Back to Journals » Infection and Drug Resistance » Volume 16
Refractory Pseudomonas aeruginosa Bronchopulmonary Infection After Lung Transplantation for Common Variable Immunodeficiency Despite Maximal Treatment Including IgM/IgA-Enriched Immunoglobulins and Bacteriophage Therapy
Authors Levêque M , Cassir N, Mathias F, Fevre C, Daviet F, Bermudez J, Brioude G, Peyron F, Reynaud-Gaubert M, Coiffard B
Received 23 March 2023
Accepted for publication 23 June 2023
Published 30 June 2023 Volume 2023:16 Pages 4265—4271
DOI https://doi.org/10.2147/IDR.S413900
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Manon Levêque,1 Nadim Cassir,2 Fanny Mathias,3 Cindy Fevre,4 Florence Daviet,5 Julien Bermudez,1 Geoffrey Brioude,6 Florence Peyron,3 Martine Reynaud-Gaubert,1 Benjamin Coiffard1
1Department of Respiratory Medicine and Lung Transplantation, APHM, Aix Marseille University, Hôpital Nord, Marseille, France; 2Department of Infectious Disease, APHM, IHU Méditerranée Infection, Aix-Marseille University, Marseille, France; 3Department of Pharmacy, APHM, Aix Marseille University, Hôpital Nord, Marseille, France; 4Research and Development, Pherecydes Pharma, Romainville, France; 5Intensive Care Medicine, APHM, Aix Marseille University, Hôpital Nord, Marseille, France; 6Department of Thoracic Surgery and Lung Transplantation, APHM, Aix Marseille University, Hôpital Nord, Marseille, France
Correspondence: Benjamin Coiffard, Department of Respiratory Medicine and Lung Transplantation, APHM, CHU Nord, Chemin des Bourrely, Marseille, 13915, France, Tel +33491966133, Email [email protected]
Abstract: Recipients transplanted for bronchiectasis in the context of a primary immune deficiency, such as common variable immunodeficiency, are at a high risk of severe infection in post-transplantation leading to poorer long-term outcomes than other transplant indications. In this report, we present a fatal case due to chronic Pseudomonas aeruginosa bronchopulmonary infection in a lung transplant recipient with common variable immunodeficiency despite successful eradication of an extensively drug-resistant (XDR) strain with IgM/IgA-enriched immunoglobulins and bacteriophage therapy. The fatal evolution despite a drastic adaptation of the immunosuppressive regimen and the maximal antibiotic therapy strategy raises the question of the contraindication of lung transplantation in such a context of primary immunodeficiency.
Keywords: primary immunodeficiency diseases, lung transplantation, Pseudomonas aeruginosa, phage therapy, immunoglobulin therapy
Introduction
Lung transplantation (LTx) for bronchiectasis in the context of a primary immune deficiency, such as common variable immunodeficiency (CIVD), are at a high risk of severe infection in post-transplantation leading to poorer long-term outcome than other transplant indications.1,2 Patients transplanted in the setting of bronchiectasis or in cystic fibrosis often have pre-transplant colonization with various infectious agents including Pseudomonas aeruginosa.3 The post-transplant maintenance immunosuppressive regimen increases the risk of serious respiratory infection, often necessitating the prescription of broad-spectrum antibiotic therapies.4 This condition favors the emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) pathogens.
The use of lytic bacteriophages, which are viruses that infect and lyse bacteria, seems to provide an effective option to fight resistant bacteria.5 Bacteriophages have in vitro activity against Pseudomonas aeruginosa6 and have already been used in personalized therapy with several examples of successful treatment of bronchopulmonary infection caused by MDR bacteria.7
In this report, we describe a case of a patient with CVID who underwent LTx and subsequently developed a chronic bronchopulmonary infection caused by XDR Pseudomonas aeruginosa. The patient was treated with IgM/IgA-enriched immunoglobulins and bacteriophage therapy.
