Back to Journals » Journal of Inflammation Research » Volume 15
Relationship Between Monocyte-to-Lymphocyte Ratio as Well as Other Leukocyte-Derived Ratios and Carotid Plaques in Patients with Coronary Heart Disease: A RCSCD-TCM Study
Authors Ma M, Liu Y, Wang L, Yang R, Li Z, Gao S, Li L, Yu C
Received 22 May 2022
Accepted for publication 26 August 2022
Published 7 September 2022 Volume 2022:15 Pages 5141—5156
DOI https://doi.org/10.2147/JIR.S375759
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Adam D Bachstetter
Mei Ma,1,* Yijia Liu,1,* Lichun Wang,2,* Rongrong Yang,1 Zhu Li,1 Sheng Gao,3 Lin Li,1 Chunquan Yu1
1Department of Graduate Schools, Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China; 2Department of Information Center, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China; 3Department of Endocrine Metabolic Diseases, Nankai Hospital, Tianjin Hospital of Integrated Traditional Chinese and Western Medicine, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lin Li, Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China, Email [email protected] Sheng Gao, Nankai Hospital, Tianjin Hospital of Integrated Traditional Chinese and Western Medicine, Tianjin, People’s Republic of China, Email [email protected]
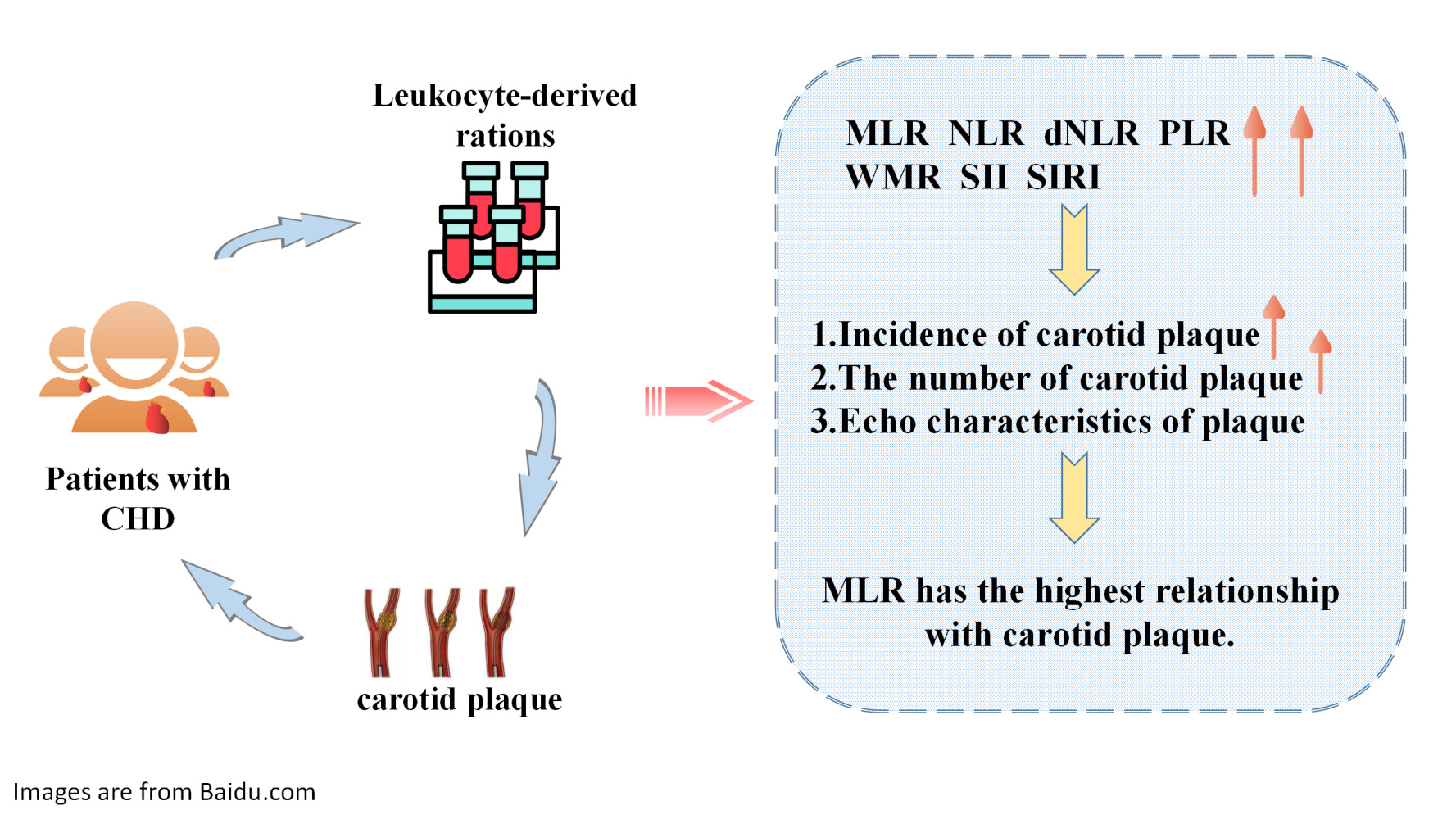
Purpose: This study explored the relationship between monocyte-to-lymphocyte ratio (MLR) as well as other leukocyte-derived ratios and carotid plaques in patients with coronary heart disease (CHD).
