Back to Journals » Journal of Multidisciplinary Healthcare » Volume 14
Remarkable Response of Toripalimab Combined with Chemotherapy in Sarcomatoid Carcinoma of Palatine Tonsil: A Case Report
Authors Huang J, Lei L, Chen B, Pan G, Fang M, Wang X
Received 9 December 2020
Accepted for publication 11 February 2021
Published 9 March 2021 Volume 2021:14 Pages 599—604
DOI https://doi.org/10.2147/JMDH.S296584
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jiahuan Huang,1,2 Lei Lei,1,2 Bo Chen,2,3 Guoqiang Pan,4 Xiaojiao Wang,1,2 Meiyu Fang2,5
1Department of Breast Medical Oncology, Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Hangzhou, 310022, People’s Republic of China; 2Institute of Cancer and Basic Medicine (IBMC), Chinese Academy of Sciences, Hangzhou, 310022, People’s Republic of China; 3Department of Pathology, Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Hangzhou, 310022, People’s Republic of China; 4Department of Thoracic Oncology, Wenzhou Medical University, Wenzhou, 325035, People’s Republic of China; 5Department of Medical Oncology of Rare Cancer and Head and Neck Cancer, Cancer Hospital of University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Key Laboratory of Head and Neck Cancer Translational Research of Zhejiang Province, Hangzhou, Zhejiang, 310022, People’s Republic of China
Correspondence: Meiyu Fang Department of Medical Oncology of Rare Cancer and Head and Neck Cancer
Cancer Hospital of University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Key Laboratory of Head and Neck Cancer Translational Research of Zhejiang Province, Hangzhou, 310022, People’s Republic of China
Tel +86-571-88122222
Email [email protected]
Xiaojiao Wang
Department of Breast Medical Oncology, Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Hangzhou, 310022, People’s Republic of China
Tel +86-571-88122222
Email [email protected]
Background: Sarcomatoid carcinoma (SaCa) of the palatine tonsil is a rare and aggressive subset of head and neck (H&N) cancer which is characterized by insensitivity to surgery and radiotherapy and a poor prognosis. Immunotherapy has led advances in the treatment of melanoma and H&N cancer, but the combined effects of immunotherapy and chemotherapy have not been sufficiently investigated.
Case Presentation: Herein, we report the case of 29-year-old Chinese women with local advanced non-resectable SaCa of the palatine tonsil who exhibited a substantial partial response to toripalimab and chemotherapy followed by radiation therapy. To the best of our knowledge, this is the first report of its successful application in this context.
Conclusion: Toripalimab combined with chemotherapy may be an effective approach for locally advanced H&N cancer in rare categories of patients, which was the first application as far as we know.
Keywords: chemotherapy, combination therapy, palatine tonsil cancer, PD-1 inhibitor, sarcomatoid carcinoma
Introduction
Sarcomatoid carcinoma (SaCa), also known as spindle cell carcinoma, carcinosarcoma and pseudosarcoma is a rare malignant variant of squamous cell carcinoma (SCC) that consists both the epithelial squamous cell component and spindle cell components.1–3 With the region of the head and neck, it has been widely reported in the larynx, esophagus, and lips.2 SaCa of the palatine tonsil is extremely rare and is characterized by high rates of recurrence and mortality.4 Recently emerging evidence supports the efficacy and safety of immune checkpoint inhibitors in patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC).5,6 Immunotherapy has proved to be a promising treatment approach with surprising survival in some patients. Toripalimab, also known as JS001 or TAB001, is a selective recombinant humanized lgG4 monoclonal antibody that blocks the interactions between programmed death receptor-1 (PD-1) and its ligands programmed death ligands 1 (PD-L1) and programmed death ligands 2 (PD-L2). It is highly recommended for the treatment of melanoma, nasopharyngeal carcinoma, and other tumors.7 Cisplatin-based chemotherapy has been the conventional systemic therapy for HNSCC for decades.8 Therefore, combining immunotherapy with standard chemotherapy may be an effective option in patients with local advanced H&N cancer. Herein, we report the efficacy and safety of toripalimab combined with chemotherapy and radiotherapy in an unusual case of SaCa in the palatine tonsil with PD-L1 overexpression.
