Back to Journals » International Journal of General Medicine » Volume 16
Relationship Between Diabetic Chorea and Timing of MRI Findings: A Systematic Review with Case Reports
Authors Otaka Y , Harada Y , Sugawara N , Shimizu T, Yasui-Furukori N
Received 7 June 2023
Accepted for publication 19 September 2023
Published 2 October 2023 Volume 2023:16 Pages 4465—4476
DOI https://doi.org/10.2147/IJGM.S423400
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Luca Testarelli
Yumi Otaka,1 Yukinori Harada,1 Norio Sugawara,2 Taro Shimizu,1 Norio Yasui-Furukori2
1Department of Diagnostic and Generalist Medicine, Dokkyo Medical University, School of Medicine, Tochigi, Japan; 2Department of Psychiatry, Dokkyo Medical University, School of Medicine, Tochigi, Japan
Correspondence: Norio Yasui-Furukori, Department of Psychiatry, Dokkyo Medical University, School of Medicine, Mibu, Shimotsuga, Tochigi, 321-0293, Japan, Tel +81-282-86-1111, Fax +81-282-86-5187, Email [email protected]
Background: Diabetic chorea is a rare complication of diabetes mellitus for which head MRI is the most common diagnostic imaging modality. Cases have been reported where CT and/or MRI findings are inconsistent or clinical symptoms and imaging findings do not appear simultaneously. We aimed to compile the cases in which imaging findings appeared on MRI retests and to examine in a systematic review whether temporal differences in the appearance of imaging findings correlate with clinical characteristics.
Case Presentation: An 80-year-old man with type 2 diabetes mellitus came to a hospital with abnormal movements of the left upper and lower extremities. Two days after the first visit, his symptoms flared up, and his head MRI showed an old cerebral infarction and no new lesion. On day 14, he retested T1-weighted imaging and showed a high signal in the right putamen, which was considered diabetic chorea. Blood glucose was controlled with insulin, and the involuntary movements disappeared.
Methods: PubMed and ICHUSHI were searched to identify patients with diabetic chorea who had undergone MRI retests. Patients grouped by the temporal change in the presence/absence of imaging findings were compared on age, sex, duration of diabetes mellitus, blood glucose level, HbA1c level, side of involuntary movement, time to first MRI, and follow-up MRI.
Results: Of the 64 cases analyzed, 43 (67.2%) were female. The mean age was 69.0 years. 16 (25.0%) had worsening findings upon MRI retesting, 37 (57.8%) had improvement, and 10 (15.6%) had unchanged findings. There were no significant differences in age, sex, mean blood glucose level or HbA1c at onset among the groups.
Conclusion: There was no association between the pattern of appearance of imaging findings over time and clinical characteristics, including glucose levels. If initial MRI findings are negative, MRI retesting after a certain time may help diagnose diabetic chorea.
Keywords: hyperglycemia, hemichorea, hemiballismus, diabetes, movement disorders
Background
Nonketotic hyperglycemia-induced chorea was first described in the 1960s,1 and diabetic chorea is a disease that causes chorea and ballism associated with hyperglycemia. Although a rare complication of diabetes, nonketotic hyperglycemia is the most common metabolic cause of hemichorea-hemiballismus.2,3 It is characterized by acute-onset chorea and hyperintense changes in the striatum on T1-weighted MRI. Its prevalence is 1 in 100,000,3 and it occurs more frequently in older women with type 2 diabetes mellitus. CT and MRI are the most common imaging modalities for detecting striatal abnormalities in diabetic chorea. Chua et al4 reported the sensitivity of CT and MRI to be 78.86% and 95.33%, respectively, with MRI being the more reliable test than CT. Furthermore, the discrepancy between CT and MRI findings was reported in 1/6 of cases, and in 1/10 of cases, clinical symptoms and imaging findings of diabetic chorea did not appear simultaneously.5 To our knowledge, few reports of MRI being repeated within a short time after negative MRI findings, with the retest showing typical findings. In addition, the characteristics of cases in which MRI is repeated and new imaging findings appear or the imaging findings worsen (increased brightness) are unknown.