Patients and Methods: A total of 12,093 patients with CHD were selected as research participants. Leukocyte-derived ratios assessed in this study included neutrophil-to-lymphocyte ratio (NLR), derived NLR (dNLR), MLR, platelet-to-lymphocyte ratio (PLR), white blood cell-to-mean platelet volume ratio (WMR), lymphocyte×neutrophil/104 ratio (MNM), systemic immune inflammation index (SII), and systemic inflammation response index (SIRI). Leukocyte-derived ratios were divided into four groups according to quarters. Logistic regression analysis was performed to evaluate the relationship between leukocyte-derived ratios and the incidence, number, and echo characteristics of carotid plaques in patients with CHD. Further analysis was performed after adjusting for confounding factors.
Results: Among the 12,093 participants, 71.7% had carotid plaques. After adjusting for confounding factors, MLR, NLR, dNLR, PLR, SII, SIRI, and WMR were found to be associated with carotid plaque formation. Among them, MLR had the strongest association with the incidence of carotid plaques (odd ratio[OR]:1.889; 95% confidence interval[CI]:1.406– 2.539) and hyperechoic plaques (OR:2.024; 95% CI:1.481– 2.767). When MLR was viewed as a categorical variable, the risk of carotid plaque formation in Q4 was 1.4 times higher than that in Q1. The relationship between MLR and carotid plaques in females (OR:2.250; 95% CI:1.458– 3.473) was stronger than that in males (OR: 1.638; 95% CI:1.102–-2.436). The relationship between MLR and carotid plaques in patients younger than 65 years (OR:3.597; 95% CI:2.379– 5.439) was stronger than that in those older than 65 years (OR:1.577; 95% CI:1.046– 2.378).
Conclusion: Leukocyte-derived ratios were related to the incidence, number, and echo characteristics of carotid plaques. In particular, MLR, an inflammatory biomarker that encompasses innate and adaptive immunity, may be of great value in revealing the incidence and echo characteristics of plaques.
Keywords: coronary heart disease, leukocyte-derived ratios, monocyte-to-lymphocyte ratio, carotid plaque
Graphical Abstract:
Introduction
The occurrence and development of atherosclerosis (AS) is a process of chronic inflammation and lipid accumulation, which is the primary cause of most cardiovascular diseases (CVD).1–4 CVD has become a significant public health problem that seriously threatens the health of residents and social development.5 In the past few decades, many studies have found that the occurrence and development of AS is often accompanied by inflammatory cells, such as macrophages and T cells. Monocytes (MO) and lymphocytes (LYM) in the leukocyte subgroup are key cells involved in the process of inflammation. A disorder of the inflammatory reaction is the driving factor for the development and instability of AS.6 In 2017, the Canakinumab Anti-inflammatory Thrombosis Outcomes Study confirmed the role of inflammation in AS, and showed that inhibiting inflammatory cytokines can significantly reduce the risk of AS.7,8 Coronary AS causes coronary heart disease (CHD) and is accompanied by carotid artery stenosis.9–11 In recent years, several leukocyte-derived ratios, including neutrophil (NE)-to-LYM ratio (NLR), derived NLR (dNLR), MO-to-LYM ratio (MLR), platelet (PLT)-to-LYM ratio (PLR), white blood cell (WBC)-to-mean PLT volume ratio (WMR), LYM × NE/104 ratio (MNM), systemic immune inflammation index (SII), and system inflammation response index (SIRI) have attracted the attention of scholars.12 Hematological indices NLR and MLR can be regarded as significant predictors of all-cause long-term mortality after Off-Pump Coronary Artery Bypass Grafting (OPCAB) revascularization, which may be applied into clinical practice for meticulous postoperative monitoring of patients in higher risk of worse prognosis.13 Leukocyte-derived ratios, which integrate information from the innate and adaptive immunity to avoid relying solely on the absolute value of a single leukocyte subtype caused by infection or dehydration, can be easily obtained and have a certain predictive value for disease outcomes.14 The changes in leukocytes around the blood vessels were reported to be significantly related to inflammatory activity in the plaques. Leukocytes are involved in plaque formation and instability, which induce acute thrombotic events.15 Other studies have shown that innate immune markers are related to the thickness and extent of plaque stenosis.16–18 Using inflammatory biomarkers to identify and predict coronary plaque stability and the occurrence and prognosis of cardiovascular events has become a popular research topic in recent years. However, the relationship between leukocyte-derived ratios and carotid plaques has rarely been studied. Therefore, this study aims to clarify the relationship between leukocyte-derived ratios and the incidence and characteristics of carotid plaques in patients with CHD. This can help inform the prevention and risk stratification of carotid plaques in patients with CHD by using simple biomarkers.
