Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Rehospitalization Risk of Receptor-Affinity Profile in Antipsychotic Drug Treatment: A Propensity Score Matching Analysis Using a Japanese Employment-Based Health Insurance Database
Authors Takekita Y, Inoue S, Baba K, Nosaka T
Received 7 August 2020
Accepted for publication 5 November 2020
Published 30 November 2020 Volume 2020:16 Pages 2871—2879
DOI https://doi.org/10.2147/NDT.S276030
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Yoshiteru Takekita,1 Sachie Inoue,2 Kenji Baba,3 Tadashi Nosaka3
1Department of Neuropsychiatry, Kansai Medical University, Osaka, Japan; 2CRECON Medical Assessment Inc, Tokyo, Japan; 3Sumitomo Dainippon Pharma Co, Ltd, Tokyo, Japan
Correspondence: Yoshiteru Takekita
Department of Neuropsychiatry, Kansai Medical University, 10-15 Fumizono-Cho, Moriguchi, Osaka 570-8506, Japan
Tel +81-6-6992-1001
Fax +81-6-6992-4846
Email [email protected]
Purpose: The aim of this study was to examine whether there is a difference in the risk of rehospitalization when antipsychotics are classified into two groups treated using drugs with a higher or lower affinity to H1 or α 1 receptors than to D2 receptors (histamine H1 receptors, adrenaline α 1 receptors [HA] high- and HA low-affinity drug group, respectively) based on affinity to receptors related to sedation using a nationwide insurance claims database in Japan.
Patients and Methods: We identified eligible patients by the following two criteria: (i) hospitalization due to schizophrenia (International Classification of Disease [ICD]-10 code: F20 or F25) in psychiatric wards between January 1st, 2005 and August 31st, 2017, and (ii) administration of HA high- or HA low-affinity drugs in the next month after discharge from the earliest hospitalization due to schizophrenia (index month). The primary endpoint was rehospitalization due to schizophrenia. The secondary endpoints were (i) involuntary rehospitalization, (ii) concomitant use of anxiolytics/hypnotics, mood stabilizers, and antiparkinsonian drugs, (iii) all-cause death, and (iv) medication discontinuation. Propensity score (PS) matching analysis was applied, and the hazard ratio (HR) of the event rate in the HA high-affinity drug group relative to the HA low-affinity drug group was calculated using Cox’s proportional hazards model.
Results: Two thousand nine hundred and forty patients were identified as eligible patients. Among PS-matched patients (819 in each group), the HR in the HA high-affinity drug group compared with the HA low-affinity drug group was 1.018 (0.822– 1.260, P = 0.870). Other outcomes did not differ significantly between the two groups.
Conclusion: No significant difference was observed in the rehospitalization risk due to schizophrenia associated with HA high-affinity antipsychotic drugs. Although this study was a retrospective PS-matched cohort study, the possibility of masking of the rehospitalization risk cannot be excluded because more than 80% of the patients were administered an anxiolytic/hypnotic at the time of admission.
Keywords: schizophrenia, antipsychotics, sedative effect, rehospitalization, claim database, propensity score matching
Introduction
Schizophrenia is a common disease with a lifetime prevalence of 0.7%. Its symptoms vary widely, including hallucination/delusion (positive symptoms), abulia/autism (negative symptoms), cognitive disorders, and emotional disorders (depressive symptoms).1 The treatment goal of schizophrenia is recovery of the patient’s social life, and medication and psychosocial interventions are performed in combination for this purpose. Antipsychotics for schizophrenia are known to have relapse-prevention effects and suppressive effects on acute symptoms, but there are multiple reports that the rehospitalization rate of patients with schizophrenia is approximately 30% in Japan.2,3 As relapse due to insufficient response to antipsychotics and poor medication adherence are suggested as factors related to rehospitalization,4 maintaining medication adherence of antipsychotics is considered one of the important conditions for the prevention of relapse and minimization of rehospitalization risk.
