Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Reintroducing Fenfluramine as a Treatment for Seizures: Current Knowledge, Recommendations and Gaps in Understanding
Authors Dini G, Di Cara G, Ferrara P, Striano P , Verrotti A
Received 13 July 2023
Accepted for publication 19 September 2023
Published 26 September 2023 Volume 2023:19 Pages 2013—2025
DOI https://doi.org/10.2147/NDT.S417676
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Gianluca Dini,1 Giuseppe Di Cara,1 Pietro Ferrara,2 Pasquale Striano,3 Alberto Verrotti1
1Department of Pediatrics, University of Perugia, Perugia, Italy; 2Department of Pediatrics, Campus Bio-Medico University, Rome, Italy; 3Pediatric Neurology and Muscular Diseases Unit, IRCCS Istituto “G. Gaslini”, Genoa, Italy
Correspondence: Gianluca Dini, Department of Pediatrics, University of Perugia, Piazzale Giorgio Menghini 1, Perugia, 06129, Italy, Tel +39 3337797213, Email [email protected]
Abstract: Despite the introduction of new anti-seizure medications in recent years, approximately one-third of the epileptic population continues to experience seizures. Recently, the anti-obesity medication fenfluramine (FFA) has been successfully repurposed, and it has received approval from various regulatory agencies for the treatment of seizures associated with Dravet syndrome and Lennox-Gastaut syndrome. The potential antiseizure effects of FFA were initially observed in patients with photosensitive epilepsy in the 1980s but it was not rigorously explored as a treatment option until 30 years later. This narrative review aims to provide an overview of the historical progression of FFA’s use, starting from initial clinical observations to preclinical studies and, ultimately, successful clinical trials in the field of epilepsy.
Keywords: fenfluramine, seizures, Dravet syndrome, drug-resistant epilepsy, serotonin
Introduction
Epilepsy is a common neurological condition, affecting over 70 million individuals worldwide.1 Despite the introduction of new anti-seizure medications (ASMs) in recent years, approximately one-third of the epileptic population continues to experience seizures.2 This drug resistance poses significant challenges in managing epilepsy and improving outcomes for affected individuals.
Recently, the anti-obesity medication fenfluramine (FFA) has been successfully repurposed, and regulatory agencies have approved its use for the treatment of seizures associated with Dravet syndrome and Lennox-Gastaut syndrome.3,4 This narrative review aims to provide a comprehensive analysis of fenfluramine’s mechanism of action as an antiseizure medication, its clinical pharmacology, and relevant clinical studies. Special emphasis is placed on evaluating its efficacy in reducing seizures and examining potential adverse effects.
Materials and Methods
A Pubmed search was conducted to identify pertinent articles published from January 1985 to July 2023. Papers were searched using the following terms: “fenfluramine”, “Dravet syndrome”, and “Lennox-Gastaut syndrome”. Priority was given to systematic reviews, followed by randomized controlled trials and observational studies, without excluding any paper for its nature. A narrative review format was chosen. Additional articles were identified through manual searches of the reference lists of relevant articles.
Fenfluramine as an Appetite Suppressant
FFA (3-trifluoromethyl-N-ethylamphetamine) is a derivative of amphetamine that was initially introduced in France in 1963. It was primarily used as an antidepressant and later gained popularity as an appetite suppressant. In 1973, FFA received approval for use in the United States where it has been prescribed both as a standalone medication and in combination with phentermine.5,6 FFA is a chiral molecule with a single stereogenic center (Figure 1). It has been marketed as a racemic mixture (Pondimin®) and as the individual d-enantiomer, also known as dexfenfluramine or (+)-fenfluramine (Redux®).7
 |
Figure 1 Structure of fenfluramine. |
Pharmacokinetics
After oral administration, FFA is rapidly absorbed from the gastrointestinal tract and reaches peak plasma concentration within 3–5 hours.8 The absolute oral bioavailability of FFA is estimated to be between 75% and 83%, and it is not significantly affected by food intake.4 FFA is metabolized primarily in the liver by CYP1A2, CYP2B6, CYP2D6, CYP2C9, CYP2C19, and CYP3A4/5 to yield the major active metabolite norfenfluramine and several other minor inactive metabolites.9,10 Both FFA and its metabolites are eliminated through renal excretion, with an elimination half-life of 20 hours for FFA and 24–48 hours for norfenfluramine. The plasma concentration of FFA and its metabolites can vary significantly among individuals. A study evaluating FFA plasma concentration in 61 patients (49 with Dravet syndrome and 7 with Lennox-Gastaut syndrome) found that younger patients had lower concentrations when adjusted for weight, suggesting faster clearance in children.11
The Fen-Phen Combination
In 1984, it was reported that the anorexic effects of FFA could be enhanced while minimizing some of its side effects by combining it with smaller doses of phentermine, another appetite suppressant that had been available in the United States since 1959.12 Phentermine is a stimulant that primarily acts on the central nervous system by promoting the release of noradrenaline and dopamine.13 Although the combined use of fenfluramine and phentermine, known as fen-phen, did not receive specific approval from the FDA, it gained significant popularity, with approximately 18 million prescriptions filled in 1996 alone, mostly for overweight women.14,15
Fenfluramine: Withdrawal from the Market
FFA was withdrawn from the market in 1997 due to accumulating evidence that its chronic use could result in a significant incidence of valvulopathy. The European literature reported two cases of primary pulmonary hypertension (PPH) associated with the use of FFA in 1981.16 Subsequent reports further suggested a potential link between FFA and PPH.17 In 1996, a case-control study compared 95 patients with PPH with 355 healthy controls. The study found that the prolonged use of anorexic drugs, particularly dexfenfluramine, for a duration of 3 months or more, was associated with an odds ratio of 23.1 for developing pulmonary hypertension.18
According to an estimate by Manson and Faich,19 the absolute risk of primary pulmonary hypertension associated with FFA was approximately 28 cases per million person-years of exposure. A year later, on July 8, 1997, the perception of fenfluramine’s benefit-to-risk balance underwent a significant shift due to a report involving 24 women who developed valvular heart disease after being exposed to the fenfluramine-phentermine combination for a period ranging from 1 to 28 months.20 In most patients, the maximum daily dose of FFA taken prior to the diagnosis of valvular disease was either 40 or 60 mg, although two patients were exposed to as little 20 mg, and two received very high doses (120 and 220 mg each). Following this report, health professionals quickly became aware of the issue, and by September 30, 1997, the FDA had received 144 individual spontaneous reports (including the 24 cases reported earlier) involving FFA or dexfenfluramine with or without phentermine in association with valvulopathy.
