Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Relationship of Vitamin-D Deficiency with Kidney Disease in Patients with Type-2 Diabetes Mellitus (T2DM) in the Makkah Region: A Cross-Sectional Study
Authors Obaid AA, Mujalli A , Farrash WF, Tayeb RH, Bougeis RJ, Aljehani AA, Alshehri BA, Sharaf SE, Alqurashi SF
Received 7 November 2023
Accepted for publication 23 December 2023
Published 3 January 2024 Volume 2024:17 Pages 11—17
DOI https://doi.org/10.2147/DMSO.S445314
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Ahmad A Obaid,1 Abdulrahman Mujalli,1 Wesam F Farrash,1 Rami Hatem Tayeb,2 Rashad Jameel Bougeis,2 Alaa Adel Aljehani,2 Bandar Ali Alshehri,3 Sulafa Ezzat Sharaf,4 Saud Fahad Alqurashi4
1Department of Clinical Laboratory Sciences, Faculty of Applied Medical Sciences, Umm Al-Qura University, Makkah, Saudi Arabia; 2Laboratory Department, King Abdulaziz University Hospital, Jeddah, Saudi Arabia; 3Laboratory Department, Dar Alzahrawi Medical, Product Specialist Diagnostic, Jeddah, Saudi Arabia; 4Laboratory Department, King Faisal Hospital, Makkah, Saudi Arabia
Correspondence: Ahmad A Obaid, Email [email protected]
Aim: Vitamin D deficiency is linked with type 2 diabetes mellitus (T2DM) and the occurrence of complications in patients with type 2 diabetes mellitus. None of the studies have focused on the association between vitamin D levels in patients with type 2 diabetes mellitus and diabetic nephropathy (DN) in the Makkah region, Saudi Arabia. Hence, the purpose of this study is to investigate the relationship of vitamin D with kidney disease in patients with T2DM in the Makkah region, of Saudi Arabia.
Materials and Methods: This descriptive cross-sectional study was conducted at different hospitals in the Makkah region on T2DM patients from 2021 to 2022. In total, 328 patients with confirmed diabetes were enrolled in this study. T2DM patients over the aged> 18 to 92 years were included in the study. General laboratory characteristics of the study population were measured, including fasting blood sugar, HbA1C (Glycated hemoglobin), vitamin D, kidney function (BUN-Blood urea nitrogen and creatinine), and lipid profiles (cholesterol, triglycerides, LDL-Low density lipoprotein, and HDL-High density lipoprotein).
Results: 46.6% (n=153) of participants had normal serum vitamin D levels. Insufficient and deficient serum vitamin D level were observed in 43.9% (n=144) and 9.5% (n=31) of participants, respectively. Of the participants, 25.9% (n=85) had good glycemic control (< 7.0%). Moderate and poor glycemic control were observed in 39.9% (n=131) and 34.1% (n=112) of the participants, respectively. A significant negative correlation (p< 0.5) was found between vitamin D levels and kidney function test results (blood urea nitrogen and serum creatinine levels). An inverse relationship was observed between HbA1c levels and vitamin D deficiency.
Conclusion: Nephropathy is more likely to develop in people with type 2 diabetes mellitus and vitamin D deficiency.
