additional studies are required to develop CGP-guided therapy in routine oncological practice, thereby assisting clinicians in identifying the appropriate biomarkers for patients with cHCC-CC.
Abbreviations
cHCC-CC, combined hepatocellular cholangiocarcinoma; CC, cholangiocarcinoma; HCC, hepatocellular carcinoma; IHC, immunohistochemical; HSA, hepatocyte-specific antigen; CT, computed tomography; AFP, alpha-fetoprotein; CGP, comprehensive genomic panel; NGS, next-generation sequencing; PBMC, peripheral blood mononuclear cell; PARP, poly ADP-ribose polymerase; ICC, intrahepatic cholangiocarcinoma; VEGF, vascular endothelial growth factor; PDFG, platelet-derived growth factor; PFS, progression-free survival; BTC, biliary tract cancer; PLC, primary liver cancer; HRD, homologous recombination deficiency.
Ethical Statement
This study was approved by the Institutional Review Board of Chang Gung Medical Foundation (202001411B0).
Consent for Publication
Written informed consent to publication of this case report was obtained from the patient. Identifiable personal information has been concealed.
Acknowledgments
We appreciated the GI pathologist, Dr Tien-Tien Liu, for the accurate histopathologic diagnosis of cHCC-CC and kind assistance in taking microscopic pictures. We thank Chang Gung Medical Foundation Kaohsiung Chang Gung Memorial Hospital Tissue Bank Core Lab and Biobank (CLRPG8I0032) for their excellent technical support. This manuscript was edited by Wallace Academic Editing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Y.L.S.: Honoraria from Amgen, AstraZeneca, Merck Serono, MSD, ONO Pharmaceutical, Roche and TTY Biopharm Company; Research funding from ONO Pharmaceutical; Travel support from Amgen, ONO Pharmaceutical and Roche. C.T.N., Y.H.J., Y.L.H., C.L.W. and K.T.T.: Employment in ACT Genomics company. The authors report no other conflicts of interest in this work.
References
1. Stavraka C, Rush H, Ross P. Combined hepatocellular cholangiocarcinoma (cHCC-CC): an update of genetics, molecular biology, and therapeutic interventions. J Hepatocell Carcinoma. 2018;6:11–21. doi:10.2147/JHC.S159805
2. Ramai D, Ofosu A, Lai JK, et al. Combined Hepatocellular Cholangiocarcinoma: a Population-Based Retrospective Study. Am J Gastroenterol. 2019;114(9):1496–1501.
3. Koh KC, Lee H, Choi MS, et al. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005;189:120–125.
4. Lee JH, Chung GE, Yu SJ, et al. Long-term prognosis of combined hepatocellular and cholangiocarcinoma after curative resection comparison with hepatocellular carcinoma and cholangiocarcinoma. J Clin Gastroenterol. 2011;45:69–75.
5. Wang J, Li E, Yang H, et al. Combined hepatocellular-cholangiocarcinoma: a population level analysis of incidence and mortality trends. World J Sur Oncol. 2019;17:43.
6. Rogers J, Bolonesi R, Rashid A, et al. Systemic therapy for unresectable, mixed hepatocellular-cholangiocarcinoma: treatment of a rare malignancy. J Gastrointest Oncol. 2017;8:347–351.
7. Kobayashi S, Terashima T, Shiba S, et al. Multicenter retrospective analysis of systemic chemotherapy for unresectable combined hepatocellular and cholangiocarcinoma. Cancer Sci. 2018;109:2549–2557.
8. Trikalinos NA, Zhou A, Doyle MBM, et al. Systemic Therapy for Combined Hepatocellular-Cholangiocarcinoma: a Single-Institution Experience. J Natl Compr Canc Netw. 2018;16:1193.
9. Borbath I, Ceratti A, Verslype C, et al. Combination of gemcitabine and cetuximab in patients with advanced cholangiocarcinoma: a Phase II study of the Belgian Group of Digestive Oncology. Ann Oncol. 2013;24:2824–2829.
10. Moehler M, Maderer A, Schimanski C, et al. Gemcitabine plus sorafenib versus gemcitabine alone in advanced biliary tract cancer: a double-blind placebo-controlled multicentre phase II AIO study with biomarker and serum programme. Eur J Cancer. 2014;50:3125–3135.
11. Valle J, Wasan H, Lopes A, et al. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): a randomised Phase 2 trial. Lancet Oncol. 2015;16:967–978.
12. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in Advanced Hepatocellular Carcinoma. N Engl J Med. 2008;359:378–390.
13. Futsukaichi Y, Tajiri K, Kobayashi S, et al. Combined hepatocellular-cholangiocarcinoma successfully treated with sorafenib: case report and review of the literature. Clin J Gastroenterol. 2019;12:128–134.
14. Golan T, Raitses-Gurevich M, Kelley RK, et al. Overall survival and clinical characteristics of BRCA-Associated cholangiocarcinoma: a multicenter retrospective study. Oncologist. 2017;22:804–810.
15. Spizzo G, Puccini A, Xiu J, et al. Molecular profile of BRCA-mutated biliary tract cancers. ESMO Open. 2020;5:e000682.
16. Kaufman B, Shapira-Frommer R, Schmutzler R, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244–250.
17. Robson M, Im S, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533.
18. De Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–2102.
19. Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381:317–327.
20. Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164.
21. Cheng Y, Zhang J, Qin SK, et al. Treatment with olaparib monotherapy for BRCA2-mutated refractory intrahepatic cholangiocarcinoma: a case report. Onco Targets Ther. 2018;11:5957–5962.
22. Lin J, Shi J, Guo H, et al. Alterations in DNA damage repair genes in primary liver cancer. Clin Cancer Res. 2019;25:4701–4711.
23. Polak P, Kim J, Braunstein L, et al. A mutational signature reveals alterations underlying deficient homologous recombination repair in breast cancer. Nat Genet. 2017;49:1476–1486.
24. Prakash R, Zhang Y, Feng W, et al. Homologous recombination and human health: the roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb Perspect Biol. 2015;7:a016600.
25. Rahman N, Scott RH. Cancer genes associated with phenotypes in monoallelic and biallelic mutation carriers: new lessons from old players. Hum Mol Genet. 2007;16:R60–6.
26. Jonsson P, Bandlamudi C, Cheng ML, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature. 2019;571:576–579.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.