Back to Journals » Cancer Management and Research » Volume 13
Referral for “Neoadjuvant Chemotherapy” for Muscle-Invasive Bladder Cancer to a Multidisciplinary Board: Patterns, Management and Outcomes
Authors Dellis A, Zakopoulou R , Kougioumtzopoulou A , Tzannis K, Koutsoukos K, Fragkoulis C, Kostouros E, Papatsoris A, Varkarakis I, Stravodimos K, Boutati E, Pagoni S, Seferlis M, Chrisofos M, Kouloulias V, Ntoumas K, Deliveliotis C, Constantinides C, Dimopoulos MA, Bamias A
Received 29 April 2021
Accepted for publication 17 June 2021
Published 30 July 2021 Volume 2021:13 Pages 5941—5955
DOI https://doi.org/10.2147/CMAR.S317500
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bilikere Dwarakanath
Athanasios Dellis,1 Roubini Zakopoulou,2 Andromahi Kougioumtzopoulou,3 Kimon Tzannis,2 Konstantinos Koutsoukos,2 Charalampos Fragkoulis,4 Efthymios Kostouros,5 Athanasios Papatsoris,6 Ioannis Varkarakis,6 Konstantinos Stravodimos,7 Eleni Boutati,8 Stamata Pagoni,5 Miltiadis Seferlis,9 Michael Chrisofos,10 Vasilios Kouloulias,3 Konstantinos Ntoumas,4 Charalambos Deliveliotis,6 Constantine Constantinides,7 Meletios A Dimopoulos,2 Aristotelis Bamias8
1 2nd Department of Surgery, National & Kapodistrian University of Athens, Aretaieion University Hospital, Athens, Greece; 2Oncology Unit, Department of Clinical Therapeutics, National & Kapodistrian University of Athens, Alexandra Hospital, Athens, Greece; 3Radiotherapy Unit, 2nd Department of Radiology, National & Kapodistrian University of Athens, Attikon University Hospital, Athens, Greece; 4Department of Urology, General Hospital of Athens “G. Gennimatas”, Athens, Greece; 5 3rd Department of Internal Medicine, General Hospital of Athens “G. Gennimatas”, Athens, Greece; 6 2nd Department of Urology, National & Kapodistrian University of Athens, Sismanoglio Hospital, Athens, Greece; 7First Department of Urology, National and Kapodistrian University of Athens, “Laiko” General Hospital, Athens, Greece; 8 2nd Propaedeutic Department of Internal Medicine, National & Kapodistrian University of Athens, Attikon University Hospital, Athens, Greece; 9Department of Urology, Thriasion General Hospital, Athens, Greece; 10 3rd Department of Urology, National & Kapodistrian University of Athens, Attikon University Hospital, Athens, Greece
Correspondence: Aristotelis Bamias
2nd Propaedeutic Department of Internal Medicine, National & Kapodistrian University of Athens, Attikon University Hospital, Athens, Greece
Tel +30 2105831256 Fax +30 210 5326454
Email [email protected]
Background: Utilization of neoadjuvant chemotherapy for the treatment of muscle invasive bladder cancer in everyday practice differs from that of clinical trials. We describe the patterns of referral for “neoadjuvant chemotherapy”, treatment and outcomes in a multidisciplinary tumor board.
Methods: This was an observational study. Patients referred for neoadjuvant chemotherapy received 4 cycles of dose-dense gemcitabine/cisplatin and were then assessed for definitive local therapy. Patients had a minimum follow-up of 2 years. Primary objective was a 3-year disease-free survival rate.
Results: Forty-six patients (clinical stages II: 28, IIIA: 9, IIIB: 4, IVA: 3, missing: 2) were included. Following chemotherapy, 30 underwent radical cystectomy, 8 radiotherapy and 8 no further therapy. Pathological downstaging was observed in 14 (46.6%) of the 30 patients who underwent radical cystectomy; clinical TNM staging was correlated with disease-free survival in the whole population, while clinical and pathological stages, as well as pathological downstaging, were correlated with disease-free survival in patients undergoing radical cystectomy. Three-year disease-free survival rates for the whole cohort and for patients undergoing radical cystectomy were 67.3% (95% confidence interval [CI]: 51– 79.2) and 65.2 (95% CI: 44.9– 79.6), respectively.
Conclusion: Real-world muscle invasive bladder cancer patients who receive neoadjuvant chemotherapy are characterized by more advanced diseases and less frequent radical surgery than those included in clinical trials. Nevertheless, outcomes were comparable and, therefore, offering patients with stage II–IVA muscle invasive bladder cancer neoadjuvant chemotherapy after assessment by multidisciplinary tumor boards should be strongly encouraged.