Patients and Methods
Study Participants
A total of 107,301 patients with CHD in six tertiary hospitals in Tianjin, China, were selected as research participants from September 1, 2014, to September 1, 2020. Patients with CHD were identified with at least one or more of the International Classification of Disease codes obtained from the hospital’s diagnosis and treatment system. We excluded patients aged less than 35 years old or older than 75 years old. We also excluded patients with severe liver or kidney failure, malignant tumor, cancer, blood system diseases, severe active infections, and acute coronary syndrome. Patients who had missing leukocyte-derived ratio data or carotid ultrasound measurements were not included in this study. Finally, a total of 12,093 participants were enrolled in the study. A flow chart of patient screening is shown in Figure 1. This study was approved by the ethics committee of Tianjin University of Traditional Chinese Medicine (TJUTCM-EC20190008) and registered in the Chinese Clinical Trial Registry (ChiCTR-1900024535) and ClinicalTrials.gov (NCT04026724).
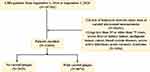 |
Figure 1 The flow chart of screening patients. |
Data Collection
We collected the clinical data of the patients from the hospital’s diagnosis and treatment system, which included the demographics of the patients, such as age, sex, ethnicity, blood pressure, medical history, and smoking and drinking history. Basic demographic data were collected and recorded by professional doctors of the hospital through standard structured questionnaires 4 h after admission. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in the right arm artery with a standard electronic sphygmomanometer after the patient was rested in the supine position for 5 min.
Fasting venous blood samples were collected in the morning from the research center. WBC, monocyte (MO), NE, LYM, and PLT counts were measured using an automatic hematology analyzer. Fasting blood glucose (FBG), glycosylated hemoglobin A1c (HbA1c), total cholesterol (TC), triglyceride (TG), high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C) levels were measured using an automatic biochemical analyzer.19 Standard laboratory procedures for quality control were strictly followed.9–11,13 Leukocyte-derived ratios were defined as follows: NLR= NE/LYM, dNLR= NE /[WBC-NE], MLR= MO/LYM, PLR= PLT/LYM, WMR= leukocyte/mean PLT volume, MNM= LYM × NE/104, SIRI= NE × MO/LYM, and SII = PLT/NE/LYM.
Hospital professionals also measured the color Doppler ultrasound of the carotid artery after the patient was rested in a supine position for approximately 15 min. Bilateral common carotid arteries, bifurcation, and the internal and external carotid arteries were scanned along the direction of the blood vessels to determine the intima-media thickness (IMT).20 The plaque was defined as an IMT that “measures ≥1.5 mm in any segment of the carotid artery.” Carotid plaques were divided into single (n=1) and multiple (n≥2) groups. Based on the morphological and acoustic characteristics of the plaques, they were categorized as hypoechoic, isoechoic, hyperechoic, and mixed echo plaques. Isoechoic and hyperechoic plaques were classified as stable plaques, whereas hypoechoic and mixed echo plaques were classified as vulnerable plaques. Professional doctors recorded the number and characteristics of plaques using strict quality-control procedures. Certified experimenters evaluated the inter-laboratory quality to maintain the consistency of monitoring and testing of image collection and analysis.
Statistical Analysis
IBM SPSS Statistics 25.0 (IBM Corp, New York, NY) was used for statistical analysis.
