Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Relationship Between Dermoscopic Capillary-Related Features of the Auricle and Earlobe and Rosacea Subtype and Disease Severity: A Cross-Sectional Analysis
Authors Zhang Y, Pan R , Gu D , Meng X, Liu T, Xu Y
Received 21 August 2023
Accepted for publication 1 November 2023
Published 9 November 2023 Volume 2023:16 Pages 3221—3228
DOI https://doi.org/10.2147/CCID.S436368
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Yue Zhang, Ruoxin Pan, Duoduo Gu, Xiaoqi Meng, Tingwei Liu, Yang Xu
Department of Dermatology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, 210029, People’s Republic of China
Correspondence: Yang Xu, Department of Dermatology, The First Affiliated Hospital of Nanjing Medical University, No. 300 Guangzhou Road, Nanjing, Jiangsu Province, 210029, People’s Republic of China, Tel/Fax +86-13851856794, Email [email protected]
Background and Aim: Clinical manifestations of rosacea include transient or persistent facial erythema, telangiectasia, papules, and pustules. The existing assessment tools primarily evaluate the facial area to assess the severity of rosacea. However, in addition to the face, telangiectasia, erythema, and flushing can occur in the ear. Here, we investigated the correlation between the dermoscopic characteristics of capillaries in the earlobe and auricle and the types and severity of rosacea.
Materials and Methods: Experienced dermatologists evaluated the clinical type, medical history, severity, and distribution pattern of facial erythema. The clinical severity of rosacea was assessed using the standard grading system (SGS), clinician’s erythema assessment (CEA), and investigator’s global assessment (IGA). Relationships of the clinical types and severity with the dermoscopic characteristics of capillaries in the earlobe and auricle were further analyzed.
Results: In total, 145 patients with rosacea were enrolled in this study. We found that SGS, CEA, and IGA correlated well with the dermoscopic features of capillaries in the earlobe (R = 0.357, 0.357, and 0.314, respectively) (p < 0.001) and auricle (R = 0.423, 0.443, and 0.374, respectively) (p < 0.001). However, there was no significant correlation between the features and types of rosacea.
Conclusion: The dermoscopic characteristics of capillaries in the earlobe and auricle can be used as indicators of the clinical severity of rosacea, regardless of the clinical subtype.
Keywords: skin conditions, ear, dermoscopy, blood vessels
Introduction
Rosacea is a chronic skin disorder characterized by transient or persistent erythema, telangiectasia, papules pustules, and sometimes sebaceous gland hypertrophy, primarily in the convex areas of the face.1 Occasionally, extrafacial rosacea has been reported, which may involve the lateral contours of the face, ears, scalp, and neck.1–3 Initially, the rosacea subtypes were described as erythematotelangiectatic rosacea (ETR), papulopustular rosacea (PPR), phymatous rosacea (PHR), and ocular rosacea, with ETR and PPR being the most common subtypes.4 Neurogenic rosacea (NR) has also been described by Scharschmidt et al5 as a new subtype of rosacea characterized by dramatic facial redness, burning, stinging, and flushing with prominent neurological symptoms; the condition fails to respond to conventional treatment. In NR, facial lesions are more common in the peripheral type than in ETR.6
The existing literature has described and evaluated rosacea lesions occurring mostly on the scalp, arms, legs, and chest, but reports on the ear are sparse.7,8 However, various patients with rosacea complain of ear flushing with or without burning at the time of presentation. In a previous study, approximately half of the patients with rosacea presented with earlobe erythema, which is obvious in the red area images acquired using the VISIA complexion analysis system.9 With disease progression, patients may develop otophyma,10 which shares similarities with the development of clinical manifestations on the face. The ear is a structure adjacent to the face, and there is also an overlap of nerve and vascular innervation between them.11 All of the above suggests that there may be a correlation between manifestations of the ear and face. However, to our knowledge, the relationship between capillary-related features of the ear and the type or severity of rosacea has never been explored.
Rosacea has been reported less frequently among individuals with darker skin than among those with lighter skin, but rosacea is not a rare disease in this population. The difficulty of discerning erythema and telangiectasia in dark skin might explain the underdiagnosis in patients of color.12 Because delayed diagnosis has been reported in substantial numbers of Asian patients, awareness of other signs to assist in evaluation is warranted in evaluating a patient with dark skin.10,13
This study aimed to explore the potential association between dermoscopic capillary-related features of the earlobe and auricle and the clinical types and severity of rosacea.
