Back to Journals » Neuropsychiatric Disease and Treatment » Volume 11
Relationship between remnant hippocampus and amygdala and memory outcomes after stereotactic surgery for mesial temporal lobe epilepsy
Authors Malikova H, Kramska L , Vojtech Z, Sroubek J , Lukavsky J , Liscak R
Received 31 August 2015
Accepted for publication 12 October 2015
Published 19 November 2015 Volume 2015:11 Pages 2927—2933
DOI https://doi.org/10.2147/NDT.S95497
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Hana Malikova,1,2,* Lenka Kramska,3,* Zdenek Vojtech,4,5 Jan Sroubek,6 Jiri Lukavsky,7 Roman Liscak8
1Department of Radiology, Na Homolce Hospital, 2Institute of Anatomy, Second Medical Faculty, Charles University in Prague, 3Department of Clinical Psychology, Na Homolce Hospital, 4Department of Neurology, Na Homolce Hospital, 5Department of Neurology, 3rd Medical Faculty, Charles University in Prague, 6Department of Neurosurgery, Na Homolce Hospital, 7Institute of Psychology, Academy of Sciences of the Czech Republic, 8Department of Radiation and Stereotactic Neurosurgery, Na Homolce Hospital, Prague, Czech Republic
*These authors contributed equally to this work
Background and purpose: Mesial temporal structures play an important role in human memory. In mesial temporal lobe epilepsy (MTLE), seizure activity is generated from the same structures. Surgery is the definitive treatment for medically intractable MTLE. In addition to standard temporal lobe microsurgical resection, stereotactic radiofrequency amygdalohippocampectomy (SAHE) is used as an alternative MTLE treatment. While memory impairments after standard epilepsy surgery are well known, it has been shown that memory decline is not a feature of SAHE. The aim of the present study was to correlate the volume of the remnant hippocampus and amygdala in patients treated by SAHE with changes in memory parameters.
Materials and methods: Thirty-seven MTLE patients treated by SAHE (ten right, 27 left) were included. Patients underwent magnetic resonance imaging examinations including hippocampal and amygdalar volumetry and neuropsychological evaluation preoperatively and 1 year after surgery.
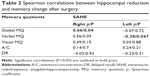
Results: Using Spearman correlation analyses, larger left-sided hippocampal reductions were associated with lower verbal memory performance (ρ=-0.46; P=0.02). On the contrary, improvement of global memory quotient (MQ) was positively correlated with larger right-sided hippocampal reduction (ρ=0.66; P=0.04). Similarly, positive correlations between the extent of right amygdalar reduction and verbal MQ (ρ=0.74; P=0.02) and global MQ change (ρ=0.69; P=0.03) were found. Thus, larger right hippocampal and amygdalar reduction was associated with higher global and verbal MQ change after SAHE.
Conclusion: Larger left-sided hippocampal reductions were associated with lower verbal memory performance. This finding is in accordance with the material-specific model of human memory, which states that the dominant hemisphere is specialized for the learning and recall of verbal information. We hypothesize that larger right-sided ablations enable the left temporal lobe to support memory more effectively, perhaps as a consequence of epileptiform discharges spreading from remnants of right mesiotemporal structures to the left.
