Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Regional Ictal Hyperperfusion in the Contralateral Occipital Area May Be a Poor Prognostic Marker of Anterior Temporal Lobectomy: A SISCOM Analysis of MTLE Cases
Authors Hwang Y , Lee HR, Jo H , Kim D , Joo EY, Seo DW, Hong SB, Shon YM
Received 28 April 2021
Accepted for publication 7 July 2021
Published 22 July 2021 Volume 2021:17 Pages 2421—2427
DOI https://doi.org/10.2147/NDT.S317915
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Yoonha Hwang,1 Hwa Reung Lee,2 Hyunjin Jo,3,4 Dongyeop Kim,3,4 Eun Yeon Joo,3,4 Dae-Won Seo,3,4 Seung Bong Hong,3,4 Young-Min Shon3– 5
1Department of Neurology, The Catholic University of Korea Eunpyeong St. Mary’s Hospital, Seoul, Republic of Korea; 2Department of Neurology, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea; 3Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea; 4Neuroscience Center, Samsung Medical Center, Seoul, Republic of Korea; 5Department of Medical Device Management and Research, Samsung Advanced Institute for Health Sciences & Technology (SAHIST), Sungkyunkwan University, Seoul, Korea
Correspondence: Young-Min Shon
Department of Neurology, Samsung Medical Center, Sungkyunkwan, University School of Medicine, 81 Irwonro, Gangnam-gu, Seoul, 06351, Republic of Korea
Tel +82-2-3410-2701
Fax +82-2-3410-0052
Email [email protected]
Background and Objective: Subtraction of ictal SPECT coregistered to MRI (SISCOM) provides complementary information for detecting the ictal onset zone, especially in patients with MRI-negative focal epilepsy, and provides additional useful information for predicting long-term postresection outcomes. This study sought to investigate the relationship between surgical failure and increased cerebral blood flow (CBF) pattern using SPECT in patients with mesial temporal lobe epilepsy with unilateral hippocampal sclerosis (MTLE-HS).
Methods: Among 42 subjects who underwent anterior temporal lobectomy with amygdalohippocampectomy (ATL-AH) for MTLE-HS, 29 (69.0%) were seizure-free (SF group). Hyperperfusion was compared in 14 ipsilateral and contralateral brain regions in SISCOM images between the two groups.
Results: The pattern of ictal hyperperfusion in temporal regions did not vary significantly between the SF and non-seizure-free (NSF) groups. However, CBF increases in the contralateral occipital area was more frequent in the NSF group than in the SF group. Furthermore, ictal hyperperfusion of the ipsilateral occipital and contralateral parietal areas tended to be more frequent in the NSF group.
Conclusion: The results indicate that poor ATL-AH surgical outcome is associated with a tendency of ictal hyperperfusion of the contralateral occipital cortex based on SISCOM analysis. The pattern of early ictal CBF changes implicating the propagation from temporal to occipital cortices can be considered a marker of poor surgical outcomes of ATL-AH in MTLE-HS patients.
Keywords: temporal lobe epilepsy, ictal hyperperfusion, SISCOM, contralateral occipital
Introduction
Epilepsy surgery is an effective treatment for medically intractable epilepsy, especially in patients with mesial temporal lobe epilepsy (MTLE). Anterior temporal lobectomy (ATL) with amygdalohippocampectomy (AH) is commonly associated with favorable outcomes in unilateral hippocampal sclerosis (HS) on MRI.1 Nevertheless, the complete seizure freedom (Engel’s class IA) rates after ATL with AH in TLE with unilateral HS patients were 85%, 77%, 74%, and 66% at 1, 2, 5, and 10 years, respectively.2
Ictal single-photon emission computed tomography (SPECT) is an effective evaluation tool to assess focal increase in cerebral blood flow (CBF) associated with an ictal increase in neuronal metabolic activity.3 Subtraction of ictal and interictal SPECT that are co-registered on MRI (SISCOM) is an established image processing technique that improves the sensitivity and specificity of seizure localization and facilitates effective presurgical evaluation of intractable focal epilepsy.4–7 Several studies have analyzed the relationship between SISCOM and postsurgical seizure outcomes, and better results were noted when the SISCOM localization was concordant with the region of surgical resection or conventional diagnostic techniques, such as intracranial EEG.8–11 A recent meta-analysis of the association between SISCOM localization and postoperative outcomes concluded that SISCOM had moderate sensitivity for localizing the epileptogenic zone and provided complementary information when the MRI was negative.12 However, the characteristics of SISCOM findings in patients with failed epilepsy surgery have not been specifically evaluated.