As factors of poor adherence, several factors, including the difference in the dosage form,5 lack of knowledge about the disease, drug abuse, negative feelings about medication, and cognitive impairment, have been suggested.6 In addition, based on a systematic literature review, Velligan et al found that there are many adverse events associated with poor adherence.6 According to a questionnaire survey of 306 patients with schizophrenia in Japan about adverse events due to antipsychotics,7 daytime drowsiness (50%) was the most common adverse event. Of the patients, 13.7% answered that daytime drowsiness was tolerable, and this percentage was lowest among other common adverse events due to antipsychotics such as weight gain (31.8%), sexual dysfunction (30.3%), akathisia (24.0%), and dry mouth (27.0%). Afonso et al also reported that medication adherence was poorer for the outpatients with more severe somnipathy.8 These results suggest that patients discontinue taking antipsychotics on their own due to drowsiness, which may lead to the relapse of psychiatric symptoms and rehospitalization.
Differences in safety and tolerability profiles of atypical antipsychotics have been summarized in several review articles.9–11 Among the atypical antipsychotics, the profile of drowsiness is different, with clozapine, olanzapine, and quetiapine summarized as very common. This adverse event is pharmacologically explained to be due to its high affinity for the H1 or α1 receptor.10 A comparative trial of antipsychotics with rehospitalization as an endpoint was performed during a randomized controlled sustained administration study (1 year)12 following a double-blind randomized controlled trial (RCT) comparing lurasidone, quetiapine, and placebo in patients with acute phase schizophrenia (6 weeks).13 In this study, the hazard ratios (HR) of medication discontinuation and rehospitalization were lower in the lurasidone-treated group than in the quetiapine-treated group. The incidence of somnolence during the 6-week double-blind RCT was 5.3% in the lurasidone group and 13.4% in the quetiapine group, with greater than a two-fold difference. Although the score of daytime drowsiness on the Epworth sleepiness scale (ESS) significantly differed between the two groups,13 the incidence of somnolence in the two groups during the 1-year period was 3.3% and 4.7%, respectively, with no marked difference.14 Although this study also suggested that the incidence of drowsiness differs among antipsychotics, the relationship between drowsiness and rehospitalization risk remains unclear, and the design of the sustained administration study, in which patients with a high tolerance were registered, was considered to be limited for confirmation of this relationship. Although there were many reports about the rehospitalization risk in schizophrenia patients, no study evaluating the relationship between drowsiness due to antipsychotics and the rehospitalization risk was found. Moreover, there is currently no report about whether the outcome of rehospitalization differs according to the development of drowsiness due to antipsychotics or the strength of the sedative effects.
The aim of this study was to evaluate whether there is a difference in the rehospitalization risk by classifying antipsychotics according to affinity to receptors related to sedation from the viewpoint of their pharmacological profile to clarify the relationship between the development of drowsiness and rehospitalization risk. Although there is no clear definition for classification of each antipsychotic drug according to the strength of their sedative effects, we assessed our hypothesis by rating the strength of sedative effects of antipsychotics from the viewpoint of their pharmacological profile using the ratio of affinity of dopamine D2 receptors and affinity to adrenaline α1 receptors or histamine H1 receptors, which are related to the sedative effects.15–17 Therefore, we examined whether the group of atypical antipsychotics with α1 or H1 receptor high affinity had a higher risk of rehospitalization than the group with α1 and H1 receptor low affinity in patients with schizophrenia using the nationwide insurance claims database in Japan.
Patients and Methods
Study Design
This study utilized a nationwide insurance claims database in Japan constructed by JMDC Inc.18 The JMDC database is an epidemiological receipt database that has accumulated receipts (inpatient, outpatient, and dispensing) and medical examination data received from multiple employer-based health insurance associations since 2005. The cumulative dataset consisted of approximately 5.6 million subjects as of June 2018. The database can track data for each patient in chronological order, even if the patient visited or was hospitalized at multiple medical institutions. As the database is composed of employer-based health insurance data, it contains insurance claims data of the employers and their families. The datasets generated and/or analyzed during the current study are not publicly available because it was purchased from a commercial provider (JMDC Inc.) but is available from the corresponding author on reasonable request.