Case Presentation
In October 2019 a 29-year-old woman was admitted to a local hospital with a mass in her right palatine tonsil and anterior cervical region that had been present for several days. She also complained of pain, fever, and dysphagia. Tonsillar biopsy established a pathological diagnosis of SaCa of palatine tonsil. The results of immunohistochemistry (IHC) showed P16 (+), CK (+), Ki67 (+,50%). She was subsequently referred to our hospital for unrelieved aching in her right tonsil after anti-inflammatory therapy. She had no history of smoking or drug abuse and no known family history of any cancer. On physical examination, a large mass was detected on the right palatine tonsil. The mass was ulcerated, and its surface was covered with white exudate. Palpable lymphadenopathy measuring approximately 1.5 x 2.5 cm was present in the anterior cervical region.
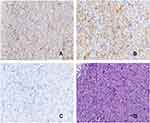
Laryngoscopy revealed malignant tumor of tonsil (Figure 1). Enhanced neck and chest computed tomography (CT) revealed soft tissue thickened within the right oropharynx and multiple swollen lymph nodes at level II of the right neck. Magnetic resonance imaging (MRI) indicted tonsillar neoplasm with dual neck lymph metastasis (Figure 2). Pathological investigations confirmed SaCa of the palatine tonsil. IHC results were PD-L1 (22C3) (TPS, +60%), hMLH1 (+), hMSH6 (+), PMS2 (+), hMSH2 (+), CK (+), EMA (-) (Figure 3). Based on the 8th edition of the American Joint committee on Cancer (AJCC) staging system the tumor was clinical stage III (cT2N3M0).9 Given the rare subtype of tumor and the patient high overexpression of PD-L1, we decided to treat her with immunotherapy combined with chemotherapy followed by radiotherapy.
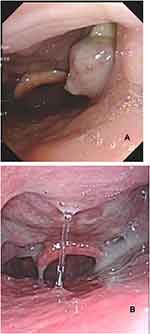 |
Figure 1 Laryngoscopy show: (A) a giant lesion in palatine tonsil which demonstrate malignant tumor. (B) After immunotherapy, chemotherapy and radiation treatment, there was no evidence of tumor. |
From October 2019 to November 2019 the patient was treated with liposomal doxorubicin (50mg), cisplatin (35mg, d1-3) and toripalimab (240mg) every 3 weeks for 2 cycles, the CT scans and MRI were performed after two cycles of treatments on 20 November 2019. At that time the target lesion and the remaining lesions were substantially reduced, and it was considered partial response (PR) as evaluated by the response evaluation criteria 1.1 in solid tumors.10 The patient suffered from immune-mediated grade 3 rash determined via the common terminology criteria for adverse events version 5.0, which improved with the application of 10 mg dexamethasone. Other toxicities such as grade 1–2 fatigue and reduced white blood cell count were well controlled. Later, local lesion-targeted radiation therapy (total dose of 70.50 Gy in the palatine tonsil and neck) was administered from November 2019 to January 2020. The response to radiotherapy was durable. Since March 2020, the patient has completed with two cycles of maintenance chemotherapy of liposomal doxorubicin (50mg) and cisplatin (35mg, d1-3). The most recent image evaluation indicated an excellent clinical response that was close to a complete response. Currently, the patient is undergoing regular review and the tumor is well controlled. As at November 2020, the duration of disease-free survival was >13 months.