Keywords: bladder cancer, neoadjuvant, chemotherapy, cystectomy, radiotherapy
Introduction
Muscle-invasive bladder cancer (MIBC) is the second most common invasive malignancy of the genito-urinary tract.1 Although a sizable proportion of patients succumb to the disease, cure is possible in 15–80% of patients without distant metastases, depending on the extent of the disease at diagnosis.2 Radical cystectomy (RC) with pelvic lymph node dissection (PLND) represents the mainstay of therapy. Pre-surgical administration of cisplatin-based chemotherapy (neoadjuvant chemotherapy-NAC) has long been studied as a means to reduce the risk of disease relapse and death from MIBC.3–5 The use of NAC has several theoretical advantages: better tolerability compared to post-surgical administration of chemotherapy, in vivo testing of chemosensitivity, and early treatment of micrometastatic disease. Importantly, there is Level I evidence that NAC prolongs survival.6 Despite the firm recommendation in both Medical Oncology and Urological guidelines2,6 for the routine use of NAC, considerable deviations from these guidelines have been observed in everyday practice.7–9
The reason for the low rates of neoadjuvant chemotherapy administration worldwide is multifactorial. The lack of specific criteria for selection of patients with MIBC for NAC may contribute to a considerable degree to its underutilization. As a result, patients with distinctly different prognoses are referred by the urological community for NAC, ranging from T2, optimally cytoreduced tumors up to patients with radiologically overt lymph node metastases. Another point of skepticism is the fact that patients included in clinical trials, which established the current position of NAC in the guidelines, are not representative of those managed in the community setting.10 Close interaction between urologists and medical oncologists could mitigate these limitations and close the gap between guidelines and everyday practice regarding the use of NAC in MIBC.11 Indeed, there is evidence from many countries that the implementation of multidisciplinary tumor boards (MDTB) for the referral of MIBC patients results in evidence-based decision-making, improved accuracy in diagnosis, improved communication between physicians of different specialties, cost-effective care and better clinician education.12–15 In certain cases, it has also improved NAC utilization.15
The institution of MDTBs in local health authorities for the referral of oncological patients was implemented in Greece by law in 2012. Although this institution is well accepted by physicians and patients, data for its impact on the management of specific tumors are lacking. We, therefore, exploited our experience with patients with MIBC who were referred to our genito-urinary MDTB, to explore the range of the definition of “neoadjuvant chemotherapy”, the management and the outcomes of patients referred with this indication.
Patients and Methods
Study Design
This was an observational study based on our MDTB records and was conducted in accordance with the Declaration of Helsinki. The MDTB is a weekly tumor board, where patients with BC are referred for evaluation and management by a multidisciplinary team that specializes in this disease. The MDTB includes physicians from two Medical Oncology Units, three Urology Depts and one Radiotherapy Dept in the area of Athens, Greece. Diagnosis, treatment details and follow-up of patients discussed at the meetings are entered by the corresponding physicians into an MDTB form to ensure homogeneity of documentation. Following completion of chemotherapy, all patients are re-staged by CT thorax, abdomen and pelvis and are assessed by MDTB to decide further management. Patients are offered definitive local therapy if no disease is detected outside the true pelvis, irrespective of initial clinical stage. Fit-for-surgery patients are offered RC+PLND. Unfit or unwilling to undergo RC patients are offered radical radiotherapy. A debulking transurethral resection of bladder tumor (TURB-T) to remove all visible tumor is strongly advised before radiotherapy. Patients treated with radiotherapy are followed cystoscopically according to institutional protocols. These normally include cystoscopy every 3 months for the first year, 4-monthly for the second year and 6-monthly for the following 3 years. Random bladder biopsy is not routinely performed. All patients, irrespective of local management, undergo a CT scan of the abdomen and pelvis every 6 months for the next 5 years.
Patients’ Selection
Patients were selected according to the following criteria: histologically confirmed MIBC; referral indication “neoadjuvant chemotherapy”; adequate staging for extravesical disease with CT of the thorax, abdomen and pelvis; minimum follow-up of 2 years after the completion of therapy (including surgery or radiotherapy); chemotherapy as the initial management for MIBC (excluding initial diagnostic procedure). Regarding the last inclusion criterion, chemotherapy was cisplatin-based for all patients, since immediate cystectomy is the MDTB recommendation for cisplatin-ineligible patients.