Here, we report our experience with a case of worsening imaging findings, and we examine through a systematic review whether changes in the presence/absence of imaging findings over time are correlated with clinical characteristics.
Case Presentation
An 80-year-old man with independent activities of daily living, cerebral infarction, and type 2 diabetes mellitus came to a hospital with a chief complaint of abnormal movements of the left upper and lower extremities. Two days after the first visit, his symptoms flared up, and his head MRI showed only an old cerebral infarction and no new lesion (Figure 1a). On day 7, his movements, including jerking of the left upper and lower extremities, worsened, and on day 11, he started saying things that did not make sense. On day 14, he visited a hospital again and was hospitalized. Physical examination revealed ballism of the left upper and lower extremities, and blood tests showed a blood glucose level of 243 mg/dl, which fluctuated little, an HbA1c of 14.1%, and no evidence of acidosis. On the same day, we suspected stroke, encephalitis or chorea, and head MRI was repeated, in which T1-weighted imaging showed a high signal in the right putamen, which was considered diabetic chorea (Figure 1b). Blood glucose was controlled with insulin, and on the 5th day in the hospital, the blood glucose level dropped below 200 mg/dl at all times, and the involuntary movements disappeared.
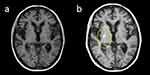 |
Figure 1 Magnetic resonance imaging (MRI) on the day of examination (a) and 14 days later (b). |
Methods
Literature Search Strategy and Selection Criteria
A systematic review was conducted based on PRISMA 2020 (Figure 2). Inclusion criteria were cases of diabetes mellitus combined with chorea and MRI, and exclusion criteria were those without MRI. A literature search on PubMed on 18 June 2022 was conducted by two authors (YO and NYF). The search formula was as follows: (hyperglycemia [TIAB] OR hyperglycemic [TIAB] OR glycemia [TIAB] OR diabetic [TIAB] OR diabetes [TIAB]) AND (chorea [TIAB] OR ballismus [TIAB] OR hemichorea [TIAB] OR hemiballismus [TIAB]). Since the handsearch resulted in many reports from Japan, a literature search was also conducted using ICHUCHI, a Japanese literature search engine that will be available until June 18, 2022. Because the above search formula was used, only abstracts written in English were retrieved.
 |
Figure 2 Study screening and selection flow chart. |
A total of 392 articles were returned, and the number of cases in the articles was 602. After screening by title and abstract, we first excluded 46 articles written in languages other than English, 55 articles not about diabetic chorea, and one article that was an update of a previous article by the same author. Then, we excluded 44 papers that did not describe the cases in detail, such as reviews and letters to editors. Reviewing all 456 remaining cases, 86 cases without any MRI and 291 cases without repeat MRI were excluded. Of the 79 cases now left, 9 had no T1-weighted MRI, which is needed to characterize diabetic chorea; 4 cases had unclear MRIs or findings other than diabetic chorea; and 2 cases had diabetic chorea but no findings on the initial MRI or the repeat MRI, leaving 64 cases in 56 papers (Table 1).6–60 We selected age, sex, duration of diabetes mellitus, blood glucose level, HbA1c level, side of involuntary movement, time to first MRI, time to follow-up MRI, plasma osmolality, hyperintense lesion of MRI (T1 weighted image), and course.
 |
Table 1 Basic Information of All Patients |
Statistical analysis was performed using ANOVA and the χ2 test with SPSS28 software, with p < 0.05 being considered significant.
Results
Of the 64 patients finally analyzed, 20 were male, 43 were female, and 1 was unspecified. The mean age was 69.0 years. First, we divided the 64 patients into 6 groups: (1) 1 case with no initial MRI findings but with retest MRI findings, (2) 15 cases with initial MRI findings and clearer findings on retest MRI (“clearer” means that the high intensity of T1-weighted imaging is more distinct), (3) 24 cases with initial MRI findings and weaker findings on retest MRI, (4) 10 cases with initial MRI findings and unchanged findings on retest MRI, (5) 13 cases with initial MRI findings and no findings on retest MRI and (6) 1 case with initial MRI findings and weaker findings on the left and clearer findings on the right on retest MRI. Afterward, the patients were consolidated into three groups: original groups (1) and (2), 16 patients made up a worsened group; original groups (3) and (5), 37 patients made up an improved group; and original group (4), 10 patients made up an unchanged group (Table 2). One case with a mismatch finding (6) was excluded from the analysis.