Keywords: prevalence, diabetes, type-2 diabetes mellitus, T2DM, vitamin-D, nephropathy
Introduction
Diabetes mellitus is an intricate metabolic disease that poses a global health burden because an alarming number of premature deaths and morbidities have been documented in recent years.1,2 Impaired insulin production and action are the manifestation of diabetes mellitus.3 Diabetes mellitus is categorized into Type-1 diabetes mellitus (T1DM) and T2DM. In recent decades, the increasing health burden of T2DM has become a major international concern. T2DM has been reported in >95% of individuals with diabetes.4 Globally, approximately 462 million individuals are affected by T2DM, which represents 6.28% of the world’s population.5 The prevalence rate in the Kingdom of Saudi Arabia ranges from 9% to-22% and there are an estimated seven million patients with diabetes in the country.6,7 Similar patterns of T2DM prevalence have been noticed in Kuwait.7,8 Risk factors for the development of T2DM include obesity, sedentary lifestyle, alcohol consumption, smoking, and fatty diet.9 The primary organs affected with T2DM are skeletal muscles, brain, and kidneys.10 Approximately 40% of patients with diabetes are diagnosed with DN, which is characterized by kidney dysfunction and proteinuria.11 The frequency of DN increases with an increase in T2DM rises.12 Vitamin D is a hormonal molecule that participates in the homeostasis of calcium and phosphate. Additionally, it is also involved in the genomic (expression of genes) and non-genomic functions (glucose metabolism, enhancing insulin resistance, insulin secretion, and stimulation of receptors associated with insulin).13,14 The metabolically active form of vitamin D is produced in the kidney.15 Several studies have reported that vitamin D deficiency contributes to the development of chronic kidney disease (CKD).16–20 In addition, vitamin D deficiency accelerates the development and genesis of T2DM because it is involved in insulin secretion.21 Many studies have revealed a link between vitamin D levels and chronic kidney disease or diabetes mellitus.22–24 Numerous studies conducted in humans and animals have indicated that vitamin D has reno-protective effects, such as anti-fibrosis, anti-inflammatory, and anti-proteinuria effects, and also prevents podocyte damage.25–28 Vitamin D supplements are beneficial in patients with CKD or diabetes mellitus.29,30 Most studies have focused on the relationship between vitamin D, CKD, and diabetes mellitus.21–24 None of the studies have investigated the relationship between vitamin D and nephropathy in T2DM patients in the Makkah region. Hence, the purpose of this study is to investigate the relationship of vitamin D with kidney disease for patients with T2DM in the Makkah region, of Saudi Arabia.
Materials and Methods
Design of the Study
The current descriptive cross-sectional study was conducted in different hospitals in the Makkah region from 2021 to 2022 on T2DM patients after obtaining permission from each hospital and informed consent from the patients. The study was conducted under the Declaration of Helsinki, and the protocol was approved by The Biomedical Research Ethics Committee at Umm Al-Qura University, Makkah, Saudi Arabia (HAPO-02-K-012-2023-03-1486). Randomly selected T2DM patients over the aged>18 to 92 years were included in the study which were sex and age matched. Patients with renal and cardiac diseases were excluded from the study. As the sun in Makkah region cities shines in both the winter and summer seasons in similar amounts, there were no major seasonal effects on the vitamin D levels in the body. Standard range for general laboratory parameters are as follows: HbA1c (normal-5.6 to 6.4, diabetes->6.4), Vitamin D (50nM), BUN (6–24mg/dL), Creatinine (for men-0.59–1.04 mg/dL and for women-0.74–1.35 mg/dL), Cholestrol (<200 mg/dL), LDL (<100 mg/dL), HDL (for men-<40 mg/dL and for women-<50 mg/dL), and HbA1c category (normal-<7.0% good glycaemic control, moderate-7-8.5% moderate glycaemic control and poor->8.5% -poor glycaemic control).
Sample Size
In total, 328 patients with confirmed diabetes were included in the study. The following parameters were collected.
- Characteristics of the study participants, which include age and gender.
- General laboratory characteristics of the study population were measured, including fasting blood sugar, HbA1C, Vitamin D, kidney function (BUN and creatinine), and lipid profiles (cholesterol, triglycerides, LDH, and HDL).
Statistical Analysis
General laboratory parameters were expressed as mean±SD deviation or median (interquartile range). Categorical variables, such as normal level, insufficiency, and deficiency, were analysed as proportions (%). SPSS package version 11.0 for Microsoft Windows was used to analyse the data collected. In addition, HbA1C was classified as normal, moderate, or poor glycaemic levels and analysed as proportions. The stacking bar chart was used to illustrate which categories of HbA1C were more likely to have vitamin D deficiency. Linear regression analysis was performed to determine the correlation between vitamin D levels and kidney function test results (BUN and creatinine).
Statistical Software
Data were analysed using SPSS (version 15.0; IBM Corp., Armonk, NY, USA), and graphs, tables, and other graphics were prepared using Microsoft Word and Excel.