Data for this analysis were retrieved from MDTB forms and were entered anonymously into a specifically designed datasheet, including surgical and medical sections. All patients had given their informed consent, which was approved by the Institutional Review Board of ALEXANDRA Hospital, Athens, Greece for the use of their medical records for research purposes. Database was locked in February 2020. Quality control was performed by the co-ordinating center (RZ, AB) by generating queries, which were resolved electronically or by site visits, if necessary.
Objectives
Primary objective was a 3-year disease-free survival (DFS) rate, which has been shown to be a valid surrogate for 5-year OS.16,17 Relapses in the bladder (for those not undergoing RC) were also considered events for this analysis. Secondary objectives were cancer-specific survival (CSS), OS, time from MIBC diagnosis to RC, perioperative mortality and morbidity, chemotherapy-related toxicity, radiation-related toxicity, and response to NAC.
Tumor staging (clinical and pathological) was reported, according to the AJCC Manual, 8th Edition, 2018.18 All CTs were reviewed by the affiliated Radiology Departments according to the local guidelines. Patients with hydronephrosis or evidence of extravesical extension on computed tomography imaging were presumed to have clinical stage T3 disease. N status was considered positive if the short axis of any LN was ≥ 1cm, since this has shown to be correlated well with pathological findings.19 DFS, CSS and OS were calculated from the date of 1st chemotherapy course. Chemotherapy-associated toxicity was assessed according to the National Cancer Institute Common Terminology Criteria v.4. Surgical complications were categorized according to the Clavien-Dindo classification.20 Radiation-related toxicity was assessed according to the RTOG-EORTC criteria.21
Response to NAC was assessed according to histopathological findings following RC or according to the post-chemotherapy cystoscopic evaluation (± TURB-T), if no RC was performed. In the case of RC, responses were classified as pathological complete response (pCR), pathological downstaging (pDS) and TNM downstaging (tnmDS). pCR was defined as ypT0N0M0. pDS and tnmDS were defined as a pathologic tumor stage (T stage) or a TNM stage that was at least 1 stage lower than the pre-NAC clinical staging (in addition to pN0 for pDS).22 The degree of pDS and tnmDS was quantified via a comparison of the difference between ypT/ypTNM stage and the pre-NAC cT/TNM stage in each patient; downstaging to ypTis/yp0is and/or ypTa/yp0a were grouped in a single category and were considered distinct from ypT1/ypI. In the case of no RC response was defined as follows: ycT0N0M0 or no visible suspicious area in cystoscopy (if biopsy was not taken) = clinical complete response (cCR); no visible tumor but microscopic disease by histology and/or urine cytology=clinical major response (cMR); macroscopic disease with unequivocal reduction compared to baseline=clinical minor response (cmR). If none of the above criteria was met, “no response” was recorded. In all cases, responses had to be confirmed by no radiological progression.
Statistical Methodology
All analyses were performed on an exploratory basis and the results will be interpreted purely descriptively. Continuous variables were summarized with the use of descriptive statistical measures [median and interquartile range (25th, 75th percentile)] and categorical variables were displayed as frequency tables (n, %). Standard statistical tests were used to check univariate associations between categorical variables and therapy (Fisher’s exact tests) or continuous variables and therapy (ANOVA). For time-to-event endpoints, Kaplan–Meier estimates were used to describe and visualize the effect of categorical variables. DFS, CSS and OS were calculated from the date of first course of chemotherapy. Patients without event (recurrence and death, respectively) were censored on the date of last contact. Log rank tests have been used to explore the prognostic value of categorical variables in clinical outcomes. Stratified version of Log rank tests was also performed to control for therapy. The level of 5% was used for statistical significance. All statistical analyses were performed using STATA/SE 16.1 software (Copyright 1985–2019; StataCorp LP, College Station, Texas, USA).
Results
Patients and Treatment
Forty-six patients, treated with chemotherapy between 01/2012 and 08/2017, were included in the analysis. Their baseline characteristics are shown in Table 1. Eight patients had clinically involved LNs: in 3 of these cases, LN disease was outside the true pelvis (M1a; paraortic:2, inguinal:1). Most patients had cT2 tumors (87%) and stage II disease (71.7%), while in situ component was reported in 5 cases.
 |
Table 1 Baseline (Pre-Chemotherapy) Characteristics of 46 Patients with Muscle-Invasive Bladder Cancer Treated with Chemotherapy |
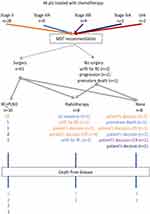
The type of post-chemotherapy management is depicted in Figure 1 and was not associated with patients’ baseline characteristics. Following completion of chemotherapy 41 patients were offered surgery, 3 radical radiotherapy (unfit for cystectomy, n=2; radiological progression after chemotherapy, n=1) and 2 received no further therapy (clinical deterioration, n=1; premature death, n=1). Eleven patients declined surgery. Therefore, 14 patients were offered cystoscopy (and TURB-T in case of residual tumor) and radiotherapy. Five patients declined cystoscopy and only 1 of them accepted the offer of radiotherapy. The remaining 9 underwent cystoscopy (±TURB-T): 2 had residual tumor and received radiotherapy; 7 had cCR and 2 of them declined radiotherapy.