Values were presented as a percentage (%) for categorical variables and as median and interquartile range (IQR) for continuous variables. The χ2 test and Kruskal–Wallis H-test were used to compare the baseline characteristics among the four groups. Four logistic regression models were constructed: Model a, unadjusted; Model b, adjusted for age and sex; Model c, adjusted for age, sex, SBP, DBP, FBG, HbA1c, smoking, and drinking; and Model d was adjusted based on Model c with TC, TG, HDL-C, LDL-C, and the use of antilipidemic medication and antihypertensive medication. The linear relationship between independent variables was evaluated to ensure that it is appropriate to include them in the same model. Missing values were calculated using the chain equation, and five complete datasets were created. P<0.05 was considered statistically significant.
Result
Baseline Characteristics
The participants’ characteristics are listed in Table 1. Among the 12,093 participants, 48.3% were male, with an average age of 64 (59–69 years), and 8,674 (71.7%) had carotid plaques. Based on the MLR quartering method, the participants were divided into four groups: Q1 (MLR ≤ 0.20), Q2 (0.20< MLR≤ 0.26), Q3 (0.26< MLR< 0.35), and Q4 (MLR ≥ 0.35). There were differences in the incidence, number, and echo characteristics of carotid plaques among the four MLR groups (P<0.001). Compared with the Q1 group, the number of patients with carotid plaques in the Q4 group was higher. They were more likely to be male, older, and had higher levels of FBG, HbA1c, WBC, NE, MO, NLR, dNLR, MLR, PLR, SII, SIRI, and WMR and lower levels of TG, TC, LDL-C, LDL-C, LYM, PLT, and MNM.
 |
Table 1 General Characteristics of Research Participants |
Relationship Between Leukocyte-Derived Ratios and the Incidence of Carotid Plaques
The relationship between univariate analysis and the risk of carotid plaques formation showed that sex, age, SBP, DBP, FBG, HbA1c, TC, HDL-C, LDL-C, smoking history, drinking history, and use of antihypertensive and antilipidemic medications were associated with the incidence of carotid plaques (Table S1). Except for MNM, all leukocyte-derived ratios were related to the incidence of carotid plaques, while MLR had the strongest relationship with the incidence of carotid plaque formation (Table 2). After further adjustment for confounding factors, the results remained consistent (Figure 2). In the unadjusted model, MLR was significantly associated with carotid plaque incidence (OR: 6.206; 95% CI: 4.621–8.334). After adjusting for age, sex, SBP, DBP, smoking, drinking, FBG, HbA1c, TC, TG, HDL-C, LDL, and use of antilipidemic and antihypertensive medication, the incidence of carotid plaque increased to 88.9% (OR: 1.889; 95% CI: 1.406–2.539). When MLR was viewed as a categorical variable (quartile), the risk of carotid plaque formation in the Q4 group was 1.4 times higher than that in the Q1 group (Figure 2). In further analysis, in the unadjusted or adjusted model, the Ptrend of MLR and carotid plaques was consistent with the results of MLR as a continuous variable (P<0.001).
 |
Table 2 Relationship Between Leukocyte-Derived Ratios and Carotid Plaques |
 |
Figure 2 Relationship between MLR as well as other leukocyte-derived ratios and carotid plaques. *P < 0.05, **P < 0.01. |
Relationship Between Leukocyte-Derived Ratios and the Number and Echo Characteristics of Carotid Plaques
Except for MNM, all leukocyte-derived ratios were related to the number of carotid plaques; MLR was the biggest risk factor for multiple carotid plaques found in patients with CHD (Tables S2 and S3), and all leukocyte-derived ratios were related to the echo characteristics of the carotid plaques (Table S4). After further adjustment for confounding factors (Table 3), MLR had the strongest relationship with hyperechoic plaques (OR: 2.024; 95% CI: 1.481–2.767). When MLR was viewed as a categorical variable (quartile), the Q4 group was 1.516 times higher than the Q1 group (Figure 3). Further analysis showed that the Ptrend of MLR and carotid plaques was consistent with the results of MLR as a continuous variable (P<0.001).