Although the risk of cardiovascular toxicity is generally believed to be dose-dependent, the presence of confounding factors makes it challenging to establish an exact relationship with dosage. The most robust evidence for a dose-response relationship comes from an evaluation of 74 fen-phen patients reported to the FDA who met the FDA’s criteria for valvulopathy.21 Among these patients, 73 were female, the median daily dose of fenfluramine was 60 mg, and the median duration of exposure was 11 months. Within this small cohort, the proportion of patients with severe valvulopathy (defined as individuals requiring valve replacement surgery or having severe aortic or mitral regurgitation) increased from 20% (3/17) for doses below 40 mg/day to 23% (3/13) at 40 mg/day, 65% (24/37) at 60 mg/day, and 71% (5/7) at doses above 60 mg/day.
The Mechanisms of Fenfluramine’s Cardiovascular Toxicity
Several lines of evidence suggest that FFA-induced valvulopathy is the result of excessive serotonergic stimulation of cardiac valve tissue. Specifically, the activation of 5-HT2B receptors by 5-HT has been shown to promote DNA synthesis and cell-cycle progression in valve interstitial cells, leading to valve damage.22,23 Recent evidence has further highlighted the crucial role of 5-HT2B receptor stimulation in the development of fenfluramine-induced valvulopathy. A study demonstrated that the increase in mitral valve thickening and endothelial cell density (which are considered early indicators of mitral valve remodelling) caused by 28-day treatment with d-norfenfluramine in mice, is fully prevented by the selective 5-HT2B receptor antagonists SB204741, as well as by the deletion of 5-HT2B receptors.24
The relationship between 5-HT2B receptor activation and cardiac valvulopathy is supported by numerous studies.23,25,26 One of these relates to the observation that cardiac valvulopathy is caused by drugs with different pharmacological profiles and chemical structures, such as FFA, benfluorex, pergolide, and cabergoline. These drugs share the common ability to bind to and activate 5-HT2B receptors.26,27 In contrast, other serotonergic drugs with low or moderate affinity for 5-HT2B receptors, such as ropinirole and lorcaserin, have not been associated with the development of valvulopathy.28–30
While 5-HT2B receptor stimulation is recognized as a crucial factor in the development of FFA-induced valvulopathy, it is important to acknowledge that other factors are likely to contribute to this condition as well. These include the stimulation of 5-HT2A receptors, serotonylation of the cytoskeletal protein filamin-A, and the interplay of genetic predisposition and individual risk factors.26
Another adverse cardiovascular effect that has been reported with high-dose FFA is pulmonary arterial hypertension (PAH).18 FFA’s serotonergic overactivity can induce proliferation of pulmonary artery smooth muscle cells, which contributes to the development of PAH.31 The increased serotonergic activity can also cause vasoconstriction of the pulmonary artery. Additionally, other factors such as nitric oxide deficiency have been suggested to play a role in the development of PAH.32
Fenfluramine Repurposing from Weight Loss to Epilepsy
The anticonvulsant activity of FFA was first recognized in the 1980s, when it was tested in individuals and in small groups of patients with treatment-resistant epilepsies. More specifically, Aicardi et al and Clemens were among the first to present data from case reports and small case series demonstrating a significant reduction in seizure frequency when FFA was added to the existing treatment regimen.33–35 It should also be noted that Gastaut et al reported the reduction of compulsive respiratory stereotypies in children with autism, when treated with FFA at doses ranging from 1.5 to 3 mg/kg/day.36
The initial pilot study conducted by Gastaut and Zifkin involved the assessment of 33 individuals with intractable epilepsy who were administered FFA at a dose ranging from 0.5 to 1.5 mg/kg/day. The results of the study revealed that almost half of the participants experienced a reduction in seizure frequency of at least 50%. Importantly, no echocardiographic findings indicating pulmonary hypertension or valvular dysfunction were reported. Among the observed side effects in this patient population, the most common were sleepiness, fatigue, and loss of appetite, with or without weight loss.37
The potential use of FFA as an antiepileptic treatment is based on the hypothesis that 5-HT receptors, which are widely distributed throughout the central nervous system, can interact with different types of ion channels, thereby influencing neuronal excitability. Both clinical and animal studies have demonstrated that serotonergic neurons possess anticonvulsant effects, and patients treated with selective serotonin reuptake inhibitors (SSRIs) may experience a reduction in seizure frequency.38–40 Furthermore, recent radioligand binding assays have revealed that FFA exhibits modulatory activity at sigma-1 receptors. These receptors are highly expressed in deeper laminae of the cortex, the olfactory bulb, nuclei of the mesencephalon, hypothalamus, and Purkinje cells in the brain. This modulation of sigma-1 receptors, in addition to FFA’s serotonergic activity, has been observed both in vitro and in vivo.41
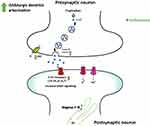
Mechanisms of Fenfluramine’s Anti-Seizure Activity
Experimental studies suggest that FFA exerts its antiseizure activity by enhancing serotonergic transmission and positively modulating sigma-1 receptors (Figure 2). The anticonvulsant effect of FFA is believed to be associated with restoring the balance between GABA-mediated inhibition and glutamatergic excitation.
Serotonergic Transmission
FFA is known to enhance serotonergic neurotransmission by disrupting the vesicle storage of 5-HT and by preventing its reuptake.42 FFA achieves this through two main mechanisms. Firstly, it interacts with the serotonin transporter, inhibiting the reuptake of 5-HT. Additionally, FFA prevents the movement of 5-HT from the cytoplasm into vesicles, resulting in the release of cytoplasmic 5-HT outside the cell via 5-HT carriers. These actions contribute to elevated levels of extracellular 5-HT. The inhibitory effects of increased brain 5-HT levels on seizures in rats were observed as early as 1957.43 Under experimental conditions, exposure to various anticonvulsants, such as phenytoin and carbamazepine, has been shown to increase extracellular 5-HT levels.44 Additionally, SSRIs, which are known to increase 5-HT levels, have been found to have a positive impact on seizure management.45
Aside from the indirect effect of increasing extracellular 5-HT levels, various in vitro and in vivo studies have demonstrated that FFA is capable of acting as an agonist of multiple serotonin receptors including 5-HT1A, 5-HT1D, 5-HT2A, 5-HT2B, and 5-HT2C.41,42,46,47
Sigma-1 Pathway
Beyond its serotonergic activity, FFA interacts with other receptors, most notably sigma-1 receptors, which are a subtype of plasma membrane-localized opioid receptors.41,47 While the serotonergic activity of FFA at 5-HT receptors primarily enhances GABAergic signaling, its interaction with sigma-1 receptors reduces glutamatergic excitability. Interestingly, sigma-1 receptors have been implicated not only in seizure control but also in the pathophysiology of non-seizure comorbidities associated with developmental and epileptic encephalopathies.41,48 In this regard, FFA ameliorated learning deficits in mouse models via sigma-1 receptor activity and improved executive functions in patients with Dravet syndrome and LGS.41,49–51
GABAergic Neurotransmission
The impairment of GABAergic neurotransmission is a major contributor to epileptogenesis in various preclinical models.52–54 FFA has been shown to enhance GABAergic neurotransmission through its effects on 5-HT release at GABAergic synapses and stimulation of 5-HT2A and 5-HT2C receptors.55–57 Additionally, FFA has demonstrated the ability to restore the dendritic arborization of GABAergic neurons in a zebrafish model of Dravet syndrome.58 These findings suggest that FFA’s effects on GABAergic neurotransmission may contribute to its antiepileptic properties.