Chemotherapy
The median time from the diagnosis of MIBC to the initiation of chemotherapy was 43.5 days (25th-75th percentile: 29–71). All patients received gemcitabine at 2500 mg/m2 and cisplatin at 70 mg/m2 with G-CSF cover, every 2 weeks23 for a planned total of 4. Forty-three patients (93.5%) received all planned 4 cycles of chemotherapy. The reasons for not administering all 4 cycles to the remaining 3 patients were gastric haemorrhage Gr 3 (n=1) and patient’s decision to proceed to definitive local therapy before completing chemotherapy. Dose reductions or delays occurred in 16 patients with thrombocytopenia and renal function impairment being the most frequent reasons. All reported toxicities are shown in Table 2. There were only 7 Grade 3/4 events and no episode of febrile neutropenia in a total of 177 cycles administered. One patient suffered a deep vein thrombosis, following the 4th cycle of chemotherapy. One patient died of a non-neutropenic infection, 4 weeks after the last course of chemotherapy.
 |
Table 2 Toxicities Associated with Chemotherapy, Surgery, Radiotherapy. Percentages are Shown in Parentheses |
Responses to chemotherapy included 14 pDS (46.6%), 5 of which were pCRs (16.6%) and 17 tnmDS (56.6%) among 30 patients who underwent RC; 5, 2, 5, and 2 patients were downstaged by 1, 2, 3, and 4 pDS categories, respectively, while 7, 2, 5, 2 and 1 were downstaged by 1, 2, 3, 4 and 5 tnmDS categories. Among the 11 evaluable patients who did not undergo cystectomy 7 cCR (63.6%), 1 cMR (9.1%), 1 cmR (9.1%) and 2 cPD (18.2%) were reported.
After a median follow-up of 57.5 months (95% CI: 40.6–70.6), 17 patients died of bladder cancer, 4 patients died without relapse (liver cirrhosis, surgical complication, sepsis and pulmonary embolism), while 1 patient died of undetermined cause with no information regarding disease relapse. This patient was not included in DFS and CSS analyses. DFS, CSS and OS of the whole cohort and according to post-chemotherapy treatment type are shown in Table 3, Figures 2 and 3. 3-year DFS, 5-year CSS and 5-year OS rates from the date of 1st chemotherapy course were 67.3% (95% CI: 51–79.2), 52.9% (95% CI: 33–69.3) and 45.3% (95% CI: 27.8–61.4), respectively. Only clinical TNM staging was correlated with DFS, OS and CSS in the whole population (Table 3).
 |
Figure 2 Disease-free survival, overall survival and cancer-specific survival for the 46 patients included in the study. |
 |
Figure 3 Disease-free survival according to post-chemotherapy treatment type. |
Surgery
Thirty patients underwent RC. The median time from diagnosis of MIBC to cystectomy was 137.5 days (25th-75th percentile: 116–222). The median time from the end of chemotherapy to surgery was 40.5 days (25th-75th percentile: 32–61). Six patients (20%) had cystectomy performed within 90 days from diagnosis.
Peri-operative complications were reported in 14 of 30 patients and are shown in Table 2. In total, 12 patients (40%) had a grade I–II event, while 3 (10%) had a grade III–V event. One patient died due to inferior vena cava rupture during cystectomy and nephroreterectomy. Urinary diversion included ureterostomies in 11 cases, ileal conduit in 10, orthotopic neobladder in 3, while details about the type of urinary diversion were not available in 6 cases.
Pathological staging following RC is shown in Table S1. In 25 cases PLND was performed, while no LNs were recovered in 5 cases. The median number of examined LNs was 5 (0–28). All patients with pT0 had no adenopathy on pre-chemotherapy imaging (cN0). The pT0 rate was 16.6%, p 40%, pDS 46.6% and tnmDS 56.6%. When cN+ patients were excluded these rates were 21.7%, 43.4%, 56.5% and 69.5%, respectively. Among the 25 patients who had LNs examined, 5 (20%) had pN+. From the 19 cN0 evaluable patients, only 2 (10.5%) had LN involvement, as opposed to 3 of 5 (60%) cN+ evaluable patients. Adjuvant chemotherapy was administered to 2 patients with pT3aN0 disease: one had small cell histology and received carboplatin/etoposide, while the other was included in a clinical trial and received nivolumab.