The ethics review committee secretariat of Kansai Medical University stated that no ethics review was necessary for this study because it was a secondary analysis of an anonymous patient database and is not based on the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects.
This study had two objectives. The primary objective was to clarify differences in the rehospitalization risk of schizophrenia patients depending on the receptor profile of atypical antipsychotics. It was a retrospective cohort study to evaluate the rehospitalization risk in two groups treated using drugs with a higher or lower affinity to H1 or α1 receptors than to D2 receptors (histamine H1 receptors, adrenaline α1 receptors [HA] high-affinity drug and HA low-affinity drug group, respectively). The secondary objective was to evaluate factors of rehospitalization by a case-control study in patients rehospitalized with schizophrenia as cases and those not rehospitalized as controls.
Study Population
From the JMDC claims database of approximately 59,994 patients with schizophrenia, schizotypal, delusional, and other non-mood psychotic disorders (International Classification of Disease [ICD]-10 code: F20-29) between January 1st, 2005 and August 31st, 2017, we identified eligible patients by the following two criteria: (i) hospitalization due to schizophrenia (ICD-10 code: F20 or F25) in psychiatric wards between January 1st, 2005 and August 31st, 2017, and (ii) administration of any of the study drugs (HA high-affinity or HA low-affinity drugs) (other than those taken irregularly as necessary and injection drugs) in the next month after the earliest hospitalization due to schizophrenia (index month). The exclusion criteria were defined as follows: (i) patients aged ≥65 years in the index month to exclude patients for whom an antipsychotic drug was used for delirium, (ii) patients to whom antipsychotics other than the study drugs (Anatomical Therapeutic Chemical [ATC] code: N05A) were administered in the index month (except those taken irregularly as necessary), or (iii) HA high- and HA low-affinity drugs were used concomitantly in the index month (except those taken only once). The study drugs were antipsychotics that contained the following ingredients. HA high-affinity drugs: asenapine, clozapine, olanzapine, quetiapine and risperidone; HA low-affinity drugs: aripiprazole, blonanserin, paliperidone and perospirone. The analysis population and study outcomes were defined by the study objectives.
Primary Objective
The analysis population of the HA high-affinity drug group and HA low-affinity drug group was defined as patients who were administered one or more tablets of HA high-affinity drugs or HA low-affinity drugs (except those taken irregularly as necessary and injection drugs), respectively, in the index month. The primary endpoint was rehospitalization due to schizophrenia in psychiatric wards. The secondary endpoints were (i) involuntary rehospitalization (treatment code: I014), (ii) concomitant use of anxiolytics/hypnotics (ATC code: N05C or N05B), mood stabilizers (lithium carbonate, sodium valproate, lamotrigine, carbamazepine), and antiparkinsonian drugs (ATC code: N04), (iii) all-cause death, and (iv) medication discontinuation.
Hospitalization due to schizophrenia in psychiatric wards was defined as any of the medical service fee points counted during the period of hospitalization (treatment code: A103, A104 3, A300, A311, A311-2, A311-3, A311-4, A312, or A318) to identify it as hospitalization due to schizophrenia by health insurance claims. Medication discontinuation was defined as a prescription interval of the study drug of 31 days or more in or after the index month, and the day of the end the last prescription before discontinuation was regarded as the day of the event.
The assessment period was defined as “up to 12 months” or the “maximum assessable period”. The maximum assessable period was defined as the shortest period from the index month to the day of the end of follow-up, time of event of each evaluation item, or time of censoring. In regard to medication discontinuation, the shortest of the periods from the index month to the day of the end of follow-up, time of censoring, or the time of event was defined as the maximum assessable period.