Fenfluramine in Experimental Models of Epilepsy
FFA has demonstrated significant antiepileptic effects in various animal models of epilepsy, including rodents and zebrafish. In the mutant scn1Lab zebrafish model of Dravet syndrome, FFA was found to greatly reduce the occurrence of seizures, as evidenced by Zhang and Sourbron.47,59
Dinday et al conducted a screening of approximately 1000 compounds and found that FFA-treated scn1Lab mutant zebrafish exhibited a suppression of spontaneous electrographic seizure activity.60 In rodent models, FFA has shown efficacy in reducing tonic seizures induced by pentylenetetrazol, as reported by Lazarova et al.61 Additionally, FFA was investigated in the DBA/1 mouse model of sudden unexpected death in epilepsy, where it demonstrated a dose-dependent reduction in seizure incidence and severity.62 These findings collectively support the antiepileptic potential of FFA in various animal models.
Fenfluramine in Dravet Syndrome
Dravet syndrome is a severe, treatment-resistant epileptic encephalopathy presenting within the first year of life and is characterized by a high seizure burden and significant behavioural, motor, and neurodevelopmental abnormalities. The long-term outcome of Dravet syndrome has been considered to be consistently poor, both in regard to developmental outcome and seizure control.63,64 Available antiepileptic drugs do not achieve adequate seizure control in most Dravet syndrome patients,65,66 making the identification of new drugs a critical need.
In 2012, Ceulemans et al conducted a retrospective study involving 12 patients with Dravet syndrome. During the course of the study, two patients discontinued treatment. However, the remaining ten patients were followed up for a period of 11 years, during which they received adjunctive FFA at a mean dosage of 0.34 mg/kg/day. Among these ten patients, seven achieved seizure freedom for at least one year.67 To date, data from two prospective, double-blind, placebo-controlled trials are available, and they both confirm the efficacy and safety of low dose FFA in Dravet syndrome.
In a first study,68 two different dosages of FFA (0.2 and 0.7 mg/kg/day) were compared to placebo. The percentage of patients who achieved a 50% reduction in seizures was 68% in the 0.7 mg/kg/day group, 38% in the 0.2 mg/kg/day group, and only 12% in the placebo group. The drug was well tolerated, with 38% of children in the highest dosage group experiencing appetite problems. Importantly, prospective cardiac follow-up did not reveal any cardiac issues in patients receiving FFA.
In another randomized clinical trial,49 a total of 87 pediatric patients were randomly assigned to receive either FFA 0.4 mg/kg day in addition to their treatment regimen or a placebo medication. The results revealed that the number of patients who achieved a 50% reduction in seizures was 54% in the FFA arm compared to only 5% in the placebo arm. These findings strongly suggest that low-dose FFA can be a safe and effective treatment option for individuals with Dravet syndrome.
Fenfluramine in Lennox-Gastaut Syndrome
Lennox-Gastaut syndrome (LGS) is a severe developmental and epileptic encephalopathy that typically begins in childhood and has a significant impact on the quality of life of patients and their families.69 It is considered a rare disease, with an estimated incidence rate of 0.1 to 0.28 per 100,000 people overall and an incidence rate of 2 per 100,000 in children. LGS accounts for approximately 2–5% of all childhood epilepsies, with a slightly higher occurrence in males.65 LGS usually manifests before the age of 12, with a peak onset between 3 and 5 years of age. Although rare, there have been reports of late-onset cases. LGS has a diverse range of clinical presentations that can evolve and change over time. Tonic seizures are a hallmark of LGS. Alongside tonic seizures, other common seizure types observed in LGS include atypical absences and tonic or atonic drop attacks.70 The treatment of LGS can be particularly challenging due to the refractory nature of seizures and the associated cognitive and behavioral impairments. Currently available treatments often have limited efficacy and may be associated with significant side effects. Therefore, there is a critical need for the development of novel and effective treatments that can effectively reduce seizures and improve the overall quality of life for individuals with LGS.
Lagae et al reported FFA’s effectiveness in LGS in an open-label prospective Phase 2 study.71 The study included 13 patients with LGS who experienced more than four convulsive seizures per month. Among them, 10 patients completed 20 weeks of FFA treatment at a dose of 0.8 mg/kg/day (maximum 30 mg/day). The results showed a median reduction of seizures by 53% in the overall patient group. Among the ten patients who completed the study, there was a 60% reduction in seizures. Additionally, >50% seizure reduction was observed in eight (62%) patients. Long-term efficacy was evaluated in nine patients, and the median reduction of seizures was 58%. Among these patients, 67% achieved a ≥50% reduction in seizures, and 33% achieved a ≥75% reduction.
A multicenter, double-blind, placebo-controlled RCT was conducted in 263 LGS patients (2–35 years old) with ≥2 drop seizures/week.72 The patients were randomly assigned to receive FFA 0.7 mg/kg/day, FFA 0.2 mg/kg/day or placebo. 25.3 and 28.1% of patients treated respectively with FFA 0.7 mg/kg/day and FFA 0.2 mg/kg/day had a ≥50% reduction in monthly drop seizures, compared with 10.3% of placebo. Overall, FFA therapy was well tolerated. Most frequent adverse events (at least 10%) included decreased appetite, somnolence, fatigue, vomiting, diarrhea and pyrexia. No cases of valvular heart disease or pulmonary arterial hypertension were reported.
Fenfluramine in Other Epileptic Syndromes
The efficacy of FFA in epilepsies other than Dravet syndrome and LGS is unknown, although recent clinical trials suggested its potential utility in other epilepsy syndromes (Table 1).