Results
A total of 328 patients with diabetes were included in this study, 43.6% of whom were male (n=143) and 56.5% of whom were female (n=185) (Table 1). A general and laboratory profile [fasting blood sugar (FBS), HbA1C, blood urea nitrogen (BUN), creatinine, vitamin-D level, triglycerides (TAG), HDL and LDL] of the participants were collected, and creatinine levels (91.5±99.81) were higher while HDL (1.2±0.39) levels were lower (Table 2). In terms of glycaemic control, 25.9% (n=85), 39.9% (n=131), and 34.1% (n=112) of the participants had good, moderate, and poor glycaemic control, respectively (Table 3). Among the participants, 46.6% (n=153), 43.9% (n=144), and 9.5% (n=31) had normal, insufficient, and deficient serum vitamin D levels, respectively (Table 3). A significant negative correlation (r=0.130, p=0.032) was found between vitamin D and BUN levels (Figure 1). There was also a significant negative correlation (r=0.0454, p=0.442) between vitamin D and creatinine (Figure 2). According to Figure 3, patients with HbA1c (7–8.5%, R2=0.0012, p=0.5353) were more likely to be vitamin-D deficient.
 |
Table 1 General Characteristics of the Study Participants |
 |
Table 2 General Laboratory Parameters of the Study Population |
 |
Table 3 Laboratory Characteristics of HbA1c & Serum Vitamin-D in the Study Population |
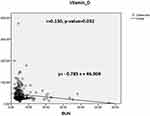 |
Figure 1 Scatter plot showing a significant correlation between vitamin-D level and BUN. |
 |
Figure 2 Scatter plot showing a significant correlation between vitamin-D levels and creatinine. |
 |
Figure 3 Stacked bar chart illustrating the relationship HbA1c and serum vitamin-D. |
Discussion
Makkah region cities receives a lot of sunlight (equal sunlight in both summer and winter) and is situated at a latitude of 21.3891° north, 39.8579° E, with an altitude of 277 m. However, approximately 63% of the patients had a vitamin-D deficiency (VDD) in the Makkah region.31 The general medical inpatient population was found to have high levels of vitamin D deficiency.32 The current study reported a 9.1% (<20ng/mL) prevalence of vitamin D deficiency in 31 T2DM patients. Several reports showed that prevalence of vitamin D deficiency ranged from 28%-75%.33–35 Sadat-Ali et al34 reported a 37% prevalence of vitamin D deficiency in healthy men in eastern Saudi Arabia. Aljabri et al36 reported a 27% prevalence of vitamin D deficiency in patients with T2DM in Saudi Arabia. The current study reported normal serum vitamin D levels and vitamin D insufficiency in 46.6% (≥30ng/mL) and 43.9% (20.1–29.9 ng/mL) of patients with T2DM, respectively. Several studies have reported a similar prevalence of normal serum vitamin D and vitamin D insufficiency in patients with T2DM in Saudi Arabia.34,37–39 Furthermore, our results showed that lower serum vitamin D was observed in patients with T2DM, which is consistent with previous findings.36,39,40 In the present study, our results showed that 25.9%, 39.9%, and 34.1% of the participants had good, moderate, and poor glycemic control, respectively. This is in agreement with several studies conducted on patients with T2DM.41–43 Interestingly, an inverse relationship has been found between the level of HbA1c and vitamin-D suggesting that patients with high HbA1c were more likely to have vitamin-D deficiency and this finding is correlated with several previous studies conducted in Saudi Arabia.43–45 Numerous studies have examined the association between lower vitamin D, CKD progression, and mortality.46–48 One study reported that patients with DN have a higher probability of suboptimal vitamin D levels, which can lead to a faster progression of both DM and CKD.46 A recent study showed that vitamin D deficiency is a crucial predictor of DN in T2DM.49 Blood urea nitrogen and serum creatinine levels are used as biomarkers to evaluate kidneys.50 In the present study, the mean BUN was 6.1±5.56 in patients with T2DM. A recent study reported the mean BUN in patients with T2DM which was 6.65±3.08.51,52 Numerous studies reported the relationship between low BUN with DN.53–55 In the current study, a significant negative correlation was also observed between serum creatinine and vitamin D levels, which correlates with several lines of evidence.56–58 The current study has many limitations associated with cross-sectional studies; in particular, the temporal link cannot be assessed between exposure and outcome because both are determined at the same time and (ii) subjects were chosen on the basis of clinical data available in previous studies.