 |
Table 2 Charceristics of Patients Between Unchanged, Worsened and Improved Groups |
There were no differences in age or sex among the three groups. The mean age of the patients in the worsened group was 67 years, and the male-to-female ratio was 4:12. In the improved group, the mean age was 71 years, and the male-to-female ratio was 11:25; in the unchanged group, the mean age was 64 years, and the male-to-female ratio was 5:5. The mean blood glucose at onset was 453 mg/dL in the worsened group, 426 mg/dL in the improved group, and 443 mg/dL in the unchanged group. The mean HbA1c at onset in the worsened group was 14.5%, 12.9% in the improved group, and 12.7% in the unchanged group, all statistically similar (Table 2).
Discussion
In reviewing the 64 patients with original and repeat MR images, there were no significant differences in age, sex, blood glucose at onset, or HbA1c at onset between the groups with improved, unchanged, or worsened MRI findings. We found only one case reported in which the patient underwent another MRI after the initial negative MRI finding and the MRI finding was positive,59 as was the case with our patient. Thus, the case we encountered is the second such case reported. In that case, the MRI findings were negative 4 days after onset, and the patient underwent another MRI approximately 1 month later, which showed a high signal in the right basal ganglia on T1-weighted imaging. The difference is that in the current case, the time until the MRI retest was approximately 1 month, and the symptoms of the initial chorea improved from constant to intermittent, but the symptoms worsened again 1 month later, so another MRI was performed.
The review of case reports revealed that while there are cases in which MRI findings are positive even on the day of onset of involuntary movements, there are also cases in which later MRI findings are positive after initial MRI findings are negative, although such reports are rare even with our case added. These results suggest that imaging findings may not always correlate with the timing of disease, intensity of symptoms, blood glucose level, or HbA1c. In addition, 41 of the 602 patients with negative initial MRI findings were not followed up with a retest MRI. It could be that some of these patients would have had positive MRI findings if the MRI had been repeated after a certain time. Therefore, it is possible that true “MRI-negative” diabetic chorea is less common than reported. When “MRI-negative” cases are difficult to distinguish from other diseases that cause involuntary movements, MRI may aid in the diagnosis of diabetic chorea if it is repeated after a certain time.
The pathophysiology of striatal abnormalities in patients with diabetic chorea and the exact mechanism of chorea onset are not fully understood. Metabolic changes secondary to disruption of the blood‒brain barrier may be involved. Hyperglycemia induces an anaerobic metabolic pathway that uses inhibitory neurotransmitters such as acetylcholinesterase and gamma-aminobutyric acid (GABA) as alternative energy sources. As a result, GABA is depleted and acetylcholine synthesis is reduced, leading to dysfunction of the basal ganglia, which is thought to clinically manifest as involuntary movements.62,63
There are several limitations to this study. First, it is a compilation of case reports and not a prospective study, which may lead to publication bias. Second, some of the case reports did not have all the information we wanted, leading to the exclusion of many cases. Third, we searched only two databases (PubMed and ICHUCHI). Since multiple search engines are usually used, the possibility that we missed something cannot be ruled out. Finally, the clinical significance of this study is limited because the topic of the study is not predictive of outcomes. Because patients with diabetic chorea are extremely rare, a national registry project would need to be developed to clarify the details.
Conclusions
This study was a case-reported systematic review of the characteristics of diabetic chorea and the pattern of MRI findings. We attempted to find associations between these patterns and some demographic and clinical characteristics but were unable to do so with the information we collected. We also tried to find associations between the characteristics of imaging findings and the time from the onset of diabetic chorea to MR imaging and the duration of diabetic chorea, but we were unable to perform a sufficient analysis because few case reports clearly described the time to MR imaging or the duration of diabetic chorea.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
We obtained signed consent from the patient for personal use. The ethics committee of the School of Medicine at Dokkyo Medical University judged that there was no need to review this case.