After a median follow-up of 47.9 months (95% CI: 38.9–64.2), 10 patients died of their disease, 1 due to surgical complication (inferior vena cava penetration during RC and nephroureterectomy for double primary) and 2 of unrelated causes with no disease progression. All relapses occurred within 20 months after RC. 3-year DFS, 5-year CSS and 5-year OS rates were 65.2% (44.9–79.6), 57.1% (95% CI: 31.2–76.3) and 46.7% (95% CI: 23.7–66.8), respectively. Clinical N stage (N0 vs N+/M1a), ypT (≤pT1 vs >T1), ypN (N0 vs N+), ypTNM (≤I vs >I) and pDS were correlated with DFS, OS and CSS, while clinical TNM and ypTNM (Table 3, Figure S1). Importantly, time to cystectomy, and pCR did not impact on DFS, OS or CSS.
No Surgery
Radiotherapy
Eight patients underwent radical radiotherapy. None of these patients had cN+ disease, while 4 had cCR, 1 cMR, 1 cmR, 1 cPD and 1 was non-evaluable in post-chemotherapy assessment. The total dose delivered to the bladder was ranged between 55 Gy and 66 Gy. The clinical target volume (CTV) was outlined in 7 cases only for the bladder, while in one case internal and external pelvic lymph nodes were also included up to 45 Gy. The planning target volume for bladder (PTV) was 1.5 cm margin around CTV. In all cases, Femoral heads, and rectum were set as Organs At Risk (OARs) and QUANTEC criteria were followed as dose constraints recommendations and met in all patients.24 All patients underwent image guided 3-dimensional conformal radiotherapy or intense modulated radiotherapy technique. The median time from the end of chemotherapy to radiotherapy initiation was 1.6 months (1.1–4.7). Two patients received concurrent chemotherapy with cisplatin. Acute and late complications are shown in Table 2. Three patients experienced progression and died of their disease, 7 months, 4 and 4 years, respectively after the end of radiotherapy (Figure 1). All 4 patients with cCR at post-chemotherapy cystoscopy remain disease-free 2 to 5 years after the end of radiotherapy.
Chemotherapy Only
Eight patients received no definitive local therapy following completion of chemotherapy. Three had cN+ disease, while 3 had cCR, 1 cPD and 4 were non-evaluable in post-chemotherapy assessment. Four patients relapsed and died of their disease 10 months to 4.5 years after chemotherapy completion, 1 died from sepsis and 1 died of undetermined cause. Two patients with clinical stages IIIA and IVA who achieved cCR in post-chemotherapy evaluation remain disease-free 4.5- and 6.5-years following completion of chemotherapy.
Discussion
Our experience shows that there are differences between patients with MIBC included in neoadjuvant trials and those who are considered for “neoadjuvant chemotherapy” in real-world. Traditionally, clinical stages II–IIIA are included in the former, while stages up to IVA were included under this term in this study. This is in concert with a recent report from the USA, also suggesting that patients who receive NAC in the community harbor more advanced disease than those who do not.8 Another important finding is that 11 of 41 patients (27%) declined surgery. Taking into consideration that 4 of these patients also declined radiation therapy, poor compliance could be advocated. The poor performance of this choice in cT2 and cN0 stage (potentially curable disease) (Table 3) suggests that this practice should be discouraged. In respect to radiotherapy, we observed that all patients treated with this modality had cN0 and cII–IIIA disease, while 4 of them had cCR in post-chemotherapy evaluation. These findings could suggest a trend towards increased use of bladder preservation in patients with favorable characteristics. Although, in concert with existing experience,25 the outcome of these patients was satisfactory, the lack of validated criteria of selection for bladder preservation does not yet justify a routine application of this strategy.