Conclusion
In conclusion, Vitamin D and kidney function tests (BUN and serum creatinine levels) also showed a negative correlation, suggesting that this could coincide with the development of different stages of kidney disease. Additionally, the study indicated that low vitamin D levels are associated with both T2DM and DN and those patients with T2DM are more likely to develop kidney disease. Vitamin D must be supplemented in individuals with pre-diabetes to reduce the incidence of diabetes. However, multi-center studies are needed to validate the correlation between vitamin D supplementation in individuals with pre-diabetes and the incidence of diabetes.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hameed I, Masoodi SR, Mir SA, Nabi M, Ghazanfar K, Ganai BA. Type 2 diabetes mellitus: from a metabolic disorder to an inflammatory condition. World J Diabetes. 2015;6(4):598. doi:10.4239/wjd.v6.i4.598
2. DeFronzo RA, Ferrannini E, Zimmet P, Alberti G. International Textbook of Diabetes Mellitus. John Wiley & Sons; 2015.
3. Kharroubi AT. Diabetes mellitus: the epidemic of the century. World J Diabetes. 2015;6(6):850. doi:10.4239/wjd.v6.i6.850
4. Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Nerurkar PV. Risk factors for type 2 diabetes mellitus: an exposure-wide umbrella review of meta-analyses. PLoS One. 2018;13(3):e0194127. doi:10.1371/journal.pone.0194127
5. Khan MA, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes–global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107. doi:10.2991/jegh.k.191028.001
6. Abdulaziz Al Dawish M, Alwin Robert A, Braham R, et al. Diabetes mellitus in Saudi Arabia: a review of the recent literature. Current Diabetes Rev. 2016;12(4):359–368. doi:10.2174/1573399811666150724095130
7. Zabetian A, Keli HM, Echouffo-Tcheugui JB, Narayan KV, Ali MK. Diabetes in the middle east and north Africa. Diabetes Res Clin Pract. 2013;101(2):106–122. doi:10.1016/j.diabres.2013.03.010
8. Alkandari A, Alarouj M, Elkum N, et al. Adult diabetes and prediabetes prevalence in Kuwait: data from the cross-sectional Kuwait diabetes epidemiology program. J Clin Med. 2020;9(11):3420. doi:10.3390/jcm9113420
9. Nasr MH, Hassan BA, Othman N, et al. Prevalence of vitamin D deficiency between type 2 diabetes mellitus patients and non-diabetics in the Arab gulf. Diabetes Metab Syndr Obes. 2022;1:647–657. doi:10.2147/DMSO.S350626
10. Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17):6275. doi:10.3390/ijms21176275
11. Gheith O, Farouk N, Nampoory N, Halim MA, Al-Otaibi T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. 2016;5(1):49.
12. Zhang XX, Kong J, Yun K. Prevalence of diabetic nephropathy among patients with type 2 diabetes mellitus in China: a meta-analysis of observational studies. J Diabetes Res. 2020;2020. doi:10.1155/2020/2315607
13. Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. In: Mayo Clinic Proceedings. Elsevier; 2013:720–755.
14. Szymczak-Pajor I, Śliwińska A. Analysis of association between vitamin D deficiency and insulin resistance. Nutrients. 2019;11(4):794. doi:10.3390/nu11040794
15. Jean G, Souberbielle JC, Chazot C. Vitamin D in chronic kidney disease and dialysis patients. Nutrients. 2017;9(4):328. doi:10.3390/nu9040328
16. Patel TV, Singh AK. Role of vitamin D in chronic kidney disease. In: Seminars in Nephrology. WB Saunders; 2009:113–121.