Consent for Publication
We obtained signed consent from the patient for the personal or clinical details to be published in this study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
Dr Yukinori Harada reports personal fees from PRECISION, Inc., outside the submitted work. The authors declare that they have no other competing interests in this work.
References
1. Ifergane G, Masalha R, Herishanu YO. Transient hemichorea/hemiballismus associated with new onset hyperglycemia. Can J Neurol Sci. 2001;28(4):365–368. doi:10.1017/S0317167100001608
2. Wang W, Tang X, Feng H, et al. Clinical manifestation of nonketotic hyperglycemia chorea: a case report and literature review. Medicine. 2020;99(22):e19801. doi:10.1097/MD.0000000000019801
3. Bendi VS, Matta A, Torres-Russotto D, Shou J. Bilateral chorea/ballismus: detection and management of a rare complication of nonketotic hyperglycemia. BMJ Case Rep. 2018;2018:bcr2018224856. doi:10.1136/bcr-2018-224856
4. Ondo WG. Hyperglycemic nonketotic states and other metabolic imbalances. Handb Clin Neurol. 2011;100:287–291.
5. Chua CB, Sun CK, Hsu CW, Tai YC, Liang CY, Tsai IT. ”Diabetic striatopathy”: clinical presentations, controversy, pathogenesis, treatments, and outcomes. Sci Rep. 2020;10(1):1594. doi:10.1038/s41598-020-58555-w
6. Nagai C, Kato T, Katagiri T, Sasaki H. Hyperintense putamen on T1-weighted MR images in a case of chorea with hyperglycemia. AJNR Am J Neuroradiol. 1995;16(6):1243–1246.
7. Broderick JP, Hagen T, Brott T, Tomsick T. Hyperglycemia and hemorrhagic transformation of cerebral infarcts. Stroke. 1995;26(3):484–487. doi:10.1161/01.STR.26.3.484
8. Lai PH, Tien RD, Chang MH, et al. Chorea-ballismus with nonketotic hyperglycemia in primary diabetes mellitus. AJNR Am J Neuroradiol. 1996;17(6):1057–1064.
9. Shan DE, Ho DM, Chang C, Pan HC, Teng MM. Hemichorea-hemiballism: an explanation for MR signal changes. AJNR Am J Neuroradiol. 1998;19(5):863–870.
10. Iwata A, Koike F, Arasaki K, Tamaki M. Blood brain barrier destruction in hyperglycemic chorea in a patient with poorly controlled diabetes. J Neurol Sci. 1999;163(1):90–93. doi:10.1016/S0022-510X(98)00325-6
11. Free T, VanderPol A, Freeman JW. Case report: hemiballismus with unusual MRI findings. S D J Med. 1999;52(4):125–126.
12. Oerlemans WG, Moll LC. Nonketotic hyperglycemia in a young woman, presenting as hemiballism-hemichorea. Acta Neurol Scand. 1999;100(6):411–414. doi:10.1111/j.1600-0404.1999.tb01062.x
13. Ohara S, Nakagawa S, Tabata K, Hashimoto T. Hemiballism with hyperglycemia and striatal T1-MRI hyperintensity: an autopsy report. Mov Disord. 2001;16(3):521–525. doi:10.1002/mds.1110
14. Lin JJ, Lin GY, Shih C, Shen WC. Presentation of striatal hyperintensity on T1-weighted MRI in patients with hemiballism-hemichorea caused by nonketotic hyperglycemia: report of seven new cases and a review of literature. J Neurol. 2001;248(9):750–755. doi:10.1007/s004150170089