Keywords: amygdalohippocampectomy, thermocoagulation, MRI, volumetry, neuropsychology
Introduction
It is well known that mesial temporal structures and particularly the hippocampus play an important role in human memory. In case of the most common epilepsy diagnosis in adults, which is mesial temporal lobe epilepsy (MTLE), seizure activity is generated from the same structures. It has repeatedly been proven that surgery is the definitive treatment for medically intractable MTLE, with approximately 50% of patients seizure-free at 10 years.1 However, memory impairment is an anticipated sequela in the surgical treatment of MTLE.2 The first case to illustrate the clear connection between memory and the temporal lobes was published by Scoville and Milner, who reported profound amnesia following bi-temporal lobectomy.3 According to the material-specific model of human memory, the dominant hemisphere is specialized for the learning and recall of verbal information, whereas the non-dominant hemisphere is specialized for the learning and recall of nonverbal information.4 Therefore, cognitive decline is usually material-specific to the side of resection. Verbal memory decline typically occurs after left-sided operations.2,5 On the contrary, visual memory deficits have been reported after right-sided operations in some studies, but not in others.6
Open microsurgical techniques, such as anterior temporal lobectomy and selective microsurgical amygdalohippocampectomy, have been used for the treatment of MTLE for many years.7 At our epilepsy center, we have also used stereotactic radiofrequency amygdalohippocampectomy (SAHE) for the treatment of MTLE since 2004.8 The stereotactic radiofrequency technique uses a Leksell stereotactic system with a coordinate frame attached to the patient’s head, as well as magnetic resonance imaging (MRI) neuronavigation. The amygdalohippocampal complex (AHC) is targeted from occipital access. Thermocoagulative lesions are created in the long axis of the AHC by a string electrode with a flexible 10 mm bold active tip. The local temperature depends on the probe thickness.8 As was published in the previous studies, long-term seizure outcomes following SAHE are comparable with open surgical approaches.9 It has been reported previously that approximately 70% of patients were assessed as Engel Class I 5 years after stereotactic surgery.9 It has also been shown that SAHE does not result in neuropsychological deficits9,10 and causes only partial destruction of target structures in the mesial temporal lobe.10 Partial destruction of target structures allows comparison between the residual hippocampal and amygdalar volumes and postoperative changes in memory parameters, which is the aim of the present study.
Materials and methods
Patient selection
Inclusion criteria for the present study were as follows: 1) patients with MTLE treated by SAHE between 2004 and 2012; 2) patients recruited, treated, and followed at our center; 3) completed preoperative and 1 year postoperative MRI; and 4) completed preoperative and 1 year postoperative neuropsychological examinations including all memory subtests. Thirty-seven MTLE patients treated by SAHE fulfilled the inclusion criteria. Prior to SAHE, all subjects provided signed, informed consent to participate in the study, including the publication of anonymized data. The study was approved by the local Ethical Committee at Na Homolce Hospital (Prague, Czech Republic).
Neuropsychological evaluation
All patients were scheduled for neuropsychological assessment preoperatively and 1 year postoperatively. Memory was tested by the Wechsler Memory Scale – Revised, which consists of 12 subtests, from which the following five composite scores were derived: general memory, attention/concentration, verbal memory, visual memory, and delayed recall.11 Hand laterality was tested preoperatively by the lateral dominance examination.12 Patients were scheduled for the WADA test (intracarotid sodium amobarbital procedure) preoperatively; however some subjects refused this invasive procedure.
MRI methods
Preoperative diagnostic MRI examinations and postoperative MRI follow-up examinations were performed on a 1.5 T whole body MRI system. In addition to common diagnostic sequences, a T1 three-dimensional (3D) volume acquisition sequence (voxel size 1.0×1.0×1.0 mm, slab one, slices per slab 176) was obtained. The diagnosis of hippocampal sclerosis was made from MRI hippocampal volumetry and visual assessment of the signal intensity of the hippocampus. Hippocampal and amygdalar volumetry was performed according to a well-defined protocol.13,14 Right and left hippocampal volumes were measured separately on preoperative T1 3D sequences obtained the day of the operation or several days before, and from the scans obtained 1 year after surgery. From the T1 3D sequence, multiplanar reconstructions in the coronal plane were performed with a slice thickness of 2 mm. The volumes were segmented manually and calculated with software developed in-house for a standard work console. Anatomical hippocampal boundaries were determined by the following criteria. The appearance of the mammillary bodies was used as a landmark to demarcate roughly where the amygdala begins to appear when navigating the brain from posterior to anterior. Moving anteriorly, amygdalar gray matter begins to extend superiorly to the hippocampus with good resolution, the lateral ventricle can be seen between the hippocampus and amygdala. Sagittal views aided in this demarcation. The posterior border was demarcated by the clearest appearance of the fornix. The lateral border was defined by the temporal horn of the lateral ventricle and/or the white matter adjacent to hippocampal gray matter. The inferior border was demarcated by the white matter of the parahippocampal gyrus. The extent of resection or tissue destruction was calculated as the percent difference between preoperative and postoperative volumes. One experienced rater did each measurement twice and the mean value of these measurements was used in analyses.