 |
Table 1 Main Results from Clinical Trials for Fenfluramine (FFA) Use in Pharmacoresistant Epilepsies |
CDKL5 deficiency disorder (CDD) is a rare neurodevelopmental and epileptic encephalopathy characterized by infantile-onset epilepsy and severe developmental delays. Most individuals with CDD develop refractory epilepsy with multiple seizure types.78 In a study conducted by Devinsky et al, the efficacy of FFA was evaluated in six patients with CDD who had failed 5–12 ASMs. These patients were treated with FFA at a dosage of 0.4 mg/kg/day (n = 2) or 0.7 mg/kg/day (n = 4). The results showed a median 90% reduction in seizure frequency, indicating a significant antiseizure effect of FFA in this patient population.76
FFA was also assessed in a study involving children and young adults with Sunflower syndrome. Nine patients were treated with FFA for a 4-month period, with a maximum dose of 0.7 mg/kg/day. After 3 months of FFA treatment, eight patients showed a ≥30% reduction in seizure activity. Notably, no cases of cardiac valvulopathy or pulmonary hypertension were observed during the study.79
Furthermore, a phase 2 clinical trial (NCT04289467) is underway to investigate the efficacy of FFA in patients with refractory infantile spasms. In this trial, patients will be treated with a dosage of 0.8 mg/kg/day FFA for a duration of 21 days.77
Efficacy of Fenfluramine Beyond Seizures
Recent data suggest that FFA confers clinical benefit beyond seizure reduction alone, including improvements in everyday executive function and a reduction of sudden unexpected death in epilepsy (SUDEP).80 Both clinical trials and animal studies have shown that FFA can improve survival rates.81,82 Notably, the SUDEP mortality rate among Dravet syndrome patients who received FFA in Phase III studies was significantly lower than pre-treatment rates (1.7 deaths per patient-years after FFA compared to 11.7 deaths per patient-years pre-FFA treatment).81
Moreover, FFA was found to improve executive functioning, including regulation of emotions and behaviour, in some patients with Dravet syndrome as well as in those with LGS.50,51,83 According to survey data from caregivers of patients with Dravet syndrome, FFA treatment is associated with meaningful improvements in cognition, focus, alertness, speech, behaviour, sleep, and motor function.84
The mechanisms behind these effects remain under investigation. However, some evidence supports a potential role for the 5-HT4 and sigma-1 receptors.82 The interaction of FFA with 5-HT4 receptors might have beneficial effects on cognitive functions, as evidence suggests that 5-HT4 receptor agonism enhances learning and memory.85
Safety of Fenfluramine
FFA was generally well tolerated as a low-dose adjunctive treatment for seizures in patients with Dravet syndrome or LGS, both in the short term (14–15 weeks)49,68,72 and in the longer term (up to 3.5 years).75,86 Common adverse effects were decreased appetite, diarrhea, pyrexia, nasopharyngitis, lethargy, and drowsiness. Cardiovascular safety is an important endpoint when evaluating patients treated with FFA. A meta-analysis reported an approximately 20-fold higher risk of aortic valve disease and approximately five-fold higher risk of mitral valve disease following exposure to FFA.87
The study conducted by Li et al demonstrated that there is an increased risk of valvulopathy with higher doses of FFA. Patients taking a dose of ≥60 mg/day had a relative risk of valvulopathy of 9.2 compared to patients taking a dose <40 mg/day.21
Additionally, the risk of valvulopathy is higher in certain populations, such as women, the elderly, and those who have been exposed to these agents for a longer duration. For example, the incidence rate of clinically symptomatic valvulopathy is 7.1 per 10,000 individuals with less than 4 months of exposure, whereas it increases to 35 per 10,000 individuals with more than 4 months of exposure.88,89
In the context of evaluating FFA for the treatment of Dravet syndrome and LGS, comprehensive monitoring programs with color Doppler echocardiographic examinations (performed at baseline, after ≈ 6–8 weeks of treatment and at study end in the core studies;49,68 and at study entry, after 4–6 weeks of treatment, and then at 3-month intervals in the open label extension studies)75,86,90,91 were implemented in all randomized controlled trials. These monitoring programs were designed to prospectively assess cardiac valve function and identify any signs of pulmonary arterial hypertension. In all these trials, no pathological functional changes in cardiac valves or signs of pulmonary arterial hypertension were observed in any patient at any point during the trials. These findings provide evidence to support the cardiovascular safety of FFA at the lower dosages used for seizure management compared to the higher dosages used in the treatment of obesity in adults.
Several factors may have contributed to the differences in the incidence of cardiac valve dysfunction between the clinical studies conducted shortly after the withdrawal of FFA in 1997 and the current studies of patients with Dravet syndrome treated with FFA: 1) Patient Population: The patients included in the clinical studies conducted after the withdrawal of fenfluramine were primarily obese adults who were using FFA as an appetite suppressant for weight loss. These individuals were older, overweight or obese, and predominantly female. In contrast, the patients in the recent studies were children and adolescents with Dravet syndrome who had normal or low body mass index (BMI). 2) Dose of fenfluramine: The dose of FFA used in the current studies of patients with Dravet syndrome was significantly lower compared to the doses used for weight loss purposes. The maximum doses used in the Dravet syndrome studies were 17 or 26 mg/day, whereas doses of 60–120 mg/day or higher were typically used for weight loss. 3) Cardiac Evaluation: In the clinical studies after the withdrawal of FFA, the adult patients were not evaluated for baseline cardiac valve function before initiating treatment. Additionally, they were not screened for risk factors for valve disease. In contrast, all patients in current studies underwent pretreatment echocardiograms to evaluate their baseline cardiac valve function. These factors, including the differences in patient population, FFA dose, and cardiac evaluation protocols, likely contributed to the variations in the incidence of cardiac valve dysfunction observed between the studies.
Dosage and Administration of Fenfluramine
Oral fenfluramine has received approval in the EU and the USA for the treatment of seizures associated with Dravet syndrome and LGS in patients aged ≥2 years.4 In the EU, it is used as an add-on therapy to other ASMs, while in the USA, it can be used both as monotherapy and as an add-on therapy.
FFA is available as an oral solution, taken twice daily with or without food. The recommended starting dosage in patients with Dravet syndrome or LGS who are not receiving stiripentol is 0.2 mg/kg/day. This dosage is gradually increased at 7-day intervals, first to 0.4 mg/kg/day and subsequently to 0.7 mg/kg/day (maximum daily dosage of 26 mg).92
For patients with Dravet syndrome or LGS who are already receiving stiripentol, the recommended starting dosage of FFA is also 0.2 mg/kg/day, and the up-titration is done after 7 days to a maintenance dosage of 0.4 mg/kg/day (maximum daily dosage of 17 mg).
Conclusion
Despite the introduction of various novel ASMs, drug resistance remains one of the major challenges in epilepsy treatment. It is important to consider that drug resistance is very probably not caused by a single mechanism in all patients but is rather more likely due to several mechanisms, which may even occur together in the same patient.93–97 The search for novel ASMs remains an active area of research, with ongoing studies focusing on identifying new molecular targets, developing innovative therapeutic approaches, and repurposing existing medications for new therapeutic indications.
FFA had been used extensively in the past as an appetite suppressant, but it was withdrawn from the market in 1997 due to its association cardiac valvulopathy. More recently, double-blind placebo-controlled trials established its efficacy in the treatment of convulsive seizures associated with Dravet syndrome and of drop seizures associated with LGS.
FFA acts via multiple receptors to exert its therapeutic effects for the treatment of seizures, although the exact mechanisms are still being elucidated. While FFA has been widely recognized as a potent serotonin-releasing agent, its pharmacological mechanism is likely to be more complex than a 5-HT-driven response alone.
It is important to highlight that FFA repositioning as an ASM and its use at lower doses in the treatment of Dravet syndrome and LGS have been shown to be beneficial for seizure management, while vigilant monitoring for cardiovascular adverse events remains crucial. The use of FFA in children with Dravet syndrome and LGS has shown an overall favourable safety profile, with mostly mild side effects. In the treatment of Dravet syndrome and LGS, it is administered at comparatively lower doses (maximum 17–26 mg/day) compared to its previous use as an appetite suppressant, where higher doses (typically 60–120 mg/day), often in combination with phentermine, were employed. Further research and long-term studies are needed to better understand the safety and tolerability of FFA in the pediatric population. Regular monitoring of cardiac function and conducting routine evaluations can help detect any potential cardiovascular issues early and guide clinical management.