Secondary Objective
The case group was defined as patients who were rehospitalized at least once due to schizophrenia in psychiatric wards during the period from 3 to 12 months after the index month, and the control group was defined as patients who were able to be followed up for 12 months after the index month and were not rehospitalized due to schizophrenia during this period. As related to rehospitalization due to schizophrenia, the following were comprehensively evaluated: the presence of medication discontinuation before rehospitalization, kinds of antipsychotics used, presence of the concomitant use of anxiolytics/hypnotics, presence of the concomitant use of mood stabilizers, presence of the concomitant use of antiparkinsonian drugs, presence of cognitive behavioral therapy, and number of outpatient visits during the 3 months prior to the evaluation point, sex, and age at the evaluation point. The evaluation point was the month of earliest hospitalization due to schizophrenia during the period from 3 to 12 months after the index month in the case group and the end of the 1-year follow-up in the control group.
Statistical Analysis
To reduce the effects of potential confounding factors in this observational study, propensity score (PS) matching analysis was applied for eligible patients (HA high-affinity drug group, N = 2120; HA low-affinity drug group, N = 820). PS matching reduces bias due to confounding factors by matching patients on baseline variables using a multivariable logistic regression model. Confounding factors of patient characteristics were examined based on clinical findings, theoretical grounds, and findings from previous studies.19–24 The candidate factors examined are shown in Appendix 1. Candidate factors were narrowed down by the following procedure: (1) high frequency of each candidate factor in both groups (2% or higher); (2) presence of biases between the two groups (p < 0.25 for between-group difference); and (3) among variables satisfying (1) and (2) above, those clinically related to each other (eg, “antidepressant use” and “depression”) were narrowed down to a single variable.
The following factors were selected: sex, age at the index month, year of hospitalization due to schizophrenia and the type of hospitalization (involuntary %), the number of antipsychotics during hospitalization, concomitant use of anxiolytics/hypnotics, mood stabilizers, and antiparkinsonian drugs during the baseline hospitalization, prescription of antidepressants, diagnosis of diabetes, hyperlipidemia, asthma, and osteoarthrosis, and allied disorders within a month before baseline hospitalization. The PS-matched pairs were created at a ratio of 1:1 based on the nearest neighbor matching algorithm with a 0.25-caliper distance with no replacements. The goodness-of-fit of the logistic regression model was evaluated by the Hosmer-Lemeshow test. Discrimination, ie, the ability to classify individuals with and without events, was evaluated by the C-statistic or the area under the receiving operating characteristic curve.25 A C-statistic >0.7 indicates good discrimination.
Regarding the primary endpoint of the primary objective, the HR of the incidence of events in the HA high-affinity drug group compared with the HA low-affinity drug group were calculated using Cox’s proportionate hazard model with rehospitalization due to schizophrenia in psychiatric wards during the 12 months from the index months and the maximum assessable period as the objective variable, and the HA high- or low-affinity drug groups as the explanatory variable. As secondary endpoints, the HR of each event in the HA high-affinity drug group compared with the HA low-affinity drug group during the period of 12 months from the index month and maximum assessable period (involuntary hospitalization, all-cause death, and medication discontinuation only) were calculated using Cox’s proportional hazard model. As for all-cause death, risk factors for death other than schizophrenia were added as covariates (Appendix 2). Regarding the secondary objective, logistic regression analysis was performed by the forced entry method using the presence of rehospitalization due to schizophrenia in psychiatric wards as the objective variable and each factor as an explanatory variable, and the odds ratio (OR) of rehospitalization for each explanatory variable were calculated. All data analyses were carried out using SAS v.9.4 and P-values <0.05 were considered significant.