Materials and Methods
Patients
Patients with rosacea were recruited from the Dermatology Department of the local university hospital between November 2022 and May 2023. This study was approved by the local ethics committee (2021-SR-326). Informed consent was obtained from all the patients. The key inclusion criterion was a clinical diagnosis of rosacea by two independent physicians according to the guidelines for rosacea.14 Information including age, sex, Fitzpatrick skin type, clinical manifestations, family history, and personal history was recorded. Facial images were acquired using the VISIA® 6.0 complexion analysis system (Canfield, NJ, USA), and the red area images were analyzed.
Subjective Assessment of Clinical Types and the Severity of Rosacea
Two senior dermatologists classified the clinical types of rosacea as NR and non-NR according to the criteria provided by Kim et al6 and further classified the non-NR cases as ETR, PPR, or PHR according to the guidelines for rosacea.14 Erythema distribution was divided into the peripheral type, in which erythema only affected the cheeks (Figure 1A–F), and the centrofacial type, in which erythema also affected the forehead, nose, and chin (Figure 1G–L), as reported in the literature.6 The severity of rosacea was evaluated according to subjective scoring systems, such as the standard grading system (SGS) for rosacea,15 clinician’s erythema assessment (CEA),16 and investigator’s global assessment (IGA),17 the details of which are provided in Tables S1–S3.
Dermoscopic Examination and Scoring of the Capillary-Related Characteristics
Dermoscopic images were obtained at the bilateral earlobes and auricles using a handheld dermoscope (DermLite® DL4, 3 Gen Inc., San Juan Capistrano, CA, USA) in contact mode with polarized light. Two blinded dermoscopists scored the dermoscopic capillary-related characteristics (no obvious capillary or erythematous background = 0, segmented dilated capillaries without obvious erythematous background = 1, and segmented or reticularly dilated capillaries with obvious erythematous background = 2) of the skin of the auricle (Figure 2A–C) and earlobe (Figure 2D–F). The grading of capillary-related characteristics was modified from the criterion of facial telangiectasia,18 adding moderate to the mild level. Dermoscopic capillary-related characteristics were classified as “none”, “mild”, or “severe”, according to the sum of the bilateral scores of 0, 1–2, or 3–4, respectively.
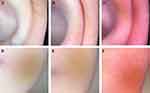 |
Figure 2 Standard dermoscopic presentations of the skin of the auricular scored as 0 (A), 1 (B), and 2 (C) and of the earlobe scored as 0 (D), 1 (E), and 2 (F). |
Statistical Analysis
Statistical analyses were conducted using the SPSS software (version 26.0; SPSS Inc., Chicago, IL, USA). Demographic and clinical data are presented as mean ± standard deviation, number (percentage), minimum, and maximum. Data according to different rosacea subtypes were compared using the Chi-squared test. The relationship between dermoscopic capillary-related characteristics and subjective grading systems was analyzed using Spearman’s rank correlation. Statistical significance was set at p < 0.05.
Results
Demographics and Clinical Characteristics
A total of 145 patients with rosacea were enrolled in the study. Their demographic characteristics are shown in Table 1. The age of the patients ranged from 16 to 56 years, and their average age was 28.9±7.2 years. Regarding the Fitzpatrick skin type, 28, 104, and 13 cases were classified as type II, III, and IV, respectively. The duration of rosacea ranged between 1 and 240 months with an average of 34.5 ± 42.9 months. Ten patients were diagnosed with NR, whereas among the remaining patients, 112 (77.2%) were subtyped as ETR, 17 (11.7%) as PPR, and 6 (4.1%) as PHR. More than half of the patients (60.0%) manifested with central facial erythema. Among all patients, 60.7% had frequent facial flushing during their teenage years and 26.9% had a positive self-reported family history of rosacea.
 |
Table 1 Demographic and Clinical Characteristics of Patients with Rosacea |
Correlation Between Rosacea Subtypes and Dermoscopic Capillary-Related Characteristics of the Earlobe and Auricle
No significant difference was found between the grades of dermoscopic capillary-related characteristics of the ears and the types of rosacea between the NR and non-NR groups (p > 0.05, Table 2). Among the non-NR patients, no correlation was found between the grades of dermoscopic capillary-related characteristics of the ears and the types of rosacea (ETR, PPR, and PHR) (p > 0.05, Table 2).