Case Presentation
Patient Background
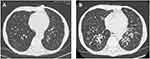
A 57-year-old man with history of double LTx in October 2019 (lung donated voluntarily with written informed consent, and that this was conducted in accordance with the Declaration of Istanbul) for bronchiectasis secondary to CVID. He was supplemented by subcutaneous immunoglobulin G and had a prior history of pan-sensitive Pseudomonas aeruginosa colonization. The immunosuppressive regimen included induction with basiliximab and maintenance with steroids, tacrolimus, and mycophenolate mofetil. After transplant, the patient rapidly developed severe recurrent bronchopulmonary infections due to Pseudomonas aeruginosa despite several repeated associative antibiotic therapies and continuous inhaled colistin (Figure 1, description of the clinical course and therapies). The patient’s respiratory function progressively deteriorated (obstructive pattern) leading to severe chronic respiratory failure requiring oxygen supplementation associated with recurrence of bronchiectasis on chest CT scan (Figure 1).
As a result of antibiotic pressure, an XDR strain emerged, which was only susceptible to colistin, cefiderocol, amikacin, and gentamicin. The use of aminoglycosides was challenging to manage due to side effects with multiple episodes of acute kidney injury that evolved to severe chronic kidney failure and hearing loss.
Immunosuppressive Regimen Management
Within the first-year post-transplant, steroids were rapidly tapered, azithromycin introduced (250 mg given once daily) as a treatment of chronic lung allograft dysfunction (CLAD), the mycophenolate mofetil stopped and never reintroduced to minimize the infection risk (antimetabolite agent), and cyclosporin A finally tapered to 10 mg per day resulting in an undetectable residual rate of blood cyclosporin A because the infection was not controlled despite the mycophenolate mofetil stopped. No acute rejection was found on the repeated lung biopsies. We enhanced the immunoglobulin supplementation with IgM/IgA-enriched immunoglobulins (Pentaglobin®, Biotest, Dreieich, Germany), Figure 2.
From that adaptation of the immunosuppressive regimen, we managed to obtain clinical stability (oxygen requirement decreased to 1 L/min, no sepsis, decreased sputum) but every attempt to stop the antibiotic treatment failed.
Bacteriophage Therapy
In this context of therapeutic impasse, a decolonization strategy with bacteriophage therapy (BT) has been considered. Pherecydes Pharma (Pherecydes Pharma, Romainville, France, https://www.pherecydes-pharma.com/en/) owns a collection of anti-Pseudomonas aeruginosa phages. In the scope of compassionate use and in agreement with the French health authorities, the strains isolated from the patient were sent to the company, which performed a phagogram (equivalent to an antibiogram but with phages). Among the phages produced in compliance with the Good Manufacturing Practices (GMP) and that were available as ready-to-use by the company, PP1450, PP1792, and PP1797 have shown activity on the patient’s strains (Figure 3). Multisite samples revealed the presence of the XDR Pseudomonas aeruginosa in the stool and the nose. For that reason, we associated inhaled phage therapy (2 inhalations a day using vibrating mesh, Aeroneb®, Aerogen, Galway, Ireland) and oral ingestion of phages 2 times a day for 7 days (Figure 4).
 |
Figure 4 Phage and antibiotic therapies to treat the Pseudomonas aeruginosa bronchopulmonary infection performed in September and October 2021. |
Treatment Preparation and Administration
The phages were supplied by Pherecydes Pharma as well as the stability data which allowed us to prepare the treatment for 2 days (4 administrations). Four mL of each phage at 10Log were collected and completed with 48 mL of a saline solution. From that mixture, nebulizations were performed with 8 mL and oral ingestion with 6 mL of the solution. A sterility test was performed with 1 mL of each prepared solution.