Discussion
SaCa of the head and neck is a poorly differentiated and comparatively aggressive variant of SCC that is relatively rare, accounting for less than 3% of all SCCs.11 It is more common in males and is positively associated with smoking, alcohol consumption, and previous irradiation.12 In our case, the patient had SaCa of palatine tonsil. However, SaCa is more aggressive that other SCCs, and may result in poorer survival.13 Differentiating the SaCa of palatine tonsil from other benign lesion or malignant neoplasm is particularly important because the relevant treatments vary substantially. Several other diseases that should be considered are discussed below.
Tuberculous retropharyngeal abscess is mainly caused by tuberculosis and is common in adults. The main symptoms are odynophagia and dysphagia. Diagnosis is mainly based on clinical features, imaging characteristics, and pathological examination of biopsies. Anti-tuberculous medication and surgical are usually effective.14 Lymphangioma of the palatine tonsil is a rare, congenital, benign proliferation of lymphatic channels. Symptoms are non-specific and include dysphagia and recurrent sore throat. Surgical is recommended for accurate histologic diagnosis, and there is usually no recurrence after radically resection.15 The parapharyngeal internal carotid artery is close to the tonsil. The etiology is unknown. Almost all patients are asymptomatic, but some exhibit symptoms such as difficulty breathing and swallowing. The gold standard for diagnosis is CT angiography or MR angiography. Surgery is the main effective treatment.16
Oropharyngeal cancer includes that of the palatine tonsil, which tends to be infiltrative and rich in lymphatics. Different p16 statues (Human papillomavirus-mediated) are associated with different outcomes. Because surgery and chemo-radiotherapy are the two vital modalities, a multidisciplinary consultation is encouraged to devise a treatment plan based on the patient’s condition. Either radical surgery or chemo-radiotherapy is recommended for early or local advanced resectable disease. Immunotherapy is only considered for first-line treatment in case of recurrence or metastatic disease.17,18
Because of its rarity, there is no consensus on the optimal treatment for SaCa. Previous reports suggest that surgery with radiotherapy and/or chemotherapy can be used to manage such tumors.
The effectiveness of radiotherapy with respect to survival is controversial, and the optimal cytotoxic chemotherapy regime remains unclear. Treatment efficacy is lower than that of SCC, and the prognosis is typically dismal.12,19,20
Considerable progress has been made in the area of immunotherapy for a variety of cancers. The PD-1 inhibitor pembrolizumab has been approved by the USA Food and Drug Administration for the treatment of recurrent or metastatic HNSCC, depending on KEYNOTE-012 and KEYNOTE-055, both of two studies exhibited clinically meaningful antitumor activity and an acceptable safety profile.21,22 In a Phase 3 study (KEYNOTE-048) pembrolizumab with chemotherapy was associated with significantly longer overall survival than cetuximab with chemotherapy (13.0 months vs 10.7 months) in patients with recurrent or metastatic HNSCC. Higher PD-L1 expression is also associated with longer overall survival. Thus, immunotherapy combined with chemotherapy becomes the first line of unresectable/recurrent/metastatic HNSCC.6 Nivolumab, another PD-1 inhibitor, has recently been approved by the FDA for the treatment of recurrent and/or metastatic HNSCC post-platinum, and in the CheckMate 141 study, it was associated with longer survival than standard therapy (7.5 m vs 5.1m).5
Toripalimab is a novel humanized IgG4 monoclonal antibody against PD-1 that differs from pembrolizumab and nivolumab. It was one of the first anti-PD-1 antibodies that was approved by the China Food and Drug Administration for the treatment of unresectable or metastatic melanoma.23 Toripalimab binds to the FG loop of the PD-1 receptor identified via three-dimensional structure analysis. In vivo, it can promote T-cell proliferation and CD8+ and CD4+ cells activation.24 Except for direct tumor lytic activity, CD4+ cells can provide protective function via cytokine secretion and inflammatory reactions.25
Chemotherapy is an ideal combination partner for immunotherapy in HNSCC for the disruption of tumor architecture and can lead to antigen shedding and the rapid induction of disease control. Cisplatin is the core component for the treatment of HNSCC.26 Tran et al,27 reported that optimal doses of cisplatin can enhance antitumor immunity and may be further improved by anti–PD-L1/PD-1 therapy. But traditional high doses also result in substantial toxicity. Syngeneic mouse model studies suggest concurrent use of suitable doses of cisplatin and anti–PD-L1/PD-1 can delay tumor growth and enhance survival.27
Combinations with other agents or radiotherapy can exhibit better efficacy.28,29 Radiation therapy alone can improve local-regional control and overall survival to some extent.30 Notably, however, traditional treatments can lead to substantial toxicity and complications, resulting in the therapy being discontinued. Therefore, based on the patient’s general condition and tumor stage, feasible and comprehensive treatments are pivotal and should be well designed.