15. Chu K, Kang DW, Kim DE, Park SH, Roh JK. Diffusion-weighted and gradient echo magnetic resonance findings of hemichorea-hemiballismus associated with diabetic hyperglycemia: a hyperviscosity syndrome? Arch Neurol. 2002;59(3):448–452. doi:10.1001/archneur.59.3.448
16. Lee EJ, Choi JY, Lee SH, Song SY, Lee YS. Hemichorea-hemiballism in primary diabetic patients: MR correlation. J Comput Assist Tomogr. 2002;26(6):905–911. doi:10.1097/00004728-200211000-00009
17. Higa M, Kaneko Y, Inokuchi T. Two cases of hyperglycemic chorea in diabetic patients. Diabet Med. 2004;21(2):196–198. doi:10.1111/j.1464-5491.2004.01096.x
18. Kang JH, Kang SY, Choi JC, Lee SS, Kim JS. Chorea triggered by hyperglycemia in a maternally inherited diabetes and deafness (MIDD) patient with the A3243G mutation of mitochondrial DNA and basal ganglia calcification. J Neurol. 2005;252(1):103–105. doi:10.1007/s00415-005-0600-y
19. Nakano N, Uchiyama T, Okuda T, Kitano M, Taneda M. Successful long-term deep brain stimulation for hemichorea-hemiballism in a patient with diabetes. Case report. J Neurosurg. 2005;102(6):1137–1141. doi:10.3171/jns.2005.102.6.1137
20. Branca D, Gervasio O, Le Piane E, Russo C, Aguglia U. Chorea induced by nonketotic hyperglycemia: a case report. Neurol Sci. 2005;26(4):275–277. doi:10.1007/s10072-005-0471-0
21. Pisani A, Diomedi M, Rum A, et al. Acanthocytosis as a predisposing factor for nonketotic hyperglycemia induced chorea-ballism. J Neurol Neurosurg Psychiatry. 2005;76(12):1717–1719. doi:10.1136/jnnp.2005.067033
22. Chung SJ, Lee JH, Lee SA, No YJ, Im JH, Lee MC. Co-occurrence of seizure and chorea in a patient with nonketotic hyperglycemia. Eur Neurol. 2005;54(4):230–232. doi:10.1159/000090717
23. Ohmori H, Hirashima K, Ishihara D, et al. Two cases of hemiballism-hemichorea with T1-weighted MR image hyperintensities. Intern Med. 2005;44(12):1280–1285. doi:10.2169/internalmedicine.44.1280
24. Felicio AC, Chang CV, Godeiro-Junior C, Okoshi MP, Ferraz HB. Hemichorea-hemiballism as the first presentation of type 2 diabetes mellitus. Arq Neuropsiquiatr. 2008;66(2A):249–250. doi:10.1590/S0004-282X2008000200022
25. Battisti C, Forte F, Rubenni E, et al. Two cases of hemichorea-hemiballism with nonketotic hyperglycemia: a new point of view. Neurol Sci. 2009;30(3):179–183. doi:10.1007/s10072-009-0039-5
26. Wang JH, Wu T, Deng BQ, Zhang YW, Zhang P, Wang ZK. Hemichorea-hemiballismus associated with nonketotic hyperglycemia: a possible role of inflammation. J Neurol Sci. 2009;284(1–2):198–202. doi:10.1016/j.jns.2009.04.005
27. Duker AP, Espay AJ. Images in clinical medicine. Hemichorea–hemiballism after diabetic ketoacidosis. N Engl J Med. 2010;363(17):e27. doi:10.1056/NEJMicm0909769
28. Higa M, Yoshida E, Yamashita K, et al. Clinical features of the four cases of hyperglycemic chorea with poorly controlled diabetes. Jpn J Med Pharm Sci. 2010;64(2):259–266.