Abbreviations
5-HT, Serotonin; ASMs, Anti-seizure medications; CDD, CDKL5 deficiency disorder; FFA, Fenfluramine; LGS, Lennox-Gastaut syndrome; PPH, Primary pulmonary hypertension; SSRIs, Selective serotonin reuptake inhibitors; SUDEP, Sudden unexpected death in epilepsy.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Falco-Walter J. Epilepsy-definition, classification, pathophysiology, and epidemiology. Semin Neurol. 2020;40(6):617–623. doi:10.1055/s-0040-1718719
2. Kalilani L, Sun X, Pelgrims B, Noack-Rink M, Villanueva V. The epidemiology of drug-resistant epilepsy: a systematic review and meta-analysis. Epilepsia. 2018;59(12):2179–2193. PMID: 30426482. doi:10.1111/epi.14596
3. Gao C, Pielas M, Jiao F, et al. Epilepsy in Dravet syndrome-current and future therapeutic opportunities. J Clin Med. 2023;12(7):2532. PMID: 37048615; PMCID: PMC10094968. doi:10.3390/jcm12072532
4. Frampton JE. Fenfluramine: a review in Dravet and Lennox-gastaut syndromes. Drugs. 2023;83(10):923–934. PMID: 37316680; PMCID: PMC10310619. doi:10.1007/s40265-023-01881-w
5. Dini G, Tulli E, Dell’Isola GB, et al. Improving therapy of pharmacoresistant epilepsies: the role of fenfluramine. Front Pharmacol. 2022;13:832929. PMID: 35668937; PMCID: PMC9164301. doi:10.3389/fphar.2022.832929
6. Odi R, Invernizzi RW, Gallily T, Bialer M, Perucca E. Fenfluramine repurposing from weight loss to epilepsy: what we do and do not know. Pharmacol Ther. 2021;226:107866. PMID: 33895186. doi:10.1016/j.pharmthera.2021.107866
7. Balagura G, Cacciatore M, Grasso EA, Striano P, Verrotti A. Fenfluramine for the treatment of Dravet syndrome and lennox-gastaut syndrome. CNS Drugs. 2020;34(10):1001–1007. PMID: 32875491. doi:10.1007/s40263-020-00755-z
8. Gammaitoni A, Smith S, Boyd B. The lack of effect of food on the pharmacokinetics of ZX008 (Fenfluramine Oral Solution): results of a single-dose, two-period crossover study. Clin Ther. 2018;40(8):1338–1346. PMID: 29941151. doi:10.1016/j.clinthera.2018.05.013
9. Haritos VS, Ching MS, Ghabrial H, et al. Metabolism of dexfenfluramine in human liver microsomes and by recombinant enzymes: role of CYP2D6 and 1A2. Pharmacogenetics. 1998;8(5):423–432. PMID: 9825834. doi:10.1097/00008571-199810000-00007
10. Boyd B, Smith S, Gammaitoni A, Galer BS, Farfel GM. A Phase I, randomized, open-label, single-dose, 3-period crossover study to evaluate the drug-drug interaction between ZX008 (fenfluramine HCl oral solution) and a regimen of stiripentol, clobazam, and valproate in healthy subjects. Int J Clin Pharmacol Ther. 2019;57(1):11–19. PMID: 30336805; PMCID: PMC6298132. doi:10.5414/CP203276
11. Schoonjans AS, Roosens L, Dewals W, Paelinck BP, Ceulemans B. Therapeutic drug monitoring of fenfluramine in clinical practice: pharmacokinetic variability and impact of concomitant antiseizure medications. Epilepsia. 2022;63(3):686–696. PMID: 35032026. doi:10.1111/epi.17162
12. Weintraub M, Hasday JD, Mushlin AI, Lockwood DH. A double-blind clinical trial in weight control. Use of fenfluramine and phentermine alone and in combination. Arch Intern Med. 1984;144(6):1143–1148. PMID: 6375610. doi:10.1001/archinte.1984.00350180055008
13. Rothman RB, Baumann MH. Serotonin releasing agents. Neurochemical, therapeutic and adverse effects. Pharmacol Biochem Behav. 2002;71(4):825–836. PMID: 11888573. doi:10.1016/s0091-3057(01)00669-4
14. Fishman AP. Aminorex to fen/phen: an epidemic foretold. Circulation. 1999;99(1):156–161. PMID: 9884392. doi:10.1161/01.cir.99.1.156
15. Schoonjans AS, Lagae L, Ceulemans B. Low-dose fenfluramine in the treatment of neurologic disorders: experience in Dravet syndrome. Ther Adv Neurol Disord. 2015;8(6):328–338. PMID: 26600876; PMCID: PMC4643872. doi:10.1177/1756285615607726
16. Douglas JG, Munro JF, Kitchin AH, Muir AL, Proudfoot AT. Pulmonary hypertension and fenfluramine. Br Med J. 1981;283(6296):881–883. PMID: 6793158; PMCID: PMC1507127. doi:10.1136/bmj.283.6296.881
17. Brenot F, Herve P, Petitpretz P, Parent F, Duroux P, Simonneau G. Primary pulmonary hypertension and fenfluramine use. Br Heart J. 1993;70(6):537–541. PMID: 8280518; PMCID: PMC1025385. doi:10.1136/hrt.70.6.537
18. Abenhaim L, Moride Y, Brenot F, et al. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International primary pulmonary hypertension study group. N Engl J Med. 1996;335(9):609–616. PMID: 8692238. doi:10.1056/NEJM199608293350901
19. Manson JE, Faich GA. Pharmacotherapy for obesity -- do the benefits outweigh the risks? N Engl J Med. 1996;335(9):659–660. PMID: 8687523. doi:10.1056/NEJM199608293350910
20. Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337(9):581–588. PMID: 9271479. doi:10.1056/NEJM199708283370901
21. Li R, Serdula MK, Williamson DF, Bowman BA, Graham DJ, Green L. Dose-effect of fenfluramine use on the severity of valvular heart disease among fen-phen patients with valvulopathy. Int J Obes Relat Metab Disord. 1999;23(9):926–928. PMID: 10490797. doi:10.1038/sj.ijo.0801020
22. Hafizi S, Taylor PM, Chester AH, Allen SP, Yacoub MH. Mitogenic and secretory responses of human valve interstitial cells to vasoactive agents. J Heart Valve Dis. 2000;9(3):454–458. PMID: 10888105.