Considering the rarity of SaCa and the patient’s PD-L1 overexpression we opted for treatment with toripalimab combined with cisplatin and liposomal doxorubicin, which achieved a substantial partial response. The formulation of the treatment scheme was based only on the medical experience of our center, not on previously reported evidence. Notably, because Chinese patients generally have a smaller body mass index and different genetic backgrounds compared with western patients, the dose of cisplatin utilized was comparatively smaller and a continuous intravenous drip was utilized for 3 days. During radiotherapy, the side effects such as mucositis were manageable and improved with the treatment of antibiotics. In the present case, there was an excellent response to immunotherapy combined with chemotherapy in a patient with local advanced SaCa of the palatine tonsil with high PD-L1 expression. However, there is a lack of previously reported evidence supporting this treatment strategy though, so further studies are needed.
Conclusion
To our knowledge, this is the first report of local advanced SaCa of the palatine tonsil with PD-L1 overexpression responding substantially to toripalimab combined with chemotherapy and local radiotherapy. This may be a promising treatment option in similar patients in the future.
Ethics Statement
This study was approved by the Ethics Committee of Zhejiang Cancer Hospital, Hangzhou, China. Written informed consent was obtained from the participant for the publication of this case report and any potentially identifying images or information.
Author Contributions
All authors made a significant contribution to the work reported, took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Thompson LDR, Wieneke JA, Miettinen M, et al. Spindle cell (sarcomatoid) carcinomas of the larynx: a clinicopathologic study of 187 cases. Am J Surg Pathol. 2002;26:153–170. doi:10.1097/00000478-200202000-00002
2. Su -H-H, Chu S-T, Hou -Y-Y, et al. Spindle cell carcinoma of the oral cavity and oropharynx: factors affecting outcome. J Chin Med Assoc. 2006;69:478–483. doi:10.1016/S1726-4901(09)70312-0
3. Tulunay O, Küçük B, Yorulmaz I, et al. Sarcomatoid carcinoma of the larynx: immunohistochemical analysis in two cases. Otolaryngol–Head Neck Surg. 2006;134:1057–1059. doi:10.1016/j.otohns.2005.03.051
4. Leventon GS, Evans HL. Sarcomatoid squamous cell carcinoma of the mucous membranes of the head and neck: a clinicopathologic study of 20 cases. Cancer. 1981;48:48. doi:10.1002/1097-0142(19810815)48:4<994::aid-cncr2820480424>3.0.co;2-m
5. Ferris RL, Blumenschein G
6. Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi:10.1016/S0140-6736(19)32591-7
7. Keam SJ. Toripalimab: first global approval. Drugs. 2019;79:573–578. doi:10.1007/s40265-019-01076-2
8. Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi:10.1056/NEJMoa032641
9. Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:122–137. doi:10.3322/caac.21389
10. Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–137. doi:10.1016/j.ejca.2016.03.081
11. Oktay M, Kokenek-Unal TD, Ocal B, et al. Spindle cell carcinoma of the tongue: a rare tumor in an unusual location. Pathology Res Int. 2011;2011:572381.