 |
Table 2 Correlation of the Grades of Dermoscopic Capillary-Related Characteristics of the Earlobe, Auricle and Rosacea Subtypes |
Correlation between the Grades of Dermoscopic Capillary-Related Characteristics of the Ears and SGS, CEA, and IGA
The correlations between SGS, CEA, and IGA and dermoscopic capillary-related characteristics of the ears are shown in Table 3. Of the 145 patients rated on the SGS, 57 were rated as mild, 61 as moderate, and 27 as severe. Statistically significant correlations were observed between the SGS and the severity of earlobe and auricle telangiectasia (p < 0.001, Table 3). Of the 145 patients rated by CEA, 12 scored 1 (almost clear), 58 scored 2 (mild), 53 scored 3 (moderate), and 22 scored 4 (severe). Statistically significant correlations were observed between CEA levels and the severity of earlobe and auricle telangiectasia (p < 0.001, Table 3).
 |
Table 3 Correlation of SGS, CEA, and IGA and Dermoscopic Capillary-Related Characteristics of the Earlobe and Auricle |
In the IGA, 16 of the 145 patients scored 1 (almost clear), 55 scored 2 (mild), 57 scored 3 (moderate), and 17 scored 4 (severe). Statistically significant correlations were observed between IGA and the severity of earlobe and auricle telangiectasias (p < 0.001, Table 3).
Discussion
In clinical practice, erythema, telangiectasia, and burning of the auricle and earlobe are usually overlooked; however, it has been noted that manifestations in the ear are worthy of clinical attention.1,2
Although the pathophysiology of rosacea is not completely understood, neurovascular dysregulation and neurogenic inflammation are presumed pathophysiologic key components.19 The density of nerve fibers in the human ear is rather high, with different nerves distributed in the external ear, including the auriculotemporal nerve (ATN), great auricular nerve, and auricular branch of the vagus nerve,20 all of which are anastomosed with the facial nerve.11 The ATN originates from the mandibular branch of the trigeminal nerve, which also divides into buccal nerves that innervate sensation in the cheek.21 In addition to the face, the sensory branch of the facial nerve receives sensations from the outer ear.22,23 The vascular supply to the ear comes from the branches of the middle temporal and posterior auricular arteries, which sensorily innervate the trigeminal nerve.24 Furthermore, the trigeminal nerve innervates the facial skin involved in rosacea.25
The facial and trigeminal nerves are thought to involved in facial vasodilation.26 Patients with gasserian lesions showed a shorter period and lower intensity of histamine-induced facial flushing than control participants.27 Gonzalez et al26 reported that stimulation of the divisions of the trigeminal nerve in cats caused an increase in skin temperature in the corresponding area of distribution. The mechanism of trigeminal nerve-induced vasodilatation may be caused by the release of substance P and a calcitonin gene-related peptide from nerve endings after stimulation, which promotes vasodilatation and extravasation of substances.28 Thus, we speculate that the ear and facial skin of patients with rosacea may be affected by a common neurovascular pathophysiology.
Patients with NR usually present with prominent neurological symptoms, remarkable erythema, and telangiectasia. However, erythema and telangiectasia of the auricle and earlobe were not associated with either NR or non-NR rosacea. A potential explanation is that the NR dose has no relationship with the telangiectasia characteristics of the auricle and earlobe. NR may not be a specific type but may simply be a class of rosacea that includes significant symptoms, such as burning, accompanied by refractory erythema.29 It may also be possible that the severity of the patient’s disease varies between the NR and non-NR groups.
Furthermore, the data presented in this study revealed no significant difference between the different subtypes of non-NR patients. Research suggests that rosacea may be a manifestation of the same underlying inflammatory continuum, which may progress in severity and to other phenotypes.14 Because of the overlap in symptoms between subtypes, rosacea may change in phenotype; for example, patients with ETR may progress to PPR and even to PHR14,30 Moreover, Lee et al31 found vascular proliferation, and telangiectasia did not differ significantly between patients classified with ETR and PPR. The conversion between subtypes and the overlap between subtypes may explain the absence of significant differences.