Results
Study Population and Analysis Population
A flow chart of the study population is shown in Figure 1. In the JMDC database, 59,994 patients having schizophrenia (F20 −29) were identified during the study period. Of them, 2940 patients who were hospitalized due to schizophrenia in psychiatric wards and for whom HA high- or low-affinity drugs were prescribed in the index month were identified as eligible patients. The numbers of patients treated using HA high- and HA low-affinity drugs in the index month were 2120 and 820, respectively (primary objective population). Of these, the numbers of patients who were rehospitalized at least once due to schizophrenia in psychiatric wards during the period from 3 to 12 months after the index month and patients who were not rehospitalized due to schizophrenia during the 12 months after the index month were 429 and 1688, respectively (Figure 1).
 |
Figure 1 Patients flowchart. |
Among the eligible patients (n = 2940), logistic regression analysis was performed for PS matching. The model was well-calibrated (Hosmer-Lemeshow test = 0.523) and demonstrated good discrimination (C-statistic = 0.601). The characteristics of PS-matched HA high- and HA low-affinity drug group patients (n = 819 of each) for the primary objective, and case and control group patients for the secondary objective (n = 429 and 1688, respectively) are summarized in Table 1. The mean age of the PS-matched patients was 35 years, and male patients accounted for approximately 38.0%. The number of days of the earliest hospitalization due to schizophrenia was approximately 80.0 days. The rate of concomitant use of anxiolytics/hypnotics in the index month exceeded 80.0%. The prescription in the index month was monotherapy in 85.8% of the patients in the HA high-affinity drug group and 95.1% in the HA low-affinity drug group, and the most frequent prescription was monotherapy of olanzapine in the HA high-affinity drug group and of aripiprazole in the HA low-affinity drug group (Appendix 3). In the secondary objective population, no marked difference was observed between the cases and controls, and no heterogeneity compared with the eligible patients was observed.
 |
Table 1 Patient Characteristics |
Primary Objective
Primary Endpoint
In the HA high- and HA low-affinity drug groups, the number of rehospitalization due to schizophrenia in psychiatric wards were 118 and 119, respectively, when the analysis period was 12 months, and 168 and 170, respectively, during the maximum assessable period. Based on Cox regression analysis, the HR in the HA high-affinity drug group compared with the HA low-affinity drug group when the analysis period was 12 months and in the maximum assessable period was 1.014 (0.786–1.308 (95% confidence interval [CI]), P = 0.913) and 1.018 (0.822–1.260, P = 0.870), respectively, and no difference in the risk of rehospitalization was observed between the two groups (Figure 2–3).
 |
Figure 2 Kaplan-Meier plot of rehospitalization (up to 12 months). |
 |
Figure 3 Kaplan-Meier plot of rehospitalization (Maximum assessment period). |
Secondary Endpoint
There were no significant differences between the two groups in involuntary rehospitalization, concomitant use, all-cause death, or medication discontinuation (Appendix 4a). Analysis using diseases related to death (Appendix 2) as covariates was also performed, but no significant difference was noted in the risk of death between the HA high- and HA low-affinity drug groups (Appendix 4b).
Secondary Objective
As a result of forced entry logistic regression analysis, “HA high-affinity drugs were prescribed” (OR 1.812; 95% CI [1.389–2.365]), “anxiolytics/hypnotics were used” (2.010; [1.535–2.630]) and “mood stabilizer was used” (1.265; [1.006–1.593]) during the 3 months before the evaluation point were found to be factors that increase the risk of rehospitalization due to schizophrenia (P <0.0001, P <0.0001 and P=0.045, respectively). “An age of ≥40 years at the evaluation point” was demonstrated to be a factor that reduces the risk of rehospitalization due to schizophrenia (0.730; [0.586–0.909]) (P=0.005) (Table 2).