29. Massaro F, Palumbo P, Falcini M, Zanfranceschi G, Pratesi A. Generalized chorea-ballism in acute non ketotic hyperglycemia: findings from diffusion-weighted magnetic resonance imaging. Parkinsonism Relat Disord. 2012;18(8):998–999. doi:10.1016/j.parkreldis.2012.04.008
30. Hashimoto T, Oguchi K, Takeuchi R. Change in striatal metabolism in diabetic haemichorea-haemiballism. BMJ Case Rep. 2012;2012:bcr2012006405. doi:10.1136/bcr-2012-006405
31. Padmanabhan S, Zagami AS, Poynten AM. A case of hemichorea-hemiballismus due to nonketotic hyperglycemia. Diabetes Care. 2013;36(4):e55–6. doi:10.2337/dc12-2048
32. Kaseda Y, Yamawaki T, Ikeda J, et al. Amelioration of persistent, nonketotic hyperglycemia-induced hemichorea by repetitive transcranial magnetic stimulation. Case Rep Neurol. 2013;5(1):68–73. doi:10.1159/000350434
33. Guo Y, Miao YW, Ji XF, Li M, Liu X, Sun XP. Hemichorea associated with nonketotic hyperglycemia: clinical and neuroimaging features in 12 patients. Eur Neurol. 2014;71(5–6):299–304. doi:10.1159/000357210
34. Lin CM, Liu CK. Bilateral hemiballism-hemi-chorea presenting in a diabetes Taiwanese woman. Neurol Int. 2014;6(3):5519. doi:10.4081/ni.2014.5519
35. Nagai J, Yamada T, Cao X, et al. Cranial magnetic resonance imaging and angiography findings in a patient with hyperglycemic hemichorea-hemiballism. J Clin Endocrinol Metab. 2015;100(1):11–12. doi:10.1210/jc.2014-2576
36. Aquino JH, Spitz M, Pereira JS. Hemichorea-hemiballismus as the first sign of type 1b diabetes during adolescence and its recurrence in the setting of infection. J Child Neurol. 2015;30(10):1362–1365. doi:10.1177/0883073814553972
37. Patel B, Ladva ZR, Khan U. Hemichorea-hemiballism: a case report. Pract Neurol. 2015;15(3):222–223. doi:10.1136/practneurol-2014-001063
38. Ray S, Howlader S, Chakraborty S, Chakraborty PP, Ghosh S. Hemichorea-hemiballism as the first presentation of type 2 diabetes. Clin Diabetes. 2015;33(2):87–89. doi:10.2337/diaclin.33.2.87
39. Teodoro T, Lobo PP, Ferreira J, et al. Delayed Parkinsonism after acute chorea due to nonketotic hyperglycemia. J Neurol Sci. 2015;354(1–2):116–117. doi:10.1016/j.jns.2015.04.039
40. Lancellotti G, Sagot C, Forest A, Greffard S, Bertrand A, Verny M. An unusual case of hemiballism-hemichorea associated with nonketotic hyperglycemia in association with a centrum semiovale stroke. J Am Geriatr Soc. 2015;63(8):1720–1721. doi:10.1111/jgs.13577
41. Suárez-Vega VM, Sánchez Almaraz C, Bernardo AI, Rodríguez-Díaz R, Díez Barrio A, Martín Gil L. CT and MR unilateral brain features secondary to nonketotic hyperglycemia presenting as hemichorea-hemiballism. Case Rep Radiol. 2016;2016:5727138. doi:10.1155/2016/5727138
42. Roy U, Das SK, Mukherjee A, et al. Irreversible hemichorea-hemiballism in a case of nonketotic hyperglycemia presenting as the initial manifestation of diabetes mellitus. Tremor Other Hyperkinet Mov. 2016;6:393. doi:10.5334/tohm.301
43. Yu F, Steven A, Birnbaum L, Altmeyer W. T2*-based MR imaging of hyperglycemia-induced hemichorea-hemiballism. J Neuroradiol. 2017;44(1):24–30. doi:10.1016/j.neurad.2016.09.005
44. Kitagawa M, Yamanaka Y, Adachi T, et al. Diabetic hemichorea-hemiballism after prompt improvement in hyperglycemia. Intern Med. 2017;56(22):3073–3076. doi:10.2169/internalmedicine.8615-16
45. Sato H, Hamano M, Fushimi E, Takahashi T, Horikawa Y, Horiguchi S. Diabetic striatopathy manifesting as severe consciousness disturbance with no involuntary movements. Diabet Med. 2017;34(12):1795–1799. doi:10.1111/dme.13526