In the current study, the severity of rosacea correlated well with the dermoscopic features of both the auricle and earlobe. It has been reported that the severity of perifollicular lymphohistiocytic inflammation and follicular inflammatory reactions correlate with the severity of rosacea.31 Drummond et al32 found that patients with severe rosacea presented with greater axon reflex vasodilatation than those with mild rosacea. Rosacea often starts with facial flushing that evolves into more persistent erythema upon exacerbation, which may be the result of the dilation or disruption of precapillary arterioles and postcapillary venules.33 Dilated skin vessels are susceptible to vasoactive substances, resulting in a vicious cycle of vasodilation and destruction.25 As patients with severe rosacea may experience more significant neurological and inflammatory responses, the complex overlap of nerve and vascular innervation between the ear and face may explain the consistency in severity between the dermoscopic features of the ear and facial symptoms.
Conclusion
Our study, to the best of our knowledge, is the first to investigate the relationship between rosacea and capillary-related characteristics of ear. The results suggest that dermoscopic capillary-related characteristics of the auricle and earlobe can be used as indicators of the clinical severity of rosacea, regardless of the clinical subtype, which will bring clinicians’ attention to the manifestations of rosacea beyond the face.
Abbreviations
ATN, auriculotemporal nerve; CEA, clinician’s erythema assessment; ETR, erythematotelangiectatic; IGA, investigator’s global assessment; NR, neurogenic rosacea; PPR, papulopustular; PHR, phymatous; SGS, standard grading system.
Data Sharing Statement
Data are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study complies with the Declaration of Helsinki and was approved by the Ethics Committee of First Affiliated Hospital of Nanjing Medical University (2021-SR-326).
Consent for Publication
The patients in this manuscript have given written informed consent to publication of their case details.
Acknowledgments
We thank Dr. Xin Liu for his initial idea for this research. The authors would also like to acknowledge the participating investigators and patients involved in the study. The patients in this manuscript have given written informed consent to publication of their case details.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Demitsu T, Tsukahara R, Umemoto N., et al. Disseminated extrafacial rosacea with papulonecrotic lesions. J Dermatol Case Rep. 2016;10(4):68–72.
2. Pereira TM, Vieira AP, Basto AS. Rosacea with extensive extrafacial lesions. Int J Dermatol. 2008;47(1):52–55. doi:10.1111/j.1365-4632.2007.03360.x
3. Bolognia JL, Jorizzo JJ, Schaffer JV, et al. Dermatology.
4. Barakji YA, Rønnstad ATM, Christensen MO, et al. Assessment of frequency of rosacea subtypes in patients with rosacea: a systematic review and meta-analysis. JAMA Dermatol. 2022;158(6):617–625. doi:10.1001/jamadermatol.2022.0526
5. Scharschmidt TC, Yost JM, Truong SV, Steinhoff M, Wang KC, Berger TG. Neurogenic rosacea: a distinct clinical subtype requiring a modified approach to treatment. Arch Dermatol. 2011;147(1):123–126. doi:10.1001/archdermatol.2010.413
6. Kim HO, Kang SY, Kim KE, Cho SY, Kim KH, Kim IH. Neurogenic rosacea in Korea. J Dermatol. 2021;48(1):49–55. doi:10.1111/1346-8138.15629
7. Bostanci O, Borelli C, Schaller M. Treatment of extrafacial rosacea with low-dose isotretinoin. Acta Derm Venereol. 2010;90(4):40910.
8. Marks R, Jones EW. Disseminated rosacea. Br J Dermatol. 1969;81(1):16–28. doi:10.1111/j.1365-2133.1969.tb15915.x
9. Wang XY, Liu YY, Liu YX, et al. A predictive model for differential diagnosis between rosacea and sensitive skin: a cross-sectional study. Chin Med J. 2020;133(17):2132–2134. doi:10.1097/CM9.0000000000001001
10. Alexis AF, Callender VD, Baldwin HE, Desai SR, Rendon MI, Taylor SC. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80(6):1722–9.e7. doi:10.1016/j.jaad.2018.08.049
11. Shoja MM, Oyesiku NM, Griessenauer CJ, et al. Anastomoses between lower cranial and upper cervical nerves: a comprehensive review with potential significance during skull base and neck operations, part I: trigeminal, facial, and vestibulocochlear nerves. Clin Anat. 2014;27(1):118–130. doi:10.1002/ca.22340
12. Marson JW, Baldwin HE. Rosacea: a wholistic review and update from pathogenesis to diagnosis and therapy. Int J Dermatol. 2020;59(6):e175–82. doi:10.1111/ijd.14757