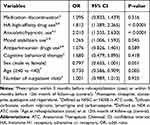 |
Table 2 Risk Factors for Rehospitalization (Secondary Objective Analysis, N = 2117) |
Discussion
In this study, we evaluated the difference in rehospitalization risk due to schizophrenia between schizophrenia patients treated using drugs with a higher affinity to H1 or α1 receptors than to D2 receptors (HA high-affinity drug group) and those treated using drugs with lower affinity (HA low-affinity drug group) using a Japanese employment-based health insurance database. Our analysis revealed that the HR of the rehospitalization risk due to schizophrenia in the HA high-affinity drug group compared with that in the HA low-affinity drug group was 1.018 (95% CI, 0.822–1.260), and there was no significant difference (P = 0.870). There was also no significant difference between the two groups in any of the secondary outcomes (involuntary rehospitalization, concomitant use of anxiolytics/hypnotics, mood stabilizers, and antiparkinsonian drugs, all-cause death, and medication discontinuation).
As the database is composed of employer-based health insurance data, it contains insurance claims data of the employers and their families, but not those of people who have left their jobs. Therefore, the population may be biased to schizophrenia patients who can maintain their jobs. This requirement raises concerns about the external validity of the analysis population. However, according to the national database open data disclosed by the Ministry of Health, Labour and Welfare,26 males are estimated to account for 43.0% of patients discharged annually from psychiatric wards (substituted by the number of psychiatric discharge guidance fees), and the value was similar to the percentage of males among the eligible patients and PS-matched patients in this analysis. As approximately 90% of the PS-matched patients were administered monotherapy prescriptions of antipsychotics in the month after the index month, ie, the month after the earliest hospitalization due to schizophrenia, patients with mild schizophrenia and a low rehospitalization risk may have been included in the analysis. Actually in this analysis, the percentage of patients hospitalized due to schizophrenia in psychiatric wards within 1 year was approximately 15% and it was lower comparing to the results reported by Uchiyama et al and Shimada et al.2,3
In this analysis, there was no difference in the risk of rehospitalization due to schizophrenia between the HA high- and HA low-affinity drug groups. One of the possible reasons is that as more than 80% of the patients were administered anxiolytics/hypnotics with sedative effects at the time of admission, the rehospitalization risk due to schizophrenia ascribed to the difference in the strength of the sedative effects between the two drug groups may have been masked. However, on stratified analysis according to the presence of the administration of anxiolytics/hypnotics, the HR (up to 12 months) of the rehospitalization risk due to schizophrenia in the HA high-affinity drug group compared with the HA low-affinity drug group was 0.944 (0.717–1.243) and 1.553 (0.784–3.074) in the groups with and without anxiolytic/hypnotic administration, respectively, demonstrating no significant difference (P = 0.681, P = 0.207, respectively). Due to the insufficient number of patients, no conclusion can be reached, and uncontrolled confounding such as duration of untreated illness and presence of tardive dyskinesia that could not be defined in the claims data used in the analysis may affect the analysis results. These are considered to be limitations of evaluation by retrospective PS-matched cohort studies, and future evaluation by well-designed prospective comparative studies is awaited. Due to concerns over the design of this study from a clinical viewpoint that the possibility of inclusion of patients administered antipsychotics not for the treatment of schizophrenia among those eligible cannot be excluded, similar analysis was carried out by eliminating patients for whom the chlorpromazine equivalent was <200 in the index month from the HA high- and HA low-affinity drug groups, but the results did not differ (HR; 0.973 [0.710–1.333], P = 0.866 [up to 12 months]). Furthermore, when the definition of rehospitalization due to schizophrenia was not limited to in psychiatric wards only, the HR (up to 12 months and maximum assessment period) of the rehospitalization risk due to schizophrenia in the HA high-affinity drug group compared with the HA low-affinity drug group was 1.009 (0.793–1.284) and 0.991 (0.808–1.215), respectively, demonstrating no significant difference (P = 0.943, P = 0.931, respectively). In the case-control study, which was the secondary objective, “prescription of HA high-affinity drugs” and “anxiolytics/hypnotics were used” during the 3 months before the evaluation point was found to be a significant factor for an increase in the rehospitalization risk due to schizophrenia (OR; 1.793, 2.115, respectively, P < 0.0001). Although these results were not shown in the analysis of the primary objective, it was suggested that prescription of HA high-affinity drugs and anxiolytics/hypnotics use might be one of the factors enhancing the rehospitalization risk due to schizophrenia.