In the present study, the characteristic ictal hyperperfusion patterns of surgery-failed patients were analyzed using SISCOM to differentiate between seizure-free (SF) and non-seizure-free (NSF) patients after ATL with AH.
Methods
Subjects
This study retrospectively reviewed 42 patients who underwent ATL and total AH for MTLE that was associated with unilateral HS between January 2010 and December 2016, at Samsung Medical Center. All patients had undergone extensive presurgical evaluation with ictal and interictal SPECT imaging. Epilepsy classification was determined based on a detailed clinical history, results of video-EEG monitoring with the Extended International 10–10 system for scalp electrodes and neuroimaging studies that included MRI, PET, and SPECT images. All seizure characteristics were compatible with mesial temporal lobe onset, such as behavioral arrest, staring, and oro-alimentary automatisms, which are frequently preceded by psychic or abdominal auras. MRI was performed using a GE Signa 1.5-Tesla scanner (GE Medical Systems, Inc., Milwaukee, WI, USA) or a 3.0-Tesla scanner (Philips, Best, Netherlands). Loss of internal structure, decreased volume, and increased signals in T2-weighted and fluid-attenuated inversion recovery (FLAIR) MRI scans were the primary supporting diagnostic images for HS. MRI diagnosis of HS was proven in all patients by postoperative histopathological examination.
Surgery and Follow-Up
Surgery and determination of the type of operation were performed independently based on discussion at a multidisciplinary patient management conference. Postoperative seizure outcome was assessed using outpatient clinic interviews or phone interviews. All patients underwent at least 2 years of postoperative follow-up. Patients were assigned to either the seizure-free group (Engel’s IA class, SF group) or non-seizure-free group (not in Engel’s IA class, NSF group) based on the outcome at the most recent follow-up.13 The study was conducted with approval from the Institutional Review Board of Samsung Medical Center, Sungkyunkwan University of Korea, according to the Declaration of Helsinki. Informed written consent was obtained from all patients or from their family members.
Interictal and Ictal SPECT Studies
Brain SPECT scans were performed 30–60 min after injection of 25 mCi 99mTc ethyl cysteinate dimer (ECD) using a three-headed Triad XLT system (Trionix Research Laboratory, Inc., Twinsburg, OH, USA). Interictal SPECT imaging was performed when the patients had no documented seizure activity for more than 24 h. For ictal studies, patients were injected with a radiotracer during seizures. The time of radiotracer injection was defined as the moment when the syringe plunger was fully depressed. Injection times were determined by reviewing a video containing this information. The SPECT injection time (time interval between EEG seizure onset and radiotracer injection) and injection time index (injection time divided by total seizure duration) were measured for all subjects. Subsequently, subjects with an injection time index on ictal SPECT > 0.50 were excluded from the analysis.
Subtraction ictal SPECT coregistered to MRI analysis was performed on an offline workstation using ANALYZE 10.0 (Biomedical Imaging Resource, Mayo Foundation, Rochester, MN, USA).
Interpretation of Subtracted SPECT
The area of ictal hyperperfusion of subtracted SPECT was considered significant only when the regional cerebral blood flow (rCBF) difference was > +2.0 SD in each pixel of the brain SPECT image between ictal and interictal states. We analyzed the presence of ictal hyperperfusion in 14 anatomic regions, based on the process in our previous study:7 temporal regions of the anterior, mesial, lateral, and temporal stem; frontal regions of dorsolateral, orbitofrontal, and mesial; insular cortex; thalamus; basal ganglia; cingulate gyrus; parietal area; occipital area; and brainstem. The location of significant ictal hyperperfusion was visually determined independently by two neurologists (YH Hwang and YM Shon), who were blinded to patient information.