17. Kim CS, Kim SW. Vitamin D and chronic kidney disease. Korean J Intern Med. 2014;29(4):416. doi:10.3904/kjim.2014.29.4.416
18. Wang Y, Deb DK, Zhang Z, et al. Vitamin D receptor signaling in podocytes protects against diabetic nephropathy. J Am Soc Nephrol. 2012;23(12):1977–1986. doi:10.1681/ASN.2012040383
19. Zhang Z, Sun L, Wang Y, et al. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int. 2008;73(2):163–171. doi:10.1038/sj.ki.5002572
20. Song Z, Xiao C, Jia X, et al. Vitamin D/VDR protects against diabetic kidney disease by restoring podocytes autophagy. Diabetes Metab Syndr Obes. 2021;14:1681. doi:10.2147/DMSO.S303018
21. Kadowaki S, Norman AW. Pancreatic vitamin D-dependent calcium binding protein: biochemical properties and response to vitamin D. Arch Biochem Biophys. 1984;233(1):228–236. doi:10.1016/0003-9861(84)90621-0
22. Nakashima A, Yokoyama K, Yokoo T, Urashima M. Role of vitamin D in diabetes mellitus and chronic kidney disease. World J Diabetes. 2016;7(5):89. doi:10.4239/wjd.v7.i5.89
23. Bosworth C, de Boer IH. Impaired vitamin D metabolism in CKD. Semin Nephrol. 2013;33(2):158–168. doi:10.1016/j.semnephrol.2012.12.016
24. Satirapoj B, Limwannata P, Chaiprasert A, Supasyndh O, Choovichian P. Vitamin D insufficiency and deficiency with stages of chronic kidney disease in an Asian population. BMC Nephrol. 2013;14(1):1–5. doi:10.1186/1471-2369-14-206
25. Zhang X, Song Z, Guo Y, Zhou M. The novel role of TRPC6 in vitamin D ameliorating podocyte injury in STZ-induced diabetic rats. Mol Cell Biochem. 2015;399(1):155–165. doi:10.1007/s11010-014-2242-9
26. Wan J, Li P, Liu DW, et al. GSK-3β inhibitor attenuates urinary albumin excretion in type 2 diabetic db/db mice, and delays epithelial-to-mesenchymal transition in mouse kidneys and podocytes. Mol Med Rep. 2016;14(2):1771–1784. doi:10.3892/mmr.2016.5441
27. Sanchez-Niño MD, Bozic M, Córdoba-Lanús E, et al. Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2012;302(6):F647–F657. doi:10.1152/ajprenal.00090.2011
28. Zhang Y, Kong J, Deb DK, Chang A, Li YC. Vitamin D receptor attenuates renal fibrosis by suppressing the renin-angiotensin system. J Am Soc Nephrol. 2010;21(6):966–973. doi:10.1681/ASN.2009080872
29. LaClair RE, Hellman RN, Karp SL, et al. Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis. 2005;45(6):1026–1033. doi:10.1053/j.ajkd.2005.02.029
30. González EA, Sachdeva A, Oliver DA, Martin KJ. Vitamin D insufficiency and deficiency in chronic kidney disease. Am j Nephrol. 2004;24(5):503–510. doi:10.1159/000081023
31. Kensarah OA, Jazar AS, Azzeh FS. Hypovitaminosis D in healthy toddlers and preschool children from western Saudi Arabia. Int J Vitam Nutr Res. 2015;85(1–2):50–60. doi:10.1024/0300-9831/a000223
32. Bokhari FF, Albaik M. Vitamin D and its deficiency in Saudi Arabia. In: Vitamin D Deficiency. IntechOpen; 2019.
33. Alsuwadia AO, Farag YM, Al Sayyari AA, et al. Prevalence of vitamin D deficiency in Saudi adults. Saudi Med J. 2013;34(8):814–818.
34. Sadat-Ali M, AlElq A, Al-Turki H, Al-Mulhim F, Al-Ali A. Vitamin D levels in healthy men in eastern Saudi Arabia. Ann Saudi Med. 2009;29(5):378–382. doi:10.4103/0256-4947.55168
35. Elshafie DE, Al-Khashan HI, Mishriky AM. Comparison of vitamin D deficiency in Saudi married couples. Eur J Clin Nutr. 2012;66(6):742–745. doi:10.1038/ejcn.2012.29
36. Aljabri KS, Bokhari SA. Severe vitamin D deficiency in Saudi patients with type 2 diabetes mellitus. J Obes. 2019;1(2):34. doi:10.14302/issn.2574-450X.jom-19-2987
37. Mansour MM, Alhadidi KM. Vitamin D deficiency in children living in Jeddah, Saudi Arabia. Indian J Endocrinol Metab. 2012;16(2):263–269. doi:10.4103/2230-8210.93746
38. Farhat KH, Arafa MA, Rabah DM, Amin HS, Ibrahim NK. Vitamin D status and its correlates in Saudi male population. BMC Public Health. 2019;19(1):1–6. doi:10.1186/s12889-019-6527-5
39. Albannawi G, Alsaif S, Alsaif G, Taher B. Vitamin D deficiency among Type 2 Diabetes patients in Saudi Arabia: a systematic review. Int J Diabetes Dev Ctries. 2020;2020:197–203.