13. Al-Dabagh A, Davis SA, McMichael AJ, Feldman SR. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;3:10.
14. Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the national rosacea society expert committee. J Am Acad Dermatol. 2018;78(1):148–155. doi:10.1016/j.jaad.2017.08.037
15. Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the national rosacea society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50(6):907–912. doi:10.1016/j.jaad.2004.01.048
16. Tan J, Liu H, Leyden JJ, Leoni MJ. Reliability of clinician erythema assessment grading scale. J Am Acad Dermatol. 2014;71(4):7603. doi:10.1016/j.jaad.2014.05.044
17. Taieb A, Ortonne JP, Ruzicka T, et al. Superiority of ivermectin 1% cream over metronidazole 0·75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015;172(4):1103–1110. doi:10.1111/bjd.13408
18. Wang SY, Yang C, J X, Wang F, Yan L, Liu G. Clinical comparison between narrow-spectrum intense pulsed light and 540nm intense pulsed light in treatment of facial telangiectasia. Chin J Med Aesth Cosmet. 2016;25(2):40–43.
19. Schwab VD, Sulk M, Seeliger S, et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15(1):53–62. doi:10.1038/jidsymp.2011.6
20. Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat. 2002;15(1):35–37. doi:10.1002/ca.1089
21. Freddi TAL, Ottaiano AC, Lucio LL, Corrêa DG, Hygino da Cruz LC. The trigeminal nerve: anatomy and pathology. Semin Ultrasound CT MR. 2022;43(5):403–413. doi:10.1053/j.sult.2022.04.002
22. Eagles K, Fralich L, Stevenson JH. Ear trauma. Clin Sports Med. 2013;32(2):303–316. doi:10.1016/j.csm.2012.12.011
23. Walker HK. Cranial nerve VII: the facial nerve and taste. In: Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. Boston: Butterworths; 1990:322–324.
24. de Amorim IL, Kauppila LA, Martins IP. Red ear: syndrome or symptom? Headache. 2018;58(6):885–891. doi:10.1111/head.13333
25. Christensen CE, Andersen FS, Wienholtz N, Egeberg A, Thyssen JP, Ashina M. The relationship between migraine and rosacea: systematic review and meta-analysis. Cephalalgia. 2018;38(7):1387–1398. doi:10.1177/0333102417731777
26. Gonzalez G, Onofrio BM, Kerr FW. Vasodilator system for the face. J Neurosurg. 1975;42(6):696–703. doi:10.3171/jns.1975.42.6.0696
27. De Marinis M, Fraioli B, Esposito V, Gagliardi FM, Agnoli A. Unilateral reduction of head pain and facial vasodilatation after gasserian ganglion lesion. Arch Neurol. 1993;50(2):203–208. doi:10.1001/archneur.1993.00540020079021
28. Drummond PD, Gonski A, Lance JW. Facial flushing after thermocoagulation of the Gasserian ganglion. J Neurol Neurosurg Psychiatry. 1983;46(7):611–616. doi:10.1136/jnnp.46.7.611
29. Wang B, Huang Y, Tang Y, et al. Paroxetine is an effective treatment for refractory erythema of rosacea: primary results from the prospective rosacea refractory erythema randomized clinical trial. J Am Acad Dermatol. 2023;88(6):1300–1307. doi:10.1016/j.jaad.2023.01.044
30. Lee WJ, Jung JM, Won KH, et al. Clinical evaluation of 368 patients with nasal rosacea: subtype classification and grading of nasal rosacea. Dermatology. 2015;230(2):177–183. doi:10.1159/000369926
31. Lee WJ, Jung JM, Lee YJ, et al. Histopathological analysis of 226 patients with rosacea according to rosacea subtype and severity. Am J Dermatopathol. 2016;38(5):347–352. doi:10.1097/DAD.0000000000000454
32. Drummond PD, Su D. Endothelial and axon reflex vasodilatation to acetylcholine in rosacea-affected skin. Arch Dermatol Res. 2012;304(2):133–137. doi:10.1007/s00403-011-1177-1
33. Steinhoff M, Schmelz M, Schauber J. Facial erythema of rosacea - aetiology, different pathophysiologies and treatment Options. Acta Derm Venereol. 2016;96(5):579–586. doi:10.2340/00015555-2335
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