Additional Measures
Because phages survival is favor by basic condition, we treated the patient with double dose of proton pump inhibitor to maximize the efficacy. We had thought of a fecal transplantation to decolonize the digestive tract. The request was refused due to a shortage of stool available in the context of the COVID19 pandemic. In June 2021, the strain was susceptible to cefiderocol and colistin. Thus, we associated these antibiotics with the BT, Figure 4. Oral and nasal decolonization were performed with chlorhexidine 4%, 4 times a day for 14 days. Daily skin decolonization was performed with chlorhexidine 2% for 14 days. The patient was isolated in a single bedroom with contact and droplet precaution. Personal belongings have been taken out of the room and isolated (including clothes, computer, and mobile phone). Daily bio-cleaning with disinfectant were performed. Finally, the patient was moved to another room at the end of the BT.
Patient Outcome
During the BT, the patient’s respiratory conditions improved (less sputum production) allowing a weaning from oxygen supplementation. At the end of the first cycle of BT, XDR Pseudomonas aeruginosa was still identified in sputum and stool. We sent new samples to Pherecydes Pharma laboratory who confirmed that the strain was still susceptible to bacteriophages initially tested. As we were waiting for the second cycle of BT, the patient presented a new acute respiratory failure related to the worsening of the bronchopulmonary infection, despite no discontinuation of the antibiotic therapy, that required mechanical ventilation. The second cycle of BT was administrated through bronchoscopy and by nasogastric tube with the same protocol as described for the first cycle. The patient presented a severe obstructive pattern on the ventilator, and despite aggressive intensive care and protective ventilation, the patient progressed to multi-organ failure and died at 24 months post-LTx. For the first time, all samples collected (rectal smear, bronchial aspiration, and bronchoalveolar lavage) were interpreted as sterile from day 5 of the second cycle of BT (and confirmed the days after till the death).
In summary, we could observe first clinical stability (low oxygen supplementation and no more sepsis) after adapting the immunosuppressive regimen and mainly after initiating the IgM/IgA-enriched immunoglobulins, but without the possibility to stop the antibiotic treatment. And second, we could obtain sterilization of the different sites that were previously infected or colonized by Pseudomonas aeruginosa after the second cycle of BT, despite the fatal outcome.
Discussion
Here, we report a chronic Pseudomonas aeruginosa bronchopulmonary infection in a lung transplant recipient with CVID. We managed to obtain clinical stability after adapting the immunosuppressive regimen, especially by initiating IgM/IgA-enriched immunoglobulins, and we were able to sterilize the different sites of infection or colonization by Pseudomonas aeruginosa after BT. Unfortunately, the patient died despite these maximal therapies.
This case highlights the difficulty of identifying the best and optimal immunosuppressive regimen in such recipients with primary immunodeficiency (CVID).1,2 The recipient had a poor outcome mainly due to chronic refractory infection and raises the question of an overimmunosuppression. We tried first to manage the immunosuppressive regimen as classically performed in the context of refractory infection after LTx. Steroids were rapidly tapered, and the mycophenolate mofetil stopped and never reintroduced. It was however insufficient, and we almost stopped all immunosuppressive therapy by tapering the cyclosporin A to an inconsiderable dose despite the risk of rejection. Finally, we were not able to completely cure the pulmonary infection despite minimizing the immunosuppressive regimen and optimizing the supplementation with enriched IV immunoglobulins. Then, indication for lung transplantation for immune deficiencies therefore remains an unresolved question.
Despite the limited effect of adapting the immune regimen, we observed an improvement in our patient condition particularly when we increased the immunoglobulins supplementation with IgM and IgA enriched therapy. Patients with antibody deficiency are usually supplemented with IgG globulins only, allowing to decrease the risk of infection. However, those patients still mainly suffer from respiratory tract infections that may evolve into chronic sinusitis and bronchiectasis.8,9 IgA plays a role in the immune mucosal defense10 which may explain why patients are still at increased risk of respiratory infection despite IgG supplementation. In our case, we started the IgA-enriched therapy quite tardily after transplant with already the occurrence of a bronchial obstructive disease and the reappearance of diffuse bronchiectasis on the allograft. IgM-IgA-enriched immunoglobulins are mainly used in the context of severe sepsis11 and scarcely reported as the immune supplementation in hypogammaglobulinemic patients.12 In such patients, early lifelong supplementation with IgM-IgA-enriched immunoglobulins could perhaps limit the risk of infection and progression to bronchiectasis or respiratory failure and mitigate the need for LTx.13
In such a situation, there is a classical risk of rejection by decreasing the immunosuppressive regimen. In our case, we never documented acute rejection on repeated transbronchial lung biopsies. However, the recipient developed an obstructive pattern over time as other cases in the literature that have reported an evolution towards early CLAD in most of the transplanted patients for X-linked agammaglobulinemia.2 Indeed, both the decrease in the immunosuppressive regimen and infections are well-known risk factors of CLAD,14 suggesting an explanation of why our patient had early poor outcomes despite azithromycin.