23. Goldberg E, Grau JB, Fortier JH, Salvati E, Levy RJ, Ferrari G. Serotonin and catecholamines in the development and progression of heart valve diseases. Cardiovasc Res. 2017;113(8):849–857. PMID: 28863437; PMCID: PMC5790145. doi:10.1093/cvr/cvx092
24. Ayme-Dietrich E, Lawson R, Côté F, et al. The role of 5-HT2B receptors in mitral valvulopathy: bone marrow mobilization of endothelial progenitors. Br J Pharmacol. 2017;174(22):4123–4139. PMID: 28806488; PMCID: PMC5680644. doi:10.1111/bph.13981
25. Rothman RB, Baumann MH. Appetite suppressants, cardiac valve disease and combination pharmacotherapy. Am J Ther. 2009;16(4):354–364. PMID: 19092640; PMCID: PMC2713386. doi:10.1097/MJT.0b013e31817fde95
26. Ayme-Dietrich E, Lawson R, Da-Silva S, Mazzucotelli JP, Monassier L. Serotonin contribution to cardiac valve degeneration: new insights for novel therapies? Pharmacol Res. 2019;140:33–42. PMID: 30208338. doi:10.1016/j.phrs.2018.09.009
27. Fitzgerald LW, Burn TC, Brown BS, et al. Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol. 2000;57(1):75–81. PMID: 10617681.
28. Papoian T, Jagadeesh G, Saulnier M, et al. Regulatory forum review*: utility of in vitro secondary pharmacology data to assess risk of drug-induced valvular heart disease in humans: regulatory considerations. Toxicol Pathol. 2017;45(3):381–388. PMID: 28421966. doi:10.1177/0192623317690609
29. Saraf TS, Felsing DE, Armstrong JL, Booth RG, Canal CE. Evaluation of lorcaserin as an anticonvulsant in juvenile Fmr1 knockout mice. Epilepsy Res. 2021;175:106677. PMID: 34130255; PMCID: PMC8296307. doi:10.1016/j.eplepsyres.2021.106677
30. Sourbron J, Lagae L. Serotonin receptors in epilepsy: novel treatment targets? Epilepsia Open. 2022;7(2):231–246. PMID: 35075810; PMCID: PMC9159250. doi:10.1002/epi4.12580
31. Adnot S, Houssaini A, Abid S, Marcos E, Amsellem V. Serotonin transporter and serotonin receptors. Handb Exp Pharmacol. 2013;218:365–380. PMID: 24092348. doi:10.1007/978-3-642-38664-0_15
32. Archer SL, Djaballah K, Humbert M, et al. Nitric oxide deficiency in fenfluramine- and dexfenfluramine-induced pulmonary hypertension. Am J Respir Crit Care Med. 1998;158(4):1061–1067. PMID: 9769261. doi:10.1164/ajrccm.158.4.9802113
33. Aicardi J, Gastaut H. Treatment of self-induced photosensitive epilepsy with fenfluramine. N Engl J Med. 1985;313(22):1419. PMID: 3932858. doi:10.1056/NEJM198511283132218
34. Aicardi J, Gastaut H, Misès J. Syncopal attacks compulsively self-induced by Valsalva’s maneuver associated with typical absence seizures. A case report. Arch Neurol. 1988;45(8):923–925. PMID: 3134879. doi:10.1001/archneur.1988.00520320125029
35. Clemens B. Dopamine agonist treatment of self-induced pattern-sensitive epilepsy. A case report. Epilepsy Res. 1988;2(5):340–343. PMID: 3197703. doi:10.1016/0920-1211(88)90044-7
36. Gastaut H, Zifkin B, Rufo M. Compulsive respiratory stereotypies in children with autistic features: polygraphic recording and treatment with fenfluramine. J Autism Dev Disord. 1987;17(3):391–406. PMID: 3654490. doi:10.1007/BF01487068
37. Gastaut H, Zifkin BG. Antiepileptic effects of fenfluramine-pilot-study. Ann Neurol. 1987;22(3):414–415.
38. Hoyer D. Serotonin 5-HT3, 5-HT4, and 5-HT-M receptors. Neuropsychopharmacology. 1990;3(5–6):371–383. PMID: 2078273.
39. Buchanan GF, Murray NM, Hajek MA, Richerson GB. Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J Physiol. 2014;592(19):4395–4410. PMID: 25107926; PMCID: PMC4215784. doi:10.1113/jphysiol.2014.277574
40. Specchio LM, Iudice A, Specchio N, et al. Citalopram as treatment of depression in patients with epilepsy. Clin Neuropharmacol. 2004;27(3):133–136. PMID: 15190237. doi:10.1097/00002826-200405000-00009
41. Martin P, de Witte PAM, Maurice T, Gammaitoni A, Farfel G, Galer B. Fenfluramine acts as a positive modulator of sigma-1 receptors. Epilepsy Behav. 2020;105:106989. PMID: 32169824. doi:10.1016/j.yebeh.2020.106989
42. Fuller RW, Snoddy HD, Robertson DW. Mechanisms of effects of d-fenfluramine on brain serotonin metabolism in rats: uptake inhibition versus release. Pharmacol Biochem Behav. 1988;30(3):715–721. PMID: 2463643. doi:10.1016/0091-3057(88)90089-5
43. Bonnycastle DD, Giarman NJ, Paasonen MK. Anticonvulsant compounds and 5-hydroxytryptamine in rat brain. Br J Pharmacol Chemother. 1957;12(2):228–231. PMID: 13446378; PMCID: PMC1509665. doi:10.1111/j.1476-5381.1957.tb00125.x
44. Jobe PC, Browning RA. The serotonergic and noradrenergic effects of antidepressant drugs are anticonvulsant, not proconvulsant. Epilepsy Behav. 2005;7(4):602–619. PMID: 16169281. doi:10.1016/j.yebeh.2005.07.014
45. Hamid H, Kanner AM. Should antidepressant drugs of the selective serotonin reuptake inhibitor family be tested as antiepileptic drugs? Epilepsy Behav. 2013;26(3):261–265. PMID: 23395350. doi:10.1016/j.yebeh.2012.10.009
46. Rodríguez-Muñoz M, Sánchez-Blázquez P, Garzón J. Fenfluramine diminishes NMDA receptor-mediated seizures via its mixed activity at serotonin 5HT2A and type 1 sigma receptors. Oncotarget. 2018;9(34):23373–23389. PMID: 29805740; PMCID: PMC5955088. doi:10.18632/oncotarget.25169
47. Sourbron J, Smolders I, de Witte P, Lagae L. Pharmacological analysis of the anti-epileptic mechanisms of fenfluramine in scn1a Mutant Zebrafish. Front Pharmacol. 2017;8:191. PMID: 28428755; PMCID: PMC5382218. doi:10.3389/fphar.2017.00191
48. Martin P, Reeder T, Sourbron J, de Witte PAM, Gammaitoni AR, Galer BS. an emerging role for sigma-1 receptors in the treatment of developmental and epileptic encephalopathies. Int J Mol Sci. 2021;22(16):8416. PMID: 34445144; PMCID: PMC8395113. doi:10.3390/ijms22168416
49. Nabbout R, Mistry A, Zuberi S, et al. Fenfluramine for treatment-resistant seizures in patients with Dravet syndrome receiving stiripentol-inclusive regimens: a randomized clinical trial. JAMA Neurol. 2020;77(3):300–308. PMID: 31790543; PMCID: PMC6902175. doi:10.1001/jamaneurol.2019.4113
50. Bishop KI, Isquith PK, Gioia GA, et al. Improved everyday executive functioning following profound reduction in seizure frequency with fenfluramine: analysis from a Phase 3 long-term extension study in children/young adults with Dravet syndrome. Epilepsy Behav. 2021;121:108024. doi:10.1016/j.yebeh.2021.108024
51. Bishop KI, Isquith PK, Gioia GA, et al. Fenfluramine treatment is associated with improvement in everyday executive function in preschool-aged children (<5 years) with Dravet syndrome: a critical period for early neurodevelopment. Epilepsy Behav. 2023;138:108994. PMID: 36463826. doi:10.1016/j.yebeh.2022.108994
52. de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495(2):387–395. PMID: 2569920. doi:10.1016/0006-8993(89)90234-5
53. Oakley JC, Kalume F, Catterall WA. Insights into pathophysiology and therapy from a mouse model of Dravet syndrome. Epilepsia. 2011;52(2):59–61. PMID: 21463282; PMCID: PMC3547637. doi:10.1111/j.1528-1167.2011.03004.x
54. Houser CR. Do structural changes in GABA neurons give rise to the epileptic state? Adv Exp Med Biol. 2014;813:151–160. PMID: 25012374; PMCID: PMC4634888. doi:10.1007/978-94-017-8914-1_12
55. Shen RY, Andrade R. 5-Hydroxytryptamine2 receptor facilitates GABAergic neurotransmission in rat hippocampus. J Pharmacol Exp Ther. 1998;285(2):805–812. PMID: 9580630.