Statistical Analyses
Statistical analyses were performed with a statistical package for a commercially available software package (PASW version 18.0; SPSS Inc., Chicago, IL, USA). Descriptive statistics were utilized to characterize the study population. Independent sample t-tests or Mann–Whitney U-tests and the χ2 test, when appropriate, were used to compare differences and frequencies of baseline characteristics between groups. The comparison of hyperperfused areas on SISCOM images was analyzed using Fisher’s exact test. To determine the regional CBF difference between the two groups, a p-value < 0.0018 in the multiple comparison using Bonferroni correction was required for statistical significance. In other tests, p-values < 0.05 were considered statistically significant.
Results
Demographics and Clinical Characteristics
Among 42 patients, 29 had SF outcomes (Engel’s IA class) and 13 subjects did not (Engel’s 2B to 4B class). The demographic and clinical characteristics of the SF and NSF groups are summarized in Table 1 and were similar between the two groups, including seizure onset age, surgery age, sex, duration of pre-operation, other temporal lesions, and history of head trauma or CNS infection. Furthermore, ictal and interictal bilateral EEG proportions were not different between the two groups. The only difference between the two groups was SPECT injection time index; the NSF group received the radiotracer injection earlier than did the SF group (p < 0.05).
 |
Table 1 Clinical Characteristics of Subjects with SF and NSF in MTLE with HS |
SISCOM Images Analysis
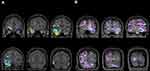
Ictal hyperperfusion in 14 ipsilateral and contralateral brain regions was evaluated and compared between the two groups using SISCOM images (Table 2). Noticeably, mesial temporal hyperperfusion was observed in almost all patients, greater than 92% in both the SF and NSF groups. The CBF increases in the ipsilateral and contralateral mesial, lateral temporal, and temporal stem areas were not significantly different; however, the contralateral occipital areas were significantly more common in the NSF group than in the SF group (61.5% vs 6.9%, p = 0.0001, Figure 1 and Table 2). Although ictal hyperperfusion of the ipsilateral occipital and contralateral parietal areas tended to be more frequent in the NSF group than in the SF group (10.3% vs 46.2%, p = 0.038; 37.9% vs 76.9%, p = 0.043, respectively), this difference was not observed after the Bonferroni correction. The ictal CBF changes in other regions of the medial frontal, dorsolateral frontal, orbitofrontal, insula, cingulum, basal ganglia, thalamus, parietal, and brain stem areas were not significantly different between the two groups.
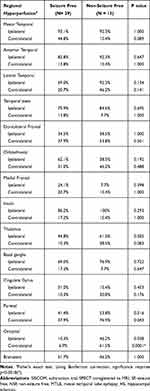 |
Table 2 Hyperperfusion Areas in SISCOM Image of Subjects with SF and NSF in MTLE with HS |
Discussion
For several decades, it has been acknowledged that perfusion changes observed during SPECT seizures are closely related to the underlying electrophysiological changes observed during semiologically matched EEG seizures.5–8,10,11,14 Based on the assumption that the nuclear tracer requires 15 to 20 seconds of transit time after intravenous injection to reach the brain,15 the peak tracer uptake interval was defined as a time period of several seconds starting 15 seconds after the patient’s individual SPECT injection.14 Therefore, the ictal hyperperfusion pattern might reflect an average of the distribution of blood flow around peak time rather than just a snapshot at the moment the blood flow reaches the corresponding brain regions.
In the present study, ictal regional hyperperfusion in the contralateral occipital areas was associated with poor surgical outcomes in patients with MTLE with unilateral HS based on SISCOM analysis. Furthermore, the NSF group showed a tendency for hyperperfusion at the ipsilateral occipital and contralateral parietal cortices. Only a few meta-analyses have investigated SISCOM and related surgical outcomes, and the studies only included patients with extra-TLE that were compared with a small number of patients12 or non-lesional TLE cases with inhomogeneous clinical features.9 To the best of our knowledge, this is the first study in which SISCOM images were used to compare surgical outcomes in homogeneous MTLE-HS patients.