Statistics
The data were analyzed using the R statistical package.15 Data were summarized using the mean ± standard deviation. Differences between preoperative and postoperative neuropsychological results were evaluated using the paired Wilcoxon test. We tested the effect of hippocampal and amygdalar volume reduction on memory performance with Spearman correlation. P-values <0.05 were considered statistically significant.
Results
Patient selection and MRI volumetry data
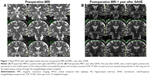
Thirty-seven MTLE patients treated by SAHE (ten right-sided, 27 left-sided) were included. Patient inclusion data and MRI volumetry results are summarized in Table 1. Figure 1 demonstrates typical MRI findings preoperatively and 1 year after SAHE.
Neuropsychological outcomes
Patients receiving right-sided SAHE improved significantly in global memory quotient (MQ) (11.4±13.6, P=0.04), verbal MQ (13.6±12.2, P=0.01), and delayed recall (16.3±14.4, P=0.004). Visual MQ and attention/concentration data remained stable. No significant changes in memory were detected following left-sided SAHE.
Hippocampal and amygdalar reduction versus neuropsychological changes
We tested the effect of hippocampal and amygdalar volume reduction on postoperative change in memory performance with Spearman correlation. Using this analysis we found significant correlation results. Larger left-sided hippocampal reduction in the SAHE group was associated with a decrease in verbal memory performance (ρ=−0.46; P=0.02). On the contrary, improvement of global MQ was positively related to right-sided hippocampal reduction (ρ=0.66; P=0.04). Similarly, positive correlations between right amygdalar reduction and verbal MQ (ρ=0.74; P=0.02) and global MQ change (ρ=0.69; P=0.03) were found. We found no correlation between the extent of left amygdalar reduction and changes in memory performance. Spearman correlation results between hippocampal reduction and memory changes are summarized in Tables 2 and 3. A series of graphs (Figure 2) illustrates the relationship between changes in a particular memory outcome and reduction of the hippocampus and amygdala.
Discussion
In the present study, we evaluated correlations between memory outcomes and remnant hippocampal and amygdalar volumes in MTLE patients treated by mini-invasive SAHE. SAHE clearly differs from open surgical procedures; the destruction of target structures after SAHE is asymmetrical and only partial. The posterior portion of the remnant hippocampus, untouched by thermocoagulation, is significantly longer than in anterior temporal resection.10 The longer posterior hippocampal remnant, together with partial destruction of the rest of the AHC and a completely spared lateral neocortex, may explain good neuropsychological outcomes.10 Although SAHE spares mesial temporal structures and causes only partial destruction of target structures, it provides comparable seizure outcomes to open surgery and does not cause postoperative memory impairment.9,10 Thus, SAHE treatment may potentially expand our understanding of human memory in MTLE patients.
We found a significant relationship between memory parameters and volumes of the remnant hippocampus and amygdala. We found significant correlation between global MQ change after SAHE and right-sided hippocampal reduction, and negative correlation in verbal MQ change and left-sided hippocampal reduction. Larger left-sided hippocampal reduction was associated with lower verbal memory performance; on the contrary, larger right-sided hippocampal reduction was associated with better global MQ. Larger right amygdalar reduction was associated with higher global and verbal MQ change after SAHE.
The significant correlation between larger left-sided hippocampal reduction and lower verbal memory performance is not surprising. According to the material-specific model of human memory, the dominant hemisphere is specialized for the learning and recall of verbal information.4 Verbal memory impairment after left-sided open surgical procedures is well known2 and it has been repeatedly proven that selective approaches in MTLE bring better cognitive outcomes then anterior temporal lobectomy.16–18 Thus, our results in patients after left-sided SAHE are in agreement with those reported previously.