Conclusion
In this claims data based retrospective cohort study, no significant difference in the rehospitalization risk due to schizophrenia associated with the use of HA high-affinity antipsychotics was observed.
Abbreviations
ATC, Anatomical Therapeutic Chemical; CI, confidence interval; ESS, Epworth sleepiness scale; HA, histamine H1 receptors, adrenaline α1 receptors; HR, hazard ratio; ICD, International Classification of Disease; OR, odds ratio; PS, propensity score; RCT, randomized controlled trial.
Acknowledgments
The authors would like to thank Hidetoshi Shibahara, an employee of CRECON Medical Assessment Inc, for his support on the data analysis under the direction of the authors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Sumitomo Dainippon Pharma Co, Ltd.
Disclosure
YT has received grant funding from Japan Society for the Promotion of Science, and speaker’s honoraria from Meiji-Seika Pharma, Sumitomo Dainippon Pharma, Janssen Pharmaceutical, Otsuka, Eisai, MSD K.K. Daiichi-Sankyo, Pfizer, UCB Japan, Novartis and Ono Pharmaceutical. KB and TN are employees of Sumitomo Dainippon Pharma Co, Ltd. SI is an employee of CRECON Medical Assessment. CRECON Medical Assessment Inc. was paid by Sumitomo Dainippon Pharma Co, Ltd to conduct analyses for the study. The authors report no other conflicts of interest in this work.
References
1. Ministry of Health, Labour and Welfare [homepage on the Internet]. [Websites on mental health, illness, support and services. Schizophrenia]. Available from: http://www.mhlw.go.jp/kokoro/speciality/detail_into.html.
2. Uchiyama N, Ikeno T, Kurihara T, et al. Predictive factors of readmission in patients with schizophrenia: a nationwide retrospective cohort study. Clin Psychiatry. 2012;54:1201–1207.
3. Shimada T, Nishi A, Yoshida T, Tanaka S, Kobayashi M. Factors Influencing Rehospitalisation of Patients with Schizophrenia in Japan: A 1-year Longitudinal Study. Hong Kong J Occup Ther. 2016;28(1):7–14. doi:10.1016/j.hkjot.2016.10.002
4. Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886–891. doi:10.1176/appi.ps.55.8.886
5. Greene M, Yan T, Chang E, Hartry A, Touya M, Broder MS. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ. 2018;21(2):127–134. doi:10.1080/13696998.2017.1379412
6. Velligan DI, Sajatovic M, Hatch A, Kramata P, Docherty JP. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449–468. doi:10.2147/PPA.S124658
7. Hatano M, Kamei H, Kato A, et al. Assessment of the Latent Adverse Events of Antipsychotic Treatment Using a Subjective Questionnaire in Japanese Patients with Schizophrenia. Clin Psychopharmacol Neurosci. 2017;15(2):132–137. doi:10.9758/cpn.2017.15.2.132
8. Afonso P, Brissos S, Cañas F, Bobes J, Bernardo-Fernandez I. Treatment adherence and quality of sleep in schizophrenia outpatients. Int J Psychiatry Clin Pract. 2014;18(1):70–76. doi:10.3109/13651501.2013.845219
9. Berardis DD, Rapini G, Olivieri L, et al. Safety of antipsychotics for the treatment of schizophrenia: a focus on the adverse effects of clozapine. Therapeutic Advances in Drug Safety. 2018;9(5):237–256. doi:10.1177/2042098618756261
10. Orsolinia L, Tomasetti C, Valchera A, et al. An update of safety of clinically used atypical antipsychotics. Expert Opin Drug Saf. 2016;15(10):1329–1347. doi:10.1080/14740338.2016.1201475
11. Orsolinia L, Berardis DD, Volpe U. Up-to-date expert opinion on the safety of recently developed antipsychotics. Expert Opin Drug Saf. 2020;19(8):981–997. doi:10.1080/14740338.2020.1795126
12. Loebel A, Cucchiaro J, Xu J, Sarma K, Pikalov A, Kane JM. Effectiveness of lurasidone vs. quetiapine XR for relapse prevention in schizophrenia: a 12-month, double-blind, noninferiority study. Schizophr Res. 2013;147(1):95–102. doi:10.1016/j.schres.2013.03.013
13. Loebel A, Cucchiaro J, Sarma K, et al. Efficacy and safety of lurasidone 80mg/day and 160mg/day in the treatment of schizophrenia: A randomized, double-blind, placebo- and active-controlled trial. Schizophr Res. 2013;145(1–3):101–109. doi:10.1016/j.schres.2013.01.009
14. Sunovion [homepage on the Internet]. Lurasidone HCl - A Long Term Phase 3 Study of Patients With Chronic Schizophrenia (PEARL 3 Ext); 2012. Available from: https://clinicaltrials.gov/ct2/show/NCT00789698.