46. Son BC, Choi JG, Ko HC. Globus pallidus internus deep brain stimulation for disabling diabetic hemiballism/hemichorea. Case Rep Neurol Med. 2017;2017:2165905. doi:10.1155/2017/2165905
47. Lucassen EB, Delfyett WT, Stahl MC. Persistent hemichorea and caudate atrophy in untreated diabetic striatopathy: a case report. Case Rep Neurol. 2017;9(3):299–303. doi:10.1159/000484201
48. Fong SL, Tan AH, Lau KF, Ramli N, Lim SY. hyperglycemia-associated hemichorea-hemiballismus with predominant ipsilateral putaminal abnormality on neuroimaging. J Mov Disord. 2019;12(3):187–189. doi:10.14802/jmd.19014
49. Lin YT, Chen SC, Yip PK, Wang V. Magnetic resonance imaging volumetric analysis for diabetic striatopathy with two episodes of hemichorea-hemiballism syndrome: a case report. Medicine. 2019;98(38):e17249. doi:10.1097/MD.0000000000017249
50. Marinelli L, Maggi D, Trompetto C, Renzetti P. Neuroradiological evolution of glycemic hemichorea-hemiballism and the possible role of brain hypoperfusion. Eur J Case Rep Intern Med. 2019;6(11):001257. doi:10.12890/2019_001257
51. Zheng W, Chen L, Chen JH, et al. Hemichorea associated with nonketotic hyperglycemia: a case report and literature review. Front Neurol. 2020;11:96. doi:10.3389/fneur.2020.00096
52. Wang DM, Su S, Lin ZZ, Lai LY, Wu YM, Wang SN. Recurrent hemichorea in a patient with diabetes and anti-phospholipid syndrome: a case report. Chin Med J. 2020;133(6):753–755. doi:10.1097/CM9.0000000000000698
53. Kammeyer RM, Orjuela KD. Rapidly progressive dementia and temporal lobe atrophy in a case of nonketotic hyperglycemic hemichorea. Neurohospitalist. 2020;10(3):229–233. doi:10.1177/1941874420902875
54. Chakales P, Park A, Amos A, Baldinger E, Sirotkin I, Frontera A. Hemiballismus in patients with poorly controlled type 2 diabetes mellitus. Fed Pract. 2020;37(6):282–287. doi:10.1016/j.parkreldis.2011.08.015
55. Currò CT, Nicocia G, Ziccone V, et al. Pimozide and pancreatic cancer in diabetic chorea: a case report. Int J Neurosci. 2021;3:1–4.
56. Zhao S, Wu S, Feng L, et al. Hemichorea induced by nonketotic hyperglycemia evaluated with 18F-FDG and 11C-CFT PET/CT. Eur J Nucl Med Mol Imaging. 2021;48(9):3001–3002. doi:10.1007/s00259-021-05240-3
57. Scamarcia PG, Agosta F, Anzalone N, Volontè MA, Filippi M. Striatal atrophy and hypometabolism in drug-resistant non-ketotic hyperglycemic chorea-ballism. Mov Disord Clin Pract. 2021;8(3):486–488. doi:10.1002/mdc3.13155
58. Kataja Knight A, Magnusson P, Sjöholm Å. Hemiballismus in hyperglycemia. Clin Case Rep. 2021;9(5):e04343. doi:10.1002/ccr3.4343
59. Rupp J, Gillespie A. A case of diabetic hemichorea hemiballismus exacerbated by hypoglycemia. AACE Clin Case Rep. 2021;7(5):327–329. doi:10.1016/j.aace.2021.04.004
60. Nelson CC, Ohnoutka C, Ulen M. Non-ketotic hyperglycemic hemichorea-hemiballismus in the setting of antipsychotics and methamphetamine. Cureus. 2021;13(10):e19094. doi:10.7759/cureus.19094
61. Maia M, Moreira AP, Gonçalves AI, Espírito Santo J, Araújo J. Hemichorea-hemiballism as a manifestation of hyperglycemia. Cureus. 2021;13. doi:10.7759/cureus.19330
62. Aggarwal A, Bansal N, Aggarwal R. Nonketotic hyperglycemia presenting as monoballism. J Emerg Med. 2016;50(3):e133–4. doi:10.1016/j.jemermed.2015.11.016
63. Shafait S, Alamgir W, Shafique M. Hyperglycemia presenting with hemichorea-hemiballismus and T-1 hyperintensity on MRI brain. J Coll Physicians Surg Pak. 2021;31(10):1228–1230.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