56. Higgins GA, Desnoyer J, Van Niekerk A, et al. Characterization of the 5-HT2C receptor agonist lorcaserin on efficacy and safety measures in a rat model of diet-induced obesity. Pharmacol Res Perspect. 2015;3(1):e00084. PMID: 25692009; PMCID: PMC4317222. doi:10.1002/prp2.84
57. Guiard BP, Di Giovanni G. Central serotonin-2A (5-HT2A) receptor dysfunction in depression and epilepsy: the missing link? Front Pharmacol. 2015;6:46. PMID: 25852551; PMCID: PMC4362472. doi:10.3389/fphar.2015.00046
58. Tiraboschi E, Martina S, van der Ent W, et al. New insights into the early mechanisms of epileptogenesis in a zebrafish model of Dravet syndrome. Epilepsia. 2020;61(3):549–560. PMID: 32096222. doi:10.1111/epi.16456
59. Zhang Y, Kecskés A, Copmans D, et al. Pharmacological characterization of an antisense knockdown zebrafish model of Dravet syndrome: inhibition of epileptic seizures by the serotonin agonist fenfluramine. PLoS One. 2015;10(5):e0125898. PMID: 25965391; PMCID: PMC4428833. doi:10.1371/journal.pone.0125898
60. Dinday MT, Baraban SC. Large-scale phenotype-based antiepileptic drug screening in a zebrafish model of Dravet syndrome. eNeuro. 2015;2(4):ENEURO.0068–15.2015. PMID: 26465006; PMCID: PMC4596025. doi:10.1523/ENEURO.0068-15.2015
61. Lazarova M, Bendotti C, Samanin R. Studies on the role of serotonin in different regions of the rat central nervous system on pentylenetetrazol-induced seizures and the effect of di-n-propylacetate. Naunyn Schmiedebergs Arch Pharmacol. 1983;322(2):147–152. PMID: 6408491. doi:10.1007/BF00512388
62. Tupal S, Faingold CL. Fenfluramine, a serotonin-releasing drug, prevents seizure-induced respiratory arrest and is anticonvulsant in the DBA/1 mouse model of SUDEP. Epilepsia. 2019;60(3):485–494. PMID: 30719703. doi:10.1111/epi.14658
63. Genton P, Velizarova R, Dravet C. Dravet syndrome: the long-term outcome. Epilepsia. 2011;52 Suppl 2:44–49. PMID: 21463279. doi:10.1111/j.1528-1167.2011.03001.x
64. Akiyama M, Kobayashi K, Yoshinaga H, Ohtsuka Y. A long-term follow-up study of Dravet syndrome up to adulthood. Epilepsia. 2010;51(6):1043–1052. PMID: 20041943. doi:10.1111/j.1528-1167.2009.02466.x
65. Dravet C. Dravet syndrome history. Dev Med Child Neurol. 2011;53(2):1–6. PMID: 21504424. doi:10.1111/j.1469-8749.2011.03964.x
66. Verrotti A, Striano P. Novel therapeutic options for Dravet and lennox-gastaut syndrome. Expert Rev Neurother. 2021;21(11):1191–1194. PMID: 33297778. doi:10.1080/14737175.2020.1862651
67. Ceulemans B, Boel M, Leyssens K, et al. Successful use of fenfluramine as an add-on treatment for Dravet syndrome. Epilepsia. 2012;53(7):1131–1139. PMID: 22554283. doi:10.1111/j.1528-1167.2012.03495.x
68. Lagae L, Sullivan J, Knupp K, et al. Fenfluramine hydrochloride for the treatment of seizures in Dravet syndrome: a randomised, double-blind, placebo-controlled trial. Lancet. 2019;394(10216):2243–2254. PMID: 31862249. doi:10.1016/S0140-6736(19)32500-0
69. Asadi-Pooya AA. Lennox-Gastaut syndrome: a comprehensive review. Neurol Sci. 2018;39(3):403–414. PMID: 29124439. doi:10.1007/s10072-017-3188-y
70. Strzelczyk A, Schubert-Bast S. Expanding the treatment landscape for lennox-gastaut syndrome: current and future strategies. CNS Drugs. 2021;35(1):61–83. PMID: 33479851; PMCID: PMC7873005. doi:10.1007/s40263-020-00784-8
71. Lagae L, Schoonjans AS, Gammaitoni AR, Galer BS, Ceulemans B. A pilot, open-label study of the effectiveness and tolerability of low-dose ZX008 (fenfluramine HCl) in Lennox-Gastaut syndrome. Epilepsia. 2018;59(10):1881–1888. PMID: 30146701. doi:10.1111/epi.14540
72. Knupp KG, Scheffer IE, Ceulemans B, et al. Efficacy and safety of fenfluramine for the treatment of seizures associated with lennox-gastaut syndrome: a randomized clinical trial. JAMA Neurol. 2022;79(6):554–564. PMID: 35499850; PMCID: PMC9062770. doi:10.1001/jamaneurol.2022.0829
73. Schoonjans A, Paelinck BP, Marchau F, et al. Low-dose fenfluramine significantly reduces seizure frequency in Dravet syndrome: a prospective study of a new cohort of patients. Eur J Neurol. 2017;24(2):309–314. PMID: 27790834; PMCID: PMC5298030. doi:10.1111/ene.13195
74. Specchio N, Pietrafusa N, Doccini V, et al. Efficacy and safety of Fenfluramine hydrochloride for the treatment of seizures in Dravet syndrome: a real-world study. Epilepsia. 2020;61(11):2405–2414. PMID: 32945537. doi:10.1111/epi.16690
75. Sullivan J, Scheffer IE, Lagae L, et al. Fenfluramine HCl (Fintepla®) provides long-term clinically meaningful reduction in seizure frequency: analysis of an ongoing open-label extension study. Epilepsia. 2020;61(11):2396–2404. PMID: 33078386; PMCID: PMC7756901. doi:10.1111/epi.16722
76. Devinsky O, King L, Schwartz D, Conway E, Price D. Effect of fenfluramine on convulsive seizures in CDKL5 deficiency disorder. Epilepsia. 2021;62(7):e98–e102. PMID: 33979451; PMCID: PMC8360137. doi:10.1111/epi.16923
77. Children’s Hospital of Orange County. Treatment of refractory infantile spasms with fenfluramine. Available from: https://www.clinicaltrials.gov/study/NCT04289467.ClinicalTrials.gov.ID:NCT04289467.