15. Correll CU. From receptor pharmacology to improved outcomes: individualising the selection, dosing, and switching of antipsychotics. Eur Psychiatry. 2010;25(Suppl S2):S12–S21. doi:10.1016/S0924-9338(10)71701-6
16. Murasaki M, Nishikawa H, Ishibashi T. [Dopamine-serotonin antagonist: receptor binding profile of a novel antipsychotic blonanserin]. Jpn J Clin Psychopharmacol. 2008;11:845–854.
17. Gray JA, Roth BL. The pipeline and future of drug development in schizophrenia. Mol Psychiatry. 2007;12(10):904–922.
18. JMDC Inc. [homepage on the Internet]. Available from: https://www.jmdc.co.jp/.
19. Limosin F, Belhadi D, Comet D, et al. Comparison of Paliperidone Palmitate and Risperidone Long-Acting Injection in Schizophrenic Patients: results From a Multicenter Retrospective Cohort Study in France. J Clin Psychopharmacol. 2018;38(1):19–26. doi:10.1097/JCP.0000000000000827
20. Lafeuille M-H, Laliberté-Auger F, Lefebvre P, Frois C, Fastenau J, Duh MS. Impact of atypical long-acting injectable versus oral antipsychotics on rehospitalization rates and emergency room visits among relapsed schizophrenia patients: a retrospective database analysis. BMC Psychiatry. 2013;13(1):221. doi:10.1186/1471-244X-13-221
21. Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative Effectiveness of Clozapine and Standard Antipsychotic Treatment in Adults With Schizophrenia. Am J Psychiatry. 2016;173(2):166–173. doi:10.1176/appi.ajp.2015.15030332
22. Lafeuille M-H, Grittner AM, Fortier J, et al. Comparison of rehospitalization rates and associated costs among patients with schizophrenia receiving paliperidone palmitate or oral antipsychotics. Am J Health Syst Pharm. 2015;72(5):378–389. doi:10.2146/ajhp140219
23. Pilon D, Amos TB, Germain G, Lafeuille M-H, Lefebvre P, Benson CJ. Treatment persistence and hospitalization rates among patients with schizophrenia: a quasi-experiment to evaluate a patient information program. Curr Med Res Opin. 2017;33(4):713–721. doi:10.1080/03007995.2016.1277989
24. MacEwan JP, Kamat SA, Duffy RA, et al. Hospital Readmission Rates Among Patients With Schizophrenia Treated With Long-Acting Injectables or Oral Antipsychotics. Psychiatr Serv. 2016;67(11):1183–1188. doi:10.1176/appi.ps.201500455
25. Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128–138. doi:10.1097/EDE.0b013e3181c30fb2
26. Ministry of Health, Labour and Welfare [homepage on the Internet]. [4th NDB Open Data Japan]. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000177221_00003.html.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