Correlations between both hippocampal and amygdalar reductions and memory changes in patients after right-sided procedures are not easily explained. The amygdala plays an important role in the regulation of emotions and behavior, as well as in the modulation of cognitive functions.19 However, we did not test emotional memory in our patients. According to the literature, the amygdala may be responsible for fear during seizures and for prolonged postictal confusion.19 Amygdalar destruction in the non-dominant hemisphere in seizure-free patients may improve emotional regulation and reduce stress and fear. These factors may positively influence the process of learning. However, this is only speculative. Amygdalar reduction was likely connected with hippocampal reduction, thus larger destruction of whole AHC would be related to better verbal and global memory outcome. These results are in line with previously published studies based on neuropsychological, noninvasive and invasive neurophysiological data, and functional and morphologic imaging.20 Temporal lobe epilepsy (TLE) is increasingly recognized as a dysfunction of large-scale neuronal networks.21 These networks are asymmetrically distributed and organized in left and right TLE. Frequently, cognitive impairment affects contralateral temporal and frontal functions.22 Thus, TLE cannot be considered a well lateralized temporal dysfunction. In right TLE patients, a global decrease in left hemisphere functional activity was found.23 Furthermore, interictal epileptiform discharges and seizures may transiently worsen these deficits.24 Recently, fast-varying behavior of epileptic networks during interictal spikes in right and left TLE at a whole-brain scale using directed connectivity has been evaluated.25 The authors found a different network pattern between both conditions. The contralateral spread was much more prominent in right TLE compared to the left TLE. In line with this observation was the finding that half of the right TLE patients and none of the left TLE patients had neuropsychological deficits consistent with contralateral temporal lobe dysfunction. This observation suggests a relationship between connectivity changes and cognitive deficits. These observations may explain our results. We hypothesized that left temporal lobe, released from epileptic activity spreading from the right, could support memory more effectively after larger right-sided destruction.
The present study has several important limitations. Due to its retrospective design, the subgroups were not comparable as most SAHE patients underwent operations on the left side. Patients underwent only standard Wechsler memory tests and their emotional memory was not tested. Moreover, not all patients underwent the WADA test. Hand laterality does not prove hemispherical dominance and substitute for the WADA test.
Conclusion
Memory outcomes likely depend on the residual volume of mesial temporal structures. Larger left-sided hippocampal reduction was associated with lower verbal memory performance. This finding is expected, according to the memory-specific model. In right-sided correlations, we hypothesize that the left temporal lobe, released from epileptic activity spreading from the right, could support memory more effectively after larger right-sided destruction.
Acknowledgments
The work was supported by Ministry of Health, Czech Republic – conceptual development of research organization (Nemocnice Na Homolce – NNH, 00023884; IG 154301). The work of JL was supported by grant number RVO 68081740 of the Academy of Sciences of the Czech Republic. We are indebted to Aaron Rulseh MD, PhD, for help with English editing.
Disclosure
The authors report no conflicts of interest in this work.