78. Leonard H, Downs J, Benke TA, Swanson L, Olson H, Demarest S. CDKL5 deficiency disorder: clinical features, diagnosis, and management. Lancet Neurol. 2022;21(6):563–576. PMID: 35483386; PMCID: PMC9788833. doi:10.1016/S1474-4422(22)00035-7
79. Geenen KR, Doshi SP, Patel S, et al. Fenfluramine for seizures associated with Sunflower syndrome. Dev Med Child Neurol. 2021;63(12):1427–1432. PMID: 34216017. doi:10.1111/dmcn.14965
80. Sourbron J, Lagae L. Fenfluramine: a plethora of mechanisms? Front Pharmacol. 2023;14:1192022. PMID: 37251322; PMCID: PMC10213522. doi:10.3389/fphar.2023.1192022
81. Cross JH, Galer BS, Gil-Nagel A, et al. Impact of fenfluramine on the expected SUDEP mortality rates in patients with Dravet syndrome. Seizure. 2021;93:154–159. PMID: 34768178. doi:10.1016/j.seizure.2021.10.024
82. Tupal S, Faingold CL. Serotonin 5-HT4 receptors play a critical role in the action of fenfluramine to block seizure-induced sudden death in a mouse model of SUDEP. Epilepsy Res. 2021;177:106777. PMID: 34601387. doi:10.1016/j.eplepsyres.2021.106777
83. Bishop KI, Isquith PK, Gioia GA, et al. Fenfluramine treatment improves everyday executive functioning in patients with lennox gastaut syndrome: analysis from a phase 3 clinical trial. Plenary Present Am Acad Neurol. 2021;2021:1.
84. Jensen MP, Gammaitoni AR, Salem R, Wilkie D, Lothe A, Amtmann D. Fenfluramine treatment for Dravet syndrome: caregiver- and clinician-reported benefits on the quality of life of patients, caregivers, and families living in Germany, Spain, Italy, and the United Kingdom. Epilepsy Res. 2023;190:107091. PMID: 36701932. doi:10.1016/j.eplepsyres.2023.107091
85. Murphy SE, Wright LC, Browning M, Cowen PJ, Harmer CJ. A role for 5-HT4 receptors in human learning and memory. Psychol Med. 2020;50(16):2722–2730. PMID: 31615585. doi:10.1017/S0033291719002836
86. Knupp KG, Scheffer IE, Ceulemans B, et al. Fenfluramine provides clinically meaningful reduction in frequency of drop seizures in patients with Lennox-Gastaut syndrome: interim analysis of an open-label extension study. Epilepsia. 2023;64(1):139–151. PMID: 36196777; PMCID: PMC10099582. doi:10.1111/epi.17431
87. Hopkins PN, Polukoff GI. Risk of valvular heart disease associated with use of fenfluramine. BMC Cardiovasc Disord. 2003;3:5. PMID: 12801402; PMCID: PMC194859. doi:10.1186/1471-2261-3-5
88. Dahl CF, Allen MR, Urie PM, Hopkins PN. Valvular regurgitation and surgery associated with fenfluramine use: an analysis of 5743 individuals. BMC Med. 2008;6:34. PMID: 18990200; PMCID: PMC2585088. doi:10.1186/1741-7015-6-34
89. Jick H, Vasilakis C, Weinrauch LA, Meier CR, Jick SS, Derby LE. A population-based study of appetite-suppressant drugs and the risk of cardiac-valve regurgitation. N Engl J Med. 1998;339(11):719–724. PMID: 9731087. doi:10.1056/NEJM199809103391102
90. Lai WW, Galer BS, Wong PC, et al. Cardiovascular safety of fenfluramine in the treatment of Dravet syndrome: analysis of an ongoing long-term open-label safety extension study. Epilepsia. 2020;61(11):2386–2395. PMID: 32809271; PMCID: PMC7754414. doi:10.1111/epi.16638
91. Agarwal A, Farfel GM, Gammaitoni AR, Wong PC, Pinto FJ, Galer BS. Long-term cardiovascular safety of fenfluramine in patients with Dravet syndrome treated for up to 3 years: findings from serial echocardiographic assessments. Eur J Paediatr Neurol. 2022;39:35–39. PMID: 35640431. doi:10.1016/j.ejpn.2022.05.006
92. European Medicine Agency. Fintepla: Summary of product characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/fintepla-epar-product-information_en.pdf.
93. Czornyj L, Auzmendi J, Lazarowski A. Transporter hypothesis in pharmacoresistant epilepsies. Is it at the central or peripheral level? Epilepsia Open. 2022;7(Suppl 1):S34–S46. PMID: 34542938; PMCID: PMC9340303. doi:10.1002/epi4.12537
94. Leandro K, Bicker J, Alves G, Falcão A, Fortuna A. ABC transporters in drug-resistant epilepsy: mechanisms of upregulation and therapeutic approaches. Pharmacol Res. 2019;144:357–376. PMID: 31051235. doi:10.1016/j.phrs.2019.04.031
95. Juvale IIA, Che Has AT. Possible interplay between the theories of pharmacoresistant epilepsy. Eur J Neurosci. 2021;53(6):1998–2026. PMID: 33306252. doi:10.1111/ejn.15079
96. Feldmann M, Koepp M. ABC transporters and drug resistance in patients with epilepsy. Curr Pharm Des. 2016;22(38):5793–5807. PMID: 27514707. doi:10.2174/1381612822666160810150416
97. Tang F, Hartz AMS, Bauer B. Drug-resistant epilepsy: multiple hypotheses, few answers. Front Neurol. 2017;8:301. PMID: 28729850; PMCID: PMC5498483. doi:10.3389/fneur.2017.00301
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.