Our findings showed that among pre-therapy characteristics, only radiological evidence of LN involvement was significantly associated with DFS among patients undergoing surgery. Nevertheless, among 5 patients with clinical LN involvement, 2 achieved pN0 after chemotherapy and one of them remained with no relapse for 4 years and died of an unrelated cause. Therefore, cN staging cannot be recommended as criterion for selection. The same is true for stages IIIB and IVa, which are usually not included in neoadjuvant trials: 2 of our 7 patients remain without disease 4 and 7 years following completion of chemotherapy. These findings justify our MDTB decision to take into account these limitations and adjust our function to the real-world attitude of a homogenous aggressive management of all MIBC without non-LN metastases, rather than limiting the routine offer of neoadjuvant chemotherapy plus surgery (or radiotherapy) only to stages II-IIIA, according to the experience from clinical trials. Thus, management of MIBC through MDTBs can ensure the most effective and up-to-date uses of our armamentarium in everyday practice. This may become more relevant in the near future, with the expected use of novel therapies, such as immunotherapy, in the perioperative setting of non-metastatic MIBC.26
The most important prognostic information was derived from post-chemotherapy staging. As consistently shown in previous studies,22,27–31 both pT and pN were strongly associated with all outcome measures. The most recent AJCC TNM classification was also useful in this respect. ypTNM was strongly associated with DFS, CSS and OS. Importantly, no relapses have been observed among ypT≤1 and ypTNM≤I. Pathological response to NAC has also been associated with survival after NAC+RC.22,32,33 We assessed pathological response using three parameters: pCR, pDS and tnmDS. pCR is the most studied of the three and has been proposed as a surrogate for DFS, CSS and OS.34 Nevertheless, this notion has been challenged, especially outside the context of clinical trials.35 We also found no such association. Although our finding is limited by the small number of patients, it is in concert with other real-world series, where survival rates comparable to those of clinical trials are usually combined with lower pCR rates,8,22,36,37 underlining the limitations of this factor. On the contrary, our results support the conclusions of recent reports22,38 that pDS, which takes into account any pT downstaging combined with pN0, may be a more powerful determinant of prognosis compared to pCR. A commonly cited reason for the limited use of NAC is that the benefit is limited only to the small percentage of patients achieving a pCR. The findings of Martini et al and our study reinforce that the benefit of NAC is not limited to this subset of patients. The proportion of patients achieving pDS in our cohort was approximately 3-fold higher than the proportion of patients achieving a pCR (46.6% vs 16.6%) and their 3-year DFS rate was excellent at 92.3%.
We used a DDGC combination, which has shown efficacy both in metastatic23 and in neoadjuvant27 settings. This regimen does not cause significant alopecia, appears equally effective but less toxic than DD MVAC23 and requires fewer visits than classic GC. Survival rates were similar to those of contemporary real-world series,8,22,29,34,35 thus confirming its efficacy, while both our results and those of Iyer et al27 do not confirm initial concerns for increased incidence of vascular events.28 Biweekly administration is expected to shorten time to definitive treatment imposed by the more protracted non-dense regimes, and this could favorably affect prognosis.39 The median time in our study exceeded 4 months, which is greater than that reported in clinical trials27,28,31 but identical to that reported in a recent retrospective analysis from the UK.29 The median time from the end of chemotherapy to RC was 40.5 days, which is identical to those reported in clinical trials27,31 and better than in other RWE series.36 It, therefore, seems that the median time of 43 days required for the initiation of chemotherapy could be a target for improvement in our practice. This time results from the generation of pathological diagnosis and indicates that earlier referral of patients for neoadjuvant chemotherapy, pending the official pathology report, should ensure reduction of these delays.
This study has several limitations. First, it is an observational, retrospective study. Additionally, the relatively small sample size limits the power of the study to show statistical differences. Moreover, the data used for this study are from a single, tertiary care center, which can limit the generalizability of findings to other centers that may have different practice patterns. In contrast, homogenous chemotherapy and long follow-up, which counteracts, to a point, the small number of patients, should be acknowledged as strengths of this report. In addition, this systematic analysis of our experience sets very specific targets for improvement, such as an increase in the percentage of patients undergoing surgery after chemotherapy and a reduction in the time from diagnosis to initiation of neoadjuvant chemotherapy.
Conclusion
The data presented here are in concert with RWE from other countries, indicating that utilization of neoadjuvant chemotherapy shares similar problems worldwide and could, therefore, be dealt with through international guidelines and consensus. In order to be widely applicable, these should take into consideration RWE and in this respect, we believe that our contribution is of a certain value. A migration towards more advanced stages is observed in everyday practice, compared to neoadjuvant clinical trials. Pre-treatment clinicopathological factors cannot aid accurate selection of patients for combined chemotherapy and definitive local therapy. Hopefully, the use of molecular markers will fulfil this unmet medical need.40,41 Until then, the combination of cisplatin-based chemotherapy followed by RC+PLND or radiotherapy, when surgery is not an option, remains the treatment with the highest probability of cure in patients with stages II–IVA BC. Routine management of these patients through MDTBs can ensure optimization of management through close collaboration between the experts involved.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The ethics committee of General Hospital Alexandra approved the study and patients have signed informed consent for the analysis and publication of their data. Additionally, all methods were carried out in accordance with European guidelines.