Two findings support our hypothesis that the ictal hyperperfusion pattern indicates the importance of a connection between the mesial temporal ictal onset zone and its contralateral occipital lobe. First, structural neuroimaging studies have shown substantial evidence that TLE-HS is widespread and impacts both gray16,17 and white matter (WM) abnormalities18–21 that extend beyond the epileptogenic zone. Moreover, recent graph theoretical studies based on diffusion tensor imaging (DTI) suggested that epileptogenic networks can be disrupted over inferior fronto-occipital fasciculus or thalamo-occipital connections in patients with TLE-HS.22–24 The underlying mechanism is unknown, but research reports reporting that robust and recurrent focal seizures can cause widespread chronic vasogenic edema that can cause progressive WM injury in tracts that radiate from the seizure focus in occipital areas.25–27 Second, diffuse or contralateral propagation of ictal EEG has been associated with poor surgical outcomes in MTLE-HS patients.28–30 Ictal electrographic seizure propagation from the occipital to temporal lobes has been described in previous studies,31,32 and the reverse direction of spread has also been reported in a thorough electrode study.33 In addition, in cases with EEG seizure pattern that propagates to the contralateral side, the outcomes were worse when the propagation time was < 10 s.28 These findings contribute to the understanding that the ictal CBF pattern of early propagation to the contralateral side, especially the occipital and parietal areas, is an indirect sign of poor surgical outcomes.
Prognostic markers of surgical outcomes in MTLE have varied from study to study and have included unilateral interictal epileptiform discharges, epilepsy duration, age at surgery, hand dystonic posturing, and presence of focal to bilateral tonic-clonic seizure (FBTCS).34–36 MTLE patients with FBTCS showed extended hypometabolism in FDG-PET compared with subjects without FBTCS,37 indicating that FBTCS affects surgical outcomes through the diffuse epileptogenic zone or secondary epileptogenesis toward the contralateral side. However, a significant difference in the history of FBTCS was not noted for the NSF and SF groups in this study. Moreover, because all the ictal injections in our patients were performed during the first half of the seizure (injection time index < 0.50), we can reasonably assume that the effect of diffuse, widespread hyperperfusion from FBTCS, which typically occurs late in the course of mesial temporal seizures, could be minimized considerably.
There were some limitations to the present study. First, the relatively small size of the study population and the difference in size between groups (13 vs 29 patients) limit the generalization of the results. Second, variability in radioisotope injection time was a possible limitation. Regional ictal CBF patterns are strongly affected by the timing of radioisotope injection in relation to seizure onset and duration. However, potential increases in the topographic variability of ictal perfusion caused by delayed injection time could not be excluded.7 Furthermore, there is no established standard for appropriate injection time for TLE in SPECT.
In conclusion, based on SISCOM analysis, poor ATL-AH surgical outcome in MTLE-unilateral HS patients was associated with ictal hyperperfusion of the contralateral occipital cortex. The rapid CBF propagation toward occipital cortices during ictal onset might play an important role in poor surgical outcomes in MTLE. The presence of these SISCOM findings may be used as a prognostic indicator of poor postoperative seizure outcomes and warrants additional consideration before proceeding to surgery. Further investigations with a larger study population and concurrent analysis of dynamic EEG changes could elucidate the detailed relationship between temporal-occipital connection and postoperative seizure outcomes in MTLE patients.
Funding
This work was supported by the Samsung Medical Center Grant (#SMX1200251), National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2018R1D1A1B07048147).
Disclosure
None of the authors have any conflicts of interest to disclose.