12. Iqbal MS, Paleri V, Brown J, et al. Spindle cell carcinoma of the head and neck region: treatment and outcomes of 15 patients. E Cancer Med Sci. 2015;9:594. doi:10.3332/ecancer.2015.594
13. Vazquez A, Khan MN, Blake DM, et al. Sinonasal squamous cell carcinoma and the prognostic implications of its histologic variants: a population-based study. Int Forum Allergy Rhinol. 2015;5:85–91. doi:10.1002/alr.21418
14. Hsu HE, Chen CY. Tuberculous retropharyngeal abscess with Pott disease and tuberculous abscess of the chest wall: a case report. Medicine. 2019;98:e16280. doi:10.1097/MD.0000000000016280
15. Mardekian S, Karp JK. Lymphangioma of the palatine tonsil. Arch Pathol Lab Med. 2013;137:1837–1842. doi:10.5858/arpa.2012-0678-RS
16. Ferlito S, Di Luca M, Maniaci A, et al. Progressive dysphagia in a patient with parapharingeal pulsating mass: a case report and literature’s review. Acta Medica Mediterranea. 2019;35:3433–3435.
17. Colevas AD, Yom SS, Pfister DG, et al. NCCN guidelines insights: head and neck cancers, version 1.2018. J Natl Compr Canc Netw. 2018;16:479–490. doi:10.6004/jnccn.2018.0026
18. Pfister DG, Spencer S, Adelstein D, et al. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18:873–898. doi:10.6004/jnccn.2020.0031
19. Onishi H, Kuriyama K, Komiyama T, et al. T1N0 laryngeal sarcomatoid carcinoma that showed rapid systemic metastases after radical radiotherapy: a case report and review of literature. Am J Otolaryngol. 2005;26:400–402. doi:10.1016/j.amjoto.2005.02.017
20. Dai L, Fang Q, Li P, et al. Oncologic outcomes of patients with sarcomatoid carcinoma of the hypopharynx. Front Oncol. 2019;9:950. doi:10.3389/fonc.2019.00950
21. Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–965. doi:10.1016/S1470-2045(16)30066-3
22. Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, Phase II study. J Clin Oncol. 2017;35:1542–1549. doi:10.1200/JCO.2016.70.1524
23. Chi ZH, Tang BX, Sheng XN, et al. A phase II study of JS001, a humanized PD-1 mAb, in patients with advanced melanoma in China. J Clin Oncol. 2018;36:2. doi:10.1200/JCO.2018.36.15_suppl.9539
24. Wei XL, Ren C, Wang FH, et al. A Phase I study of toripalimab, an anti-PD-1 antibody, in patients with refractory malignant solid tumors. Cancer Commun. 2020;40:345–354. doi:10.1002/cac2.12068
25. Merhi M, Raza A, Inchakalody VP, et al. Squamous cell carcinomas of the head and neck cancer response to programmed cell death protein-1 targeting and differential expression of immunological markers: a case report. Front Immunol. 2018;9:1769. doi:10.3389/fimmu.2018.01769
26. Forastiere AA, Zhang Q, Weber RS, et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin oncol. 2013;31:845–852.
27. Tran L, Allen CT, Xiao R, et al. Cisplatin alters antitumor immunity and synergizes with PD-1/PD-L1 inhibition in head and neck squamous cell carcinoma. Cancer Immunol Res. 2017;5:1141–1151. doi:10.1158/2326-6066.CIR-17-0235
28. De Andrés L, Brunet J, López-Pousa A, et al. Randomized trial of neoadjuvant cisplatin and fluorouracil versus carboplatin and fluorouracil in patients with stage IV-M0 head and neck cancer. J Clin oncol. 1995;13:1493–1500. doi:10.1200/JCO.1995.13.6.1493
29. Epstein JB, Thariat J, Bensadoun R-J, et al. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin. 2012;62:400–422.
30. Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2014;89:13–20. doi:10.1016/j.ijrobp.2013.12.027
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.