 |
Table 3 Relationship Between MLR and Echo Characteristics of Carotid Plaques |
 |
Figure 3 Relationship between MLR as well as other leukocyte-derived ratios and the echo characteristics of carotid plaque. **P < 0.01. |
Relationship Between MLR and Carotid Plaques Based on Sex and Age
We also observed a significant relationship between MLR and carotid plaques in both sex (Table 4) and age (Table 5). The relationship between MLR and carotid plaques in females (OR: 2.250; 95% CI: 1.458–3.473) was stronger than that in males (OR:1.638; 95% CI: 1.102–2.436). The relationship between MLR and carotid plaques in patients younger than 65 years (OR: 3.597; 95% CI: 2.379–5.439) was stronger than that in those older than 65 years (OR: 1.577; 95% CI: 1.046–2.378). There is no interaction between sex and MLR, but there is a multiplication interaction between age and MLR (Table S5).
 |
Table 4 Relationship Between MLR and the Risk of Carotid Plaques in Different Sex |
 |
Table 5 Relationship Between MLR and the Risk of Carotid Plaques in Different Age |
Discussion
Our study found that leukocyte-derived ratios, including NLR, dNLR, MLR, PLR, WMR, SII, and SIRI, were significantly related to carotid plaque formation, especially MLR. In particular, when MLR was ≥0.35, patients with CHD were more likely to have carotid plaques.21 Studies have confirmed that NLR is related to the incidence and vulnerability of carotid plaques detected using carotid ultrasound in patients with acute ischemic stroke. However, all the participants in our study were patients with CHD, and the relationship between MLR and carotid plaque was stronger than that between MLR and NLR.22–24 More recently, some clinical studies showed that MLR was related to CHD severity; MLR > 0.3 has predictive values for colleterial carotid stenosis and may be used as an easily accessible indicator for AS severity. These findings were consistent with the results of the present study.
MLR is an inflammatory marker that factors in LYM and MO counts. It reflects the state of systemic inflammation and represents the degree of immune response activation in vivo.24,25 Low-level inflammatory reactions attract innate and adaptive immune cells into the atherosclerotic plaques. The decrease in absolute LYM count and increase in absolute MO count lead to increased MLR, resulting in an imbalance in the innate and adaptive immunity. This imbalance may be the main cause of arterial plaque formation.26 There is evidence that increased MO levels are a risk factor for coronary artery plaque formation and cardiovascular death, and they are closely related to the pathogenesis of thrombus-related organ infarction. A lower LYM count increases the risk of cardiovascular events and mortality. The relevant mechanism may be as follows:27 MO can accumulate, adhere, and differentiate into inflammatory dendritic cells, macrophages, and foam cells under chemotaxis, and then activate proinflammatory cytokine secretion, matrix metalloproteinases, and reactive oxidation substances, which play a key role in the formation, development, and rupture of AS plaques.24,28 LYM can regulate the phenotype of MO; induce the expression of tissue inhibitors of metalloproteinases; and inhibit the growth, rupture, and thrombosis of atherosclerotic plaques, which are protective factors against AS. MLR combines the increase in MO risk factors and the decrease in LYM protective factors, which has a double risk effect on carotid plaque formation.
Notably, this study found that MLR had the highest correlation with hyperechoic plaques formation.29,30 The hypoechoic plaque contains relatively more lipid components and more inflammatory substances, and the plaque ruptures easily and is more unstable, whereas hyperechoic plaques have the opposite characteristics. Fortunately, higher relationship between high MLR and the more stable plaque characteristics, although patients with elevated MLR levels were at a higher risk for carotid plaque development.31,32 Evidence suggests that plaque calcification reflects the active stage of inflammation-related AS and that the degree of calcification is closely related to macrophage infiltration. Sheet calcification is highly prevalent in stable plaques, whereas microcalcifications, punctate, and fragmented calcifications are more frequent in unstable lesions. We speculate that the effect of MLR on plaque homeostasis depends not only on the calcification characteristics of macrophages but also on the phenotype of macrophages.33,34 MO are recruited to the site of ongoing inflammation and differentiate into macrophages of different phenotypes. Both M1 and M2 macrophages contribute to plaque establishment; M1 macrophages are associated with unstable plaques, while M2 macrophages have anti-inflammatory and fibrogenic properties and are particularly abundant in stable zones of the plaque.
The incidence and complications of AS differ between sexes.35 Experiments conducted in animals have shown that male animals appear to have more inflamed yet smaller plaques than that in female animals.36 Recent research shows that NLR is closely related to the presence and severity of coronary artery disease in men but not in women. However, our research found that the relationship between MLR and carotid plaques in women was stronger than that in men.37 This may be related to the hormone levels in females. We also found that the relationship between MLR and carotid plaques in patients younger than 65 years was stronger than that in patients older than 65 years. However, this may be due to the inclusion of more patients that were younger than 65 years of age in this study, thus, creating a bias toward age. Therefore, future research should include individuals of different ages to determine the association between MLR and carotid plaques according to age.