40. Dabbour IR, Jazar AS, Azzeh FS. Vitamin D status in patients with type 2 diabetes mellitus in Makkah region of Saudi Arabia. Pak J Nutr. 2016;15(3):203. doi:10.3923/pjn.2016.203.210
41. Kostoglou-Athanassiou I, Athanassiou P, Gkountouvas A, Kaldrymides P. Vitamin D and glycemic control in diabetes mellitus type 2. Ther Adv Endocrinol Metab. 2013;4(4):122–128. doi:10.1177/2042018813501189
42. Al Slamah T, Nicholl BI, Alslail FY, Harris L, Kinnear D, Melville CA. Correlates of type 2 diabetes and glycaemic control in adults in Saudi Arabia a secondary data analysis of the Saudi health interview survey. BMC Public Health. 2020;20(1):1–3. doi:10.1186/s12889-020-08597-6
43. Aljabri KS. Vitamin D deficiency in female Saudis with type 2 diabetes mellitus. Age. 2019;53:16.
44. Mogahed MM. Vitamin D status in patients with type-2 diabetes mellitus in Riyadh city, Saudi Arabia. Kasr Al Ainy Med J. 2018;24(1):19. doi:10.4103/kamj.kamj_30_17
45. Al Hewishel MA. Vitamin D level in non-diabetic and type ii diabetic patients KFU health center: a cross sectional study. Egypt J Hosp Med. 2018;72(3):4067–4074. doi:10.21608/ejhm.2018.9118
46. Gembillo G, Cernaro V, Salvo A, et al. Role of vitamin D status in diabetic patients with renal disease. Medicina. 2019;55(6):273. doi:10.3390/medicina55060273
47. Kantas T, Capriles CA, Babor S, et al. Relationship between chronic kidney disease staging and vitamin D deficiency: a retrospective study. Cureus. 2022;14(1):1.
48. Ali M, Ejaz A, Solangi SA, et al. Vitamin D deficiency in end stage renal disease patients with diabetes mellitus undergoing hemodialysis. Cureus. 2020;12(11):1.
49. Xie S, Huang L, Cao W, et al. Association between serum 25-hydroxyvitamin D and diabetic kidney disease in Chinese patients with type 2 diabetes. PLoS One. 2019;14(4):e0214728. doi:10.1371/journal.pone.0214728
50. Lopez-Giacoman S. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J Nephrol. 2015;4(1):57. doi:10.5527/wjn.v4.i1.57
51. Zhao WJ, Xia XY, Yin J. Relationship of serum vitamin D levels with diabetic microvascular complications in patients with type 2 diabetes mellitus. Chinese Med J. 2021;134(07):814–820. doi:10.1097/CM9.0000000000001364
52. Bentli R, Taskapan H, Toktaş H, Ulutas O, Ozkahraman A, Comert M. Significant independent predictors of vitamin D deficiency in inpatients and outpatients of a nephrology unit. Int J Endocrinol. 2013;2013:1–5. doi:10.1155/2013/237869
53. Xiao X, Wang Y, Hou Y, Han F, Ren J, Hu Z. Vitamin D deficiency and related risk factors in patients with diabetic nephropathy. J Int Med Res. 2016;44(3):673–684. doi:10.1177/0300060515593765
54. Seki M, Nakayama M, Sakoh T, et al. Blood urea nitrogen is independently associated with renal outcomes in Japanese patients with stage 3–5 chronic kidney disease: a prospective observational study. BMC Nephrol. 2019;20(1):1. doi:10.1186/s12882-019-1306-1
55. Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 2018;93(3):741–752. doi:10.1016/j.kint.2017.08.033
56. Restrepo Valencia CA, Aguirre Arango JV. Vitamin D (25 (OH) D) in patients with chronic kidney disease stages 2-5. Colomb Med. 2016;47(3):160–166. doi:10.25100/cm.v47i3.2148
57. Diniz HF, Romão MF, Elias RM, Romão Júnior JE. Vitamin D deficiency and insufficiency in patients with chronic kidney disease. Braz J Nephrol. 2012;34(1):58–63. doi:10.1590/S0101-28002012000100009
58. El Din US, Fayed A, El Nokeety MM, Abdulazim DO, Salem MM, Behalf of the Vascular Calcificati Group ON. Vitamin-D deficiency is encountered in almost all Egyptian stage 3-5 chronic kidney disease patients in spite of the sunny weather. Saudi J Kidney Dis Transpl. 2019;30(6):1389. doi:10.4103/1319-2442.275483
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