References
1. Radhakrishnan K, So EL, Silbert PL, et al. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: a multivariate study. Neurology. 1998;51(2):465–471. doi:10.1212/wnl.51.2.465
2. Paglioli E, Palmini A, Paglioli E, et al. Survival analysis of the surgical outcome of temporal lobe epilepsy due to hippocampal sclerosis. Epilepsia. 2004;45(11):1383–1391. doi:10.1111/j.0013-9580.2004.22204.x
3. Schwartz TH, Bonhoeffer T. In vivo optical mapping of epileptic foci and surround inhibition in ferret cerebral cortex. Nat Med. 2001;7(9):1063–1067. doi:10.1038/nm0901-1063
4. Kaiboriboon K, Lowe VJ, Chantarujikapong SI, Hogan RE. The usefulness of subtraction ictal SPECT coregistered to MRI in single- and dual-headed SPECT cameras in partial epilepsy. Epilepsia. 2002;43(4):408–414. doi:10.1046/j.1528-1157.2002.21201.x
5. Kaminska A, Chiron C, Ville D, et al. Ictal SPECT in children with epilepsy: comparison with intracranial EEG and relation to postsurgical outcome. Brain. 2003;126(Pt 1):248–260. doi:10.1093/brain/awg013
6. O’Brien TJ, O’Connor MK, Mullan BP, et al. Subtraction ictal SPET co-registered to MRI in partial epilepsy: description and technical validation of the method with phantom and patient studies. Nucl Med Commun. 1998;19(1):31–45. doi:10.1097/00006231-199801000-00006
7. Park HR, Seong MJ, Shon YM, Joo EY, Seo DW, Hong SB. SPECT perfusion changes during ictal automatisms with preserved responsiveness in patients with right temporal lobe epilepsy. Epilepsy Behav. 2018;80:11–14. doi:10.1016/j.yebeh.2017.12.030
8. Ahnlide JA, Rosen I, Linden-Mickelsson Tech P, Kallen K. Does SISCOM contribute to favorable seizure outcome after epilepsy surgery? Epilepsia. 2007;48(3):579–588. doi:10.1111/j.1528-1167.2007.00998.x
9. Bell ML, Rao S, So EL, et al. Epilepsy surgery outcomes in temporal lobe epilepsy with a normal MRI. Epilepsia. 2009;50(9):2053–2060. doi:10.1111/j.1528-1167.2009.02079.x
10. Kudr M, Krsek P, Marusic P, et al. SISCOM and FDG-PET in patients with non-lesional extratemporal epilepsy: correlation with intracranial EEG, histology, and seizure outcome. Epileptic Disord. 2013;15(1):3–13. doi:10.1684/epd.2013.0560
11. O’Brien TJ, So EL, Mullan BP, et al. Subtraction peri-ictal SPECT is predictive of extratemporal epilepsy surgery outcome. Neurology. 2000;55(11):1668–1677. doi:10.1212/wnl.55.11.1668
12. Chen T, Guo L. The role of SISCOM in preoperative evaluation for patients with epilepsy surgery: a meta-analysis. Seizure. 2016;41:43–50. doi:10.1016/j.seizure.2016.06.024
13. Engel J
14. Krishnan B, Tousseyn S, Nayak CS, et al. Neurovascular networks in epilepsy: correlating ictal blood perfusion with intracranial electrophysiology. Neuroimage. 2021;231:117838. doi:10.1016/j.neuroimage.2021.117838
15. Bartolini A. Regional arm-brain mean transit time in the diagnostic evaluation of patients with cerebral vascular disease. Stroke. 1981;12(2):241–245. doi:10.1161/01.str.12.2.241
16. Coan AC, Campos BM, Yasuda CL, et al. Frequent seizures are associated with a network of gray matter atrophy in temporal lobe epilepsy with or without hippocampal sclerosis. PLoS One. 2014;9(1):e85843. doi:10.1371/journal.pone.0085843
17. Mueller SG, Laxer KD, Cashdollar N, Buckley S, Paul C, Weiner MW. Voxel-based optimized morphometry (VBM) of gray and white matter in temporal lobe epilepsy (TLE) with and without mesial temporal sclerosis. Epilepsia. 2006;47(5):900–907. doi:10.1111/j.1528-1167.2006.00512.x
18. Bernasconi N, Duchesne S, Janke A, Lerch J, Collins DL, Bernasconi A. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. Neuroimage. 2004;23(2):717–723. doi:10.1016/j.neuroimage.2004.06.015
19. Campos BM, Coan AC, Beltramini GC, et al. White matter abnormalities associate with type and localization of focal epileptogenic lesions. Epilepsia. 2015;56(1):125–132. doi:10.1111/epi.12871