BT has been used for a long time to treat drug-resistant infections, however, evidence-based medicine is still required despite plenty of case reports5,6,15 and a recent meta-analysis of small clinical trials demonstrating a significant efficacy.16 Some clinical cases of BT were reported in lung transplant recipients but with very heterogeneous clinical contexts, pathogens, and protocols.7,17 In our case, our first attempt was inconclusive despite an effective clinical improvement during the treatment period. The 7-day course of treatment was possibly insufficient, or the phage dose was too low (8 Log of Phage/mL were required to decrease the strain growth in vitro) as the patient worsened rapidly a few days after the end of the therapy. The method of administration is also questionable. We did not use intravenous administration due to an infection predominantly localized on the bronchi and sinus colonization with no significant systemic symptoms. One can consider that intravenous administration could be the method of choice, as antibiotics are usually administered in that way as the basic therapeutic strategy for pulmonary infection in clinical practice. Inhalations were performed using vibrating mesh according to previous reports,7,18 but the number of viable phages delivered in the lungs may be insufficient by this method. We changed to instillations through bronchoscopy and to an administration through the gastric tube for our second attempt of BT, as the patient was under mechanical ventilation, with success as all samples were sterile from day 5 of treatment. A more direct administration in the low respiratory tract and the digestive tube, with no damage related to the method of nebulization, could explain a better efficacy.
Conclusion
Here, we describe a fatal case favored by Pseudomonas aeruginosa bronchopulmonary infection in a lung transplant recipient with CVID despite successfully eradicating an XDR strain with BT. Due to chronic bronchopulmonary inflammation and decreased immunosuppression, the recipient likely developed CLAD which may account for the lethal outcome. This case also highlights multiple issues related to BT methods (timing, mode of administration, duration of treatment, etc.). The fatal outcome, despite maximal treatment, including IgM/IgA-enriched immunoglobulins and bacteriophage therapy, raises questions about an early immune supplementation in CVID and the indication of lung transplantation in such a context of primary immunodeficiency. Further research is needed to address these issues.
Abbreviations
BT, bacteriophage therapy; CVID, common variable immunodeficiency; LTx, lung transplantation; MDR, multidrug-resistant; XDR, extensively drug-resistant.
Data Sharing Statement
The data is available upon request.
Ethics and Consent
The patient was informed of the publication of this case report and signed a letter of consent. The present case did not require ethics committee approval based on the French ethical guidelines for clinical research to publish case details.
Author Contributions
All authors meet the ICMJE authorship criteria. All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors and co-workers did not receive any specific funding for this study.