This study was a cross-sectional study in a large-scale, multi-center retrospective study encompassing a large amount of data. We established several models of confounding factors and adjusted them, and the results were convincing. However, several limitations should be considered when interpreting the results of this study. First, this was a multicenter study; thus, there may be bias in the measurement methods at different research centers. However, practitioners conducted external quality assessments between the clinical laboratories in each center. Second, due to the missing data on body mass index (BMI) and the medicine records affecting white blood cell count in this study; thus, the results may be biased. Additionally, this was a cross-sectional study. Without considering time as a factor, it was difficult to draw a causal conclusion; hence, this relationship needs to be verified in future prospective studies. Nevertheless, to our knowledge, the current study is novel in that it comprehensively evaluated the relationship between leukocyte-derived ratios and the occurrence and characteristics of carotid plaques in patients with CHD. From a clinical perspective, our research allows us to reconsider the value of leukocyte-derived ratios, particularly MLR, as simple biomarkers for diseases.
Conclusion
In brief, this study provided a reference value for the prevention and risk stratification of carotid plaques in patients with CHD by studying the relationship between leukocyte-derived ratios and the incidence, number, and echo characteristics of carotid artery plaques. In particular, as an inflammatory biomarker that encompasses both the innate and adaptive immunity, MLR may be more valuable than other leukocyte-derived ratios for revealing the occurrence and echo characteristics of plaques.
Abbreviations
RCSCD-TCM, Retrospective Cohort Study on Adjuvant Treatment of Coronary Heart Disease Angina Pectoris With Chinese Patent Medicine; CHD, coronary heart disease; AS, atherosclerosis; CVD, cardiovascular diseases; OPCAB, Off-Pump Coronary Artery Bypass Grafting; IMT, intima-media thickness; NLR, Neutrophil-to-Lymphocyte ratio; dNLR, derived Neutrophil-to-Lymphocyte ratio; MLR, Monocyte-to-Lymphocyte ratio; PLR, Platelet-to-Lymphocyte ratio; WMR, White blood cell-to-Mean platelet volume ratio; MNM, Lymphocyte×Neutrophil/104 ratio; SII, systemic immune inflammation index; SIRI, system inflammation response index; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; FBG, Fasting blood glucose; HbA1c, Glycosylated hemoglobin A1c; TC, Total cholesterol; TG, Triglycerides; HDL-C, High-density lipoprotein cholesterol; LDL-C, Low-density lipoprotein cholesterol; WBC, white blood cell; NE, Neutrophils; MO, monocyte; LYM, Lymphocyte; PLT, Platelet; BMI, body mass index; IQR, Interquartile range; OR, odds ratio; CI, confidence interval; β, regression coefficient.
Data Sharing Statement
The data supporting the research results can be obtained from the corresponding author.
Ethics Approval and Informed Consent
All research data were obtained from the hospital’s medical record system, and informed consent was not obtained. To protect patients’ privacy, the information included in our research was anonymous, and all traceable personal identifiers were deleted from the analysis dataset; hence, the need for patients’ informed consent was waived. All researchers involved in this study have the responsibility to protect patient data. All research data of this study should be kept strictly confidential, and the leaker should bear any consequences caused by leakage. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Tianjin University of Traditional Chinese Medicine (TJUTCM-EC2019008). It has been registered in the Clinical Trials.gov (registration number: NCT04026724), and the Chinese Clinical Trial Registration Centre (registration number: ChiCTR1900024535).
Acknowledgments
We would like to thank six hospitals in Tianjin that provided data from the First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Second Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin Chest Hospital, Tianjin Nankai Hospital, Tianjin Medical University General Hospital, and Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital. Mei Ma, Yijia Liu, and Lichun Wang are co-first authors for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Basic Research Program of China [973 project, grant number: 2014CB542902].
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this article.