References
de Tisi J, Bell GS, Peacock JL, et al. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet. 2011;378(9800):1388–1395. | ||
Lee TM, Yip JT, Jones-Gotman M. Memory deficits after resection from left or right anterior temporal lobe in humans: a meta-analytic review. Epilepsia. 2002;43(3):283–291. | ||
Scoville WB, Milner M. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11–21. | ||
Glosser G, Saykin AJ, Deutsch GK, O’Connor MJ, Sperling MR. Neural organization of material-specific memory functions in temporal lobe epilepsy patients as assessed by the intracarotid amobarbital test. Neuropsychology. 1995;9(4):449–456. | ||
Bonelli SB, Thompson PJ, Yogarajah M, et al. Memory reorganization following anterior temporal lobe resection: a longitudinal functional MRI study. Brain. 2013;136(Pt 6):1889–1900. | ||
Vaz SA. Nonverbal memory functioning following right anterior temporal lobectomy: a meta-analytic review. Seizure. 2004;13(7):446–452. | ||
Niemeyer P. The transventricular amygdalohippocampectomy in temporal lobe epilepsy. In: Baldwin M, Bailey P, editors. Temporal lobe epilepsy. Springfield: Charles C Thomas; 1958:461–482. | ||
Liscak R, Malikova H, Kalina M, et al. Stereotactic Radiofrequency Amygdalohippocampectomy in the Treatment of Mesial Temporal Lobe Epilepsy. Acta Neurochir (Wien). 2010;152(8):1291–1298. | ||
Vojtěch Z, Malíková H, Krámská L, Liščák R, Vladyka V. MRI-guided stereotactic amygdalohippocampectomy: a single center experience. Neuropsychiatr Dis Treat. 2015;11:359–374. | ||
Malikova H, Kramska L, Vojtech Z, et al. Different surgical approaches for mesial temporal epilepsy: resection extent, seizure and neuropsychological outcomes. Stereotact Funct Neurosurg. 2014;92(6):372–380. | ||
Wechsler D. Manual for the Wechsler memory scale revised. San Antonio: The Psychological Corporation; 1987. | ||
Matejcek Z, Zlab Z. Test manual: Lateral dominance examination. Brno a Bratislava: Psychodiagnostika; 1972. | ||
Cook MJ, Fish DR, Shorvon SD, Straughan K, Stevens JM. Hippocampal volumetric and morphometric study in frontal and temporal lobe epilepsy. Brain. 1992;115(Pt 4):1001–1115. | ||
Watson C, Jack CR, Cendes F. Volumetric magnetic resonance imaging. Clinical applications and contributions to the understanding of temporal lobe epilepsy. Arch Neurol. 1997;54(12):1521–1531. | ||
R Development Core Team. A language and environment for statistical computing. Vienna: Foundation for Statistical Computing; 2013. Available from: http://www.rproject.org. Accessed October 21, 2015. | ||
Alpherts WC, Vermeulen J, van Rijen PC, Da Silva FH, van Veelen CW; Dutch Collaborative Epilepsy Surgery Program. Standard versus tailored left temporal lobe resections: differences in cognitive outcome? Neuropsychologia. 2008;46(2):455–460. | ||
Schramm J. Temporal lobe epilepsy surgery and the quest for optimal extent of resection: a review. Epilepsia. 2008;49(8):1296–1307. | ||
Helmstaedter C, Roeske S, Kaaden S, Elger CE, Schramm J. Hippocampal resection length and memory outcome in selective epilepsy surgery. J Neurol Neurosurg Psychiatry. 2011;82(12):1375–1381. | ||
Aroniadou-Anderjaska V, Fritsch B, Qashu F, Braga MF. Pathology and pathophysiology of the amygdala in epileptogenesis and epilepsy. Epilepsy Res. 2008;78(2–3):102–116. | ||
Bettus G, Ranjeva JP, Wendling F, et al. Interictal functional connectivity of human epileptic networks assessed by intracerebral EEG and BOLD signal fluctuations. PLoS One. 2011;6(5):e20071. | ||
Richardson MP. Large scale brain models of epilepsy: dynamics meets connectomics. J Neurol Neurosurg Psychiatry. 2012;83(12):1238–1248. | ||
Stretton J, Thompson PJ. Frontal lobe function in temporal lobe epilepsy. Epilepsy Res. 2012;98(1):1–13. | ||
Dupont S, Samson Y, Van de Moortele PF, et al. Bilateral hemispheric alteration of memory processes in right medial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2002;73(5):478–485. | ||
Holmes GL, Lenck-Santini PP. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav. 2006;8(3):504–515. | ||
Coito A, Plomp G, Genetti M, et al. Dynamic directed interictal connectivity in left and right temporal lobe epilepsy. Epilepsia. 2015;56(2):207–217. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.