Disclosure
Dr Cindy Fevre reports personal fees from Pherecydes Pharma, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Nathan JA, Sharples LD, Exley AR, Sivasothy P, Wallwork J. The outcomes of lung transplantation in patients with bronchiectasis and antibody deficiency. J Heart Lung Transplant. 2005;24(10):1517–1521. doi:10.1016/j.healun.2004.11.013
2. Barnes S, Kotecha S, Douglass JA, et al. Evolving practice: x-linked agammaglobulinemia and lung transplantation. Am J Transplant. 2015;15(4):1110–1113. doi:10.1111/ajt.13084
3. Lay C, Law N, Holm AM, Benden C, Aslam S. Outcomes in cystic fibrosis lung transplant recipients infected with organisms labeled as pan-resistant: an ISHLT registry‒based analysis. J Heart Lung Transplant. 2019;38(5):545–552. doi:10.1016/j.healun.2019.01.1306
4. Coiffard B, Prud’Homme E, Hraiech S, et al. Worldwide clinical practices in perioperative antibiotic therapy for lung transplantation. BMC Pulm Med. 2020;20(1):109. doi:10.1186/s12890-020-1151-9
5. Aranaga C, Pantoja LD, Martínez EA, Falco A. Phage therapy in the era of multidrug resistance in bacteria: a systematic review. Int J Mol Sci. 2022;23(9):4577. doi:10.3390/ijms23094577
6. Nordstrom HR, Evans DR, Finney AG, et al. Genomic characterization of lytic bacteriophages targeting genetically diverse Pseudomonas aeruginosa clinical isolates. iScience. 2022;25(6):104372. doi:10.1016/j.isci.2022.104372
7. Aslam S, Courtwright AM, Koval C, et al. Early clinical experience of bacteriophage therapy in 3 lung transplant recipients. Am J Transplant. 2019;19(9):2631–2639. doi:10.1111/ajt.15503
8. Syed MN, Kutac C, Miller JM, et al. Risk factors of Pneumonia in primary antibody deficiency patients receiving immunoglobulin therapy: data from the US Immunodeficiency Network (USIDNET). J Clin Immunol. 2022;42(7):1545–1552. doi:10.1007/s10875-022-01317-2
9. Huwyler C, Lin SY, Liang J. Primary immunodeficiency and rhinosinusitis. Immunol Allergy Clin North Am. 2020;40(2):233–249. doi:10.1016/j.iac.2019.12.003
10. Corthésy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol. 2013;4:185. doi:10.3389/fimmu.2013.00185
11. Nierhaus A, Berlot G, Kindgen-Milles D, Müller E, Girardis M. Best-practice IgM- and IgA-enriched immunoglobulin use in patients with sepsis. Ann Intensive Care. 2020;10(1):132. doi:10.1186/s13613-020-00740-1
12. Borleffs JCC, Schellekens JF, Brouwer E, Rozenberg-Arska M. Use of an immunoglobulin M containing preparation for treatment of two hypogammaglobulinemic patients with PersistentCampylobacter jejuni infection. Eur J Clin Microbiol Infect Dis. 1993;12(10):772–775. doi:10.1007/BF02098467
13. Langereis JD, van der Flier M, de Jonge MI. Limited innovations after more than 65 years of immunoglobulin replacement therapy: potential of IgA- and IgM-enriched formulations to prevent bacterial respiratory tract infections. Front Immunol. 2018;9:1925. doi:10.3389/fimmu.2018.01925
14. Parulekar AD, Kao CC. Detection, classification, and management of rejection after lung transplantation. J Thorac Dis. 2019;11(S14):S1732–S1739. doi:10.21037/jtd.2019.03.83
15. Maddocks S, Fabijan AP, Ho J, et al. Bacteriophage therapy of ventilator-associated Pneumonia and empyema caused by Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2019;200(9):1179–1181.
16. Marongiu L, Burkard M, Lauer UM, Hoelzle LE, Venturelli S. Reassessment of historical clinical trials supports the effectiveness of phage therapy. Clin Microbiol Rev. 2022;35(4):e0006222. doi:10.1128/cmr.00062-22
17. Lebeaux D, Merabishvili M, Caudron E, et al. A case of phage therapy against pandrug-resistant achromobacter xylosoxidans in a 12-year-old lung-transplanted cystic fibrosis patient. Viruses. 2021;13(1):60. doi:10.3390/v13010060
18. Guillon A, Pardessus J, L’Hostis G, et al. Inhaled bacteriophage therapy in a porcine model of pneumonia caused by Pseudomonas aeruginosa during mechanical ventilation. Br J Pharmacol. 2021;178(18):3829–3842. doi:10.1111/bph.15526
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.