References
1. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–e639. doi:10.1161/CIR.0000000000001052
2. Chan YH, Ramji DP. A perspective on targeting inflammation and cytokine actions in atherosclerosis. Future Med Chem. 2020;12(7):613–626. doi:10.4155/fmc-2019-0301
3. Song P, Fang Z, Wang H, et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Health. 2020;8(5):e721–e729. doi:10.1016/S2214-109X(20)30117-0
4. Xing DM, Zhu MJ, Liu CX, Wang H. Outcome measures in clinical trials of traditional Chinese medicine for stable angina pectoris. Acupunct Herb Med. 2021;1(2):99–106. doi:10.1097/HM9.0000000000000014
5. Lin P, Ji HH, Li YJ, Guo SD. Macrophage plasticity and atherosclerosis therapy. Front Mol Biosci. 2021;8:679797. doi:10.3389/fmolb.2021.679797
6. Ortega-Paz L, Capodanno D, Angiolillo DJ. Canakinumab for secondary prevention of coronary artery disease. Future Cardiol. 2021;17(3):427–442. doi:10.2217/fca-2020-0211
7. Shah PK. Inflammation, infection and atherosclerosis. Trends Cardiovasc Med. 2019;29(8):468–472. doi:10.1016/j.tcm.2019.01.004
8. Tuñón J, Bäck M, Badimón L, et al. Interplay between hypercholesterolaemia and inflammation in atherosclerosis: translating experimental targets into clinical practice. Eur J Prev Cardiol. 2018;25(9):948–955. doi:10.1177/2047487318773384
9. Manoochehri H, Gheitasi R, Pourjafar M, Amini R, Yazdi A. Investigating the relationship between the severity of coronary artery disease and inflammatory factors of MHR, PHR, NHR, and IL-25. Med J Islam Repub Iran. 2021;35:85. doi:10.47176/mjiri.35.85
10. Adamstein NH, MacFadyen JG, Rose LM, et al. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. 2021;42(9):896–903. doi:10.1093/eurheartj/ehaa1034
11. Klisic A, Radoman Vujačić I, Vučković LJ, Ninic A. Total leukocyte count, leukocyte subsets and their indexes in relation to cardiovascular risk in adolescent population. Eur Rev Med Pharmacol Sci. 2021;25(7):3038–3044. doi:10.26355/eurrev_202104_25557
12. Urbanowicz T, Olasińska-Wiśniewska A, Michalak M, et al. The prognostic significance of Neutrophil to Lymphocyte Ratio (NLR), Monocyte to Lymphocyte Ratio (MLR) and Platelet to Lymphocyte Ratio (PLR) on long-term survival in Off-Pump Coronary Artery Bypass Grafting (OPCAB) procedures. Biology. 2021;11(1):34. doi:10.3390/biology11010034
13. Lombardi G, Paganelli R, Abate M, et al. Leukocyte-derived ratios are associated with late-life any type dementia: a cross-sectional analysis of the Mugello study. Geroscience. 2021;43(6):2785–2793. doi:10.1007/s11357-021-00474-3
14. Luo X, Li W, Bai Y, Du L, Wu R, Li Z. Relation between carotid vulnerable plaques and peripheral leukocyte: a case-control study of comparison utilizing multi-parametric contrast-enhanced ultrasound. BMC Med Imaging. 2019;19(1):74. doi:10.1186/s12880-019-0374-9
15. Fani L, van Dam-Nolen DHK, Vernooij M, Kavousi M, van der Lugt A, Bos D. Circulatory markers of immunity and carotid atherosclerotic plaque. Atherosclerosis. 2021;325:69–74. doi:10.1016/j.atherosclerosis.2021.03.040
16. Zhang TY, Zhao Q, Liu ZS, Zhang CY, Yang J, Meng K. Relationship between monocyte/lymphocyte ratio and non-culprit plaque vulnerability in patients with acute coronary syndrome: an optical coherence tomography study. Medicine. 2020;99(41):e21562. doi:10.1097/MD.0000000000021562
17. Corriere T, Di marca S, Cataudella E, et al. Neutrophil-to-Lymphocyte Ratio is a strong predictor of atherosclerotic carotid plaques in older adults. Nutr Metab Cardiovasc Dis. 2018;28(1):23–27. doi:10.1016/j.numecd.2017.10.022
18. Zhang N, Tse G, Liu T. Neutrophil-lymphocyte ratio in the immune checkpoint inhibitors-related atherosclerosis. Eur Heart J. 2021;42(22):2215. doi:10.1093/eurheartj/ehab158
19. Ma M, Liu Y, Liu F, Li Z, Cheng Q, Liu Z, et al. Relationship Between Prognostic Nutrition Index and New York Heart Association Classification in Patients with Coronary Heart Disease: A RCSCD-TCM Study. J Inflamm Res. 2022;15(July):4303–4314. doi: 10.2147/JIR.S371045