Consent for Publication
All patients participating in this study signed consent to use their data.
Acknowledgments
The authors would like to thank our study participants for their permission.
Funding
There was no financial support in this research.
Disclosure
Professor Meletios A Dimopoulos report personal fees from AMGEN, personal fees from TAKEDA, personal fees from JANSSEN, personal fees from BEIGENE, personal fees from BMS, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Key Statistics for Bladder Cancer. Available from: https://www.cancer.org/cancer/bladder-cancer/about/key-statistics.html.
2. Witjes JA, Bruins HM, Cathomas R, et al. EAU Guidelines on Muscle-invasive and metastatic bladder cancer 2020. Available from: https://uroweb.org/guideline/bladder-cancer-muscle-invasive-and-metastatic.
3. Zagouri F, Peroukidis S, Tzannis K, Kouloulias V, Bamias A. Hellenic Genito-Urinary Cancer Group (HCUCG). Current clinical practice guidelines on chemotherapy and radiotherapy for the treatment of non-metastatic muscle-invasive urothelial cancer: a systematic review and critical evaluation by the Hellenic Genito-Urinary Cancer Group (HGUCG). Crit Rev Oncol Hematol. 2015;93(1):36–49.
4. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866.
5. International Collaboration of trialists. Neoadjuvant methotrexate, vinblastine, and cisplatin chemotherapy for muscle-invasive bladder cancer: a randomized controlled trial. Lancet. 1999;354:533–540.
6. Bellmunt J, Orsola A, Leow JJ, Wiegel T, De Santis M, Horwich A. On behalf of the ESMO guidelines working Group. Bladder cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii40–iii48.
7. Zaid HB, Patel SG, Stimson CJ, et al. Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: results from the National Cancer Database. Urology. 2014;83:75–80.
8. Reardon ZD, Patel SG, Zaid HB, et al. Trends in the use of perioperative chemotherapy for localized and locally advanced muscle-invasive bladder cancer: a sign of changing tides. Eur Urol. 2015;67:165–170.
9. Burger M, Mulders P, Witjes W. Use of neoadjuvant chemotherapy for muscle-invasive bladder cancer is low among major European centres: results of a feasibility questionnaire. Eur Urol. 2012;61:1070–1071.
10. Hanna N, Trinh QD, Seisen T, et al. Effectiveness of neoadjuvant chemotherapy for muscle-invasive bladder cancer in the current real world setting in the USA. Eur Urol Oncol. 2018;1:83–90.
11. Prades J, Remue E, van Hoof E, Borras JM. Is it worth reorganising cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organisation of MDTs and their impact on patient outcomes. Health Policy (New York). 2015;119(4):464–474.
12. Scarberry K, Ponsky L, Cherullo E, et al. Evaluating the impact of the genitourinary multidisciplinary tumour board: should every cancer patient be discussed as standard of care? Can Urol Assoc J. 2018;12(9):E403–8.
13. Harshman LC, Tripathi A, Kaag M, et al. Contemporary patterns of multidisciplinary care in patients with muscle-invasive bladder cancer. Clin Genitourin Cancer. 2018;16(3):213–218.
14. Kinnear N, Smith R, Hennessey DB, Bolton D, Sengupta S. Implementation rates of uro-oncology multidisciplinary meeting decisions. BJU Int. 2017;120(Supplement 3):15–20.
15. Gronostaj K, Czech AK, Fronczek J, et al. Changes in neoadjuvant chemotherapy utilization in muscle invasive bladder cancer treatment: a tertiary center retrospective study. Cent European J Urol. 2020;73:13–18.
16. Nuhn P, May M, Fritsche HM, et al. External validation of disease-free survival at 2 or 3 years as a surrogate and new primary endpoint for patients undergoing radical cystectomy for urothelial carcinoma of the bladder. EJSO. 2012;38:637–642.
17. Sonpavde G, Khan MM, Lerner SP, et al. Disease-free survival at 2 or 3 years correlates with 5-year overall survival of patients undergoing radical cystectomy for muscle invasive bladder cancer. J Urol. 2011;185(2):456–461.
18. Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual.