20. Yasuda CL, Valise C, Saude AV, et al. Dynamic changes in white and gray matter volume are associated with outcome of surgical treatment in temporal lobe epilepsy. Neuroimage. 2010;49(1):71–79. doi:10.1016/j.neuroimage.2009.08.014
21. Keller SS, Ahrens T, Mohammadi S, et al. Voxel-based statistical analysis of fractional anisotropy and mean diffusivity in patients with unilateral temporal lobe epilepsy of unknown cause. J Neuroimaging. 2013;23(3):352–359. doi:10.1111/j.1552-6569.2011.00673.x
22. Gonzalez HFJ, Chakravorti S, Goodale SE, et al. Thalamic arousal network disturbances in temporal lobe epilepsy and improvement after surgery. J Neurol Neurosurg Psychiatry. 2019;90(10):1109–1116. doi:10.1136/jnnp-2019-320748
23. Lin H, Leng X, Qin C, Wang W, Zhang C, Qiu S. Altered white matter structural network in frontal and temporal lobe epilepsy: a Graph-Theoretical Study. Front Neurol. 2020;11:561. doi:10.3389/fneur.2020.00561
24. Bernhardt BC, Chen Z, He Y, Evans AC, Bernasconi N. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb Cortex. 2011;21(9):2147–2157. doi:10.1093/cercor/bhq291
25. Holtkamp M, Schuchmann S, Gottschalk S, Meierkord H. Recurrent seizures do not cause hippocampal damage. J Neurol. 2004;251(4):458–463. doi:10.1007/s00415-004-0356-9
26. Liu RS, Lemieux L, Bell GS, et al. Cerebral damage in epilepsy: a population-based longitudinal quantitative MRI study. Epilepsia. 2005;46(9):1482–1494. doi:10.1111/j.1528-1167.2005.51603.x
27. Vaughan DN, Raffelt D, Curwood E, et al. Tract-specific atrophy in focal epilepsy: disease, genetics, or seizures? Ann Neurol. 2017;81(2):240–250. doi:10.1002/ana.24848
28. Wieser HG, Siegel AM. Analysis of foramen ovale electrode-recorded seizures and correlation with outcome following amygdalohippocampectomy. Epilepsia. 1991;32(6):838–850. doi:10.1111/j.1528-1157.1991.tb05540.x
29. Spencer SS, Spencer DD. Implications of seizure termination location in temporal lobe epilepsy. Epilepsia. 1996;37(5):455–458. doi:10.1111/j.1528-1157.1996.tb00591.x
30. Schulz R, Luders HO, Hoppe M, Tuxhorn I, May T, Ebner A. Interictal EEG and ictal scalp EEG propagation are highly predictive of surgical outcome in mesial temporal lobe epilepsy. Epilepsia. 2000;41(5):564–570. doi:10.1111/j.1528-1157.2000.tb00210.x
31. Palmini A, Andermann F, Dubeau F, et al. Occipitotemporal epilepsies: evaluation of selected patients requiring depth electrodes studies and rationale for surgical approaches. Epilepsia. 1993;34(1):84–96. doi:10.1111/j.1528-1157.1993.tb02380.x
32. Williamson PD, Thadani VM, Darcey TM, Spencer DD, Spencer SS, Mattson RH. Occipital lobe epilepsy: clinical characteristics, seizure spread patterns, and results of surgery. Ann Neurol. 1992;31(1):3–13. doi:10.1002/ana.410310103
33. Jacobs J, Dubeau F, Olivier A, Andermann F. Pathways of seizure propagation from the temporal to the occipital lobe. Epileptic Disord. 2008;10(4):266–270. doi:10.1684/epd.2008.0217
34. Hennessy MJ, Elwes RD, Rabe-Hesketh S, Binnie CD, Polkey CE. Prognostic factors in the surgical treatment of medically intractable epilepsy associated with mesial temporal sclerosis. Acta Neurol Scand. 2001;103(6):344–350. doi:10.1034/j.1600-0404.2001.103006344.x
35. Janszky J, Janszky I, Schulz R, et al. Temporal lobe epilepsy with hippocampal sclerosis: predictors for long-term surgical outcome. Brain. 2005;128(Pt 2):395–404. doi:10.1093/brain/awh358
36. Aull-Watschinger S, Pataraia E, Czech T, Baumgartner C. Outcome predictors for surgical treatment of temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2008;49(8):1308–1316. doi:10.1111/j.1528-1167.2008.01732.x
37. Savic I, Altshuler L, Baxter L, Engel J
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.