20. Addis DR, Townsley MM. Implications of carotid arterial plaque assessment by ultrasound for the cardiothoracic anesthesiologist: an overview of the 2020 American society of echocardiography recommendations for the assessment of carotid arterial plaque by ultrasound for the characterization of atherosclerosis and evaluation of cardiovascular risk. J Cardiothorac Vasc Anesth. 2021;35(4):987–990. doi:10.1053/j.jvca.2020.12.006
21. Li X, Wu J. Relationship of Neutrophil-to-Lymphocyte ratio with carotid plaque vulnerability and occurrence of vulnerable carotid plaque in patients with acute ischemic stroke. Biomed Res Int. 2021;2021:6894623. doi:10.1155/2021/6894623
22. Kose N, Akin F, Yildirim T, Ergun G, Altun I. The association between the lymphocyte-to-monocyte ratio and coronary artery disease severity in patients with stable coronary artery disease. Eur Rev Med Pharmacol Sci. 2019;23(6):2570–2575. doi:10.26355/eurrev_201903_17406
23. Urbanowicz T, Michalak M, Olasińska-Wiśniewska A, et al. Monocyte/lymphocyte ratio and MCHC as predictors of collateral carotid artery disease-preliminary report. J Pers Med. 2021;11(12):1266. doi:10.3390/jpm11121266
24. Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–327. doi:10.1161/CIRCRESAHA.118.313591
25. Marcuzzi A, Melloni E, Zauli G, et al. Autoinflammatory diseases and cytokine storms-imbalances of innate and adaptative immunity. Int J Mol Sci. 2021;22(20):11241. doi:10.3390/ijms222011241
26. Zeynalova S, Bucksch K, Scholz M, et al. Monocyte subtype counts are associated with 10-year cardiovascular disease risk as determined by the Framingham Risk Score among subjects of the LIFE-Adult study. PLoS One. 2021;16(3):e0247480. doi:10.1371/journal.pone.0247480
27. Shah AD, Denaxas S, Nicholas O, Hingorani AD, Hemingway H. Neutrophil counts and initial presentation of 12 cardiovascular diseases: a CALIBER Cohort Study [published correction appears in. J Am Coll Cardiol. 2017;69(9):1160–1169. doi:10.1016/j.jacc.2016.12.022
28. Kounis NG, Koniari I, Plotas P, et al. Inflammation, thrombosis, and platelet-to-lymphocyte ratio in acute coronary syndromes. Angiology. 2021;72(1):6–8. doi:10.1177/0003319720946213
29. Johri AM, Nambi V, Naqvi TZ, et al. Recommendations for the assessment of carotid arterial plaque by ultrasound for the characterization of atherosclerosis and evaluation of cardiovascular risk: from the American society of echocardiography. J Am Soc Echocardiogr. 2020;33(8):917–933. doi:10.1016/j.echo.2020.04.021
30. Fedak A, Chrzan R, Chukwu O, Urbanik A. Ultrasound methods of imaging atherosclerotic plaque in carotid arteries: examinations using contrast agents. J Ultrason. 2020;20(82):e191–e200. doi:10.15557/JoU.2020.0032
31. Shi X, Gao J, Lv Q, et al. Calcification in atherosclerotic plaque vulnerability: friend or foe? Front Physiol. 2020;11:56. doi:10.3389/fphys.2020.00056
32. Jinnouchi H, Sato Y, Sakamoto A, et al. Calcium deposition within coronary atherosclerotic lesion: implications for plaque stability. Atherosclerosis. 2020;306:85–95. doi:10.1016/j.atherosclerosis.2020.05.017
33. Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. 2015;12(1):10–17. doi:10.1038/nrcardio.2014.173
34. Farahi L, Sinha SK, Lusis AJ. Roles of macrophages in atherogenesis. Front Pharmacol. 2021;12:785220. doi:10.3389/fphar.2021.785220
35. Man JJ, Beckman JA, Jaffe IZ. Sex as a biological variable in atherosclerosis. Circ Res. 2020;126(9):1297–1319. doi:10.1161/CIRCRESAHA.120.315930
36. Pan Q, Zhang W, Li X, et al. Sex difference in the association between neutrophil to lymphocyte ratio and severity of coronary artery disease. Angiology. 2022;73(5):470–477. doi:10.1177/00033197211070884
37. Cortés YI, Barinas-Mitchell E, Suder Egnot N, et al. Associations of endogenous sex hormones with carotid plaque burden and characteristics in midlife women. J Clin Endocrinol Metab. 2020;105(4):1126–1136. doi:10.1210/clinem/dgz327
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