19. Ho PL, Willis DL, Patil J, et al. Outcome of patients with clinically node-positive bladder cancer undergoing consolidative surgery after preoperative chemotherapy: the M.D. Anderson Cancer Center Experience. Urol Oncol. 2016;34:
20. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213.
21. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485–1489.
22. Martini A, Jia R, Ferket BS, et al. Tumor downstaging as an intermediate endpoint to assess the activity of neoadjuvant systemic therapy in patients with muscle-invasive bladder cancer. Cancer. 2019;125(18):3155–3163.
23. Bamias Α, Dafni U, Karadimou A, et al. Prospective, randomized Phase III study comparing two intensified regimens (methotrexate/vinblastine/doxorubicin hydrochloride/cisplatin [MVAC] versus gemcitabine/cisplatin) in patients with inoperable or recurrent urothelial cancer. Ann Oncol. 2013;24(4):1011–1017.
24. Bentzen SM, Constine LS, Deasy JO, et al. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. Int J Rad Oncol Biol Phys. 2010;76:S3–S9.
25. Kulkarni GS, Hermanns T, Wei Y, et al. Propensity score analysis of radical cystectomy versus bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol. 2017;35:2299–2305.
26. Rouanne M, Bajorin DF, Hannan R, et al. Rationale and outcomes for neoadjuvant immunotherapy in urothelial carcinoma of the bladder. Eur Urol Oncol. 2020;3(6):728–738.
27. Iyer G, Balar AV, Milowsky MI, et al. Multicenter prospective Phase II trial of neoadjuvant dose-dense gemcitabine plus cisplatin in patients with muscle-invasive bladder cancer. J Clin Oncol. 2018;36:1949–1956.
28. Anari F, O’Neill J, Choi W, et al. Neoadjuvant dose-dense gemcitabine and cisplatin in muscle-invasive bladder cancer: results of a Phase 2 trial. Eur Urol Oncol. 2018;1:54–60.
29. Blick C, Hall P, Pwint T, et al. Accelerated Methotrexate, Vinblastine, Doxorubicin, and Cisplatin (AMVAC) as neoadjuvant chemotherapy for patients with muscle-invasive transitional cell carcinoma of the bladder. Cancer. 2012;118:3920–3927.
30. Bamias A, Deliveliotis C, Karayiannis A, et al. Neoadjuvant chemotherapy with docetaxel and cisplatin in patients with high-risk resectable bladder carcinoma: long term results. Eur Urol. 2004;46:344–350.
31. Choueiri TK, Jacobus S, Bellmunt J, et al. Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol. 2014;32(18):1889–1894.
32. Rosenblatt R, Sherif A, Rintala E, et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol. 2012;61:1229–1238.
33. Sonpavde G, Goldman BH, Speights VO, et al. Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer. 2009;115:4104–4109.
34. Lavery HJ, Stensland KD, Niegisch G, Albers P, Droller MJ. Pathological T0 following radical cystectomy with or without neoadjuvant chemotherapy: a useful surrogate. J Urol. 2014;191:898–906.
35. Weight CJ, Garcia JA, Hansel DE, et al. Lack of pathologic down-staging with neoadjuvant chemotherapy for muscle-invasive urothelial carcinoma of the bladder. A contemporary series. Cancer. 2009;115(4):792–799.
36. Alva AS, Tallman CT, He C, et al. Efficient delivery of radical cystectomy after neoadjuvant chemotherapy for muscle-invasive bladder cancer a multidisciplinary approach. Cancer. 2012;118:44–53.
37. Zargar H, Espiritu PN, Fairey AS, et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2015;67(2):241–249.
38. Teramukai S, Nishiyama H, Matsui Y, Ogawa O, Fukushima M. Evaluation for surrogacy of end points by using data from observational studies: tumor downstaging for evaluating neoadjuvant chemotherapy in invasive bladder cancer. Clin Cancer Res. 2006;12:139–143.
39. Lee CT, Madii R, Daignault S, et al. Cystectomy delay more than 3 months from initial bladder cancer diagnosis results in decreased disease specific and overall survival. J Urol. 2006;175:1262–1267.
40. Liu D, Plimack ER, Hoffman-Censits J, et al. Clinical validation of chemotherapy response biomarker ERCC2 in muscle-invasive urothelial bladder carcinoma. JAMA Oncol. 2016;2(8):1094–1096.
41. Pietzak EJ, Zabor EC, Bagrodia A, et al. Genomic differences between “primary” and “secondary” muscle-invasive bladder cancer as a basis for disparate outcomes to cisplatin-based neoadjuvant chemotherapy. Eur Urol. 2019;75(2):231–239.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.