Back to Journals » International Journal of General Medicine » Volume 15
Reference Intervals for Common Renal and Liver Function Clinical Chemistry Parameters Among Apparently Healthy Pregnant and Non-pregnant Women in South Wollo Zone, Amhara National Regional State, Northeast Ethiopia
Authors Mohammed M , Fiseha M , Belay G, Kindie S, Tsegaye A
Received 24 February 2022
Accepted for publication 10 May 2022
Published 24 May 2022 Volume 2022:15 Pages 5145—5157
DOI https://doi.org/10.2147/IJGM.S363129
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Miftah Mohammed,1 Mesfin Fiseha,1 Getachew Belay,2 Samuel Kindie,3 Aster Tsegaye3
1Department of Medical Laboratory Science, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia; 2Department of Medical laboratory science, College of Health Sciences, Adigrat University, Adigrat, Ethiopia; 3Department of Medical Laboratory Science, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Miftah Mohammed, Email [email protected]
Abstract:
Background: Physiological changes during pregnancy cause alterations in concentration of biochemical analytes. Thus, locally established pregnancy-specific reference intervals are important for accurate diagnosis, treatment, and prognosis of diseases. The objective of the study was to establish reference interval for the common renal and liver function clinical chemistry parameters among pregnant and non-pregnant women of South Wollo zone, Ethiopia.
Methods: A community-based cross-sectional study was conducted on a total of 323 apparently healthy study participants randomly selected from South Wollo zone, Ethiopia, from April to June 2019. Medical history, physical examination and sociodemography were collected by using questionnaire. Liver and renal function clinical chemistry tests were done using A25 Biosystems, clinical chemistry analyzer. After the exclusion of outliers, Kolmogorov–Smirnov test was used to check its normality. The 95% RI with 95% confidence interval was established using the nonparametric method. The significance of differences was evaluated using Mann–Whitney U test.
Result: There was statistically significant variation between pregnant and non-pregnant women in values of albumin, T. protein, ALP, urea and creatinine, but not for AST, ALT, bilirubin (direct) and bilirubin (total). Reference intervals established for pregnant women includes albumin 26.14– 42.87g/L, total protein 48.52– 74.71 g/L, AST 2.4– 43.6 U/L, ALT 0.94– 28.35 U/L, ALP 21.2– 337 U/L, bilirubin (direct) 0.03– 0.32 mg/dL, bilirubin (total) 0.26– 0.94 mg/dL, creatinine 0.29– 0.87 mg/dL, urea 7.17– 20.82 mg/dL. Albumin: 32.81– 47.87, total protein: 56.71– 83.9 U/L, AST: 4.2– 37.1 U/L, ALT: 2.69– 41.18 U/L, ALP: 3.22– 278.7 U/L, bilirubin (direct) 0.1– 0.51 mg/dL, bilirubin (total) 0.24– 1.06 mg/dL, creatinine 0.44– 1.00 mg/dL, urea 8.07– 27.87 mg/dL for non-pregnant women.
Conclusion: The study showed marked difference in albumin, total protein, alkaline phosphatase, urea and creatinine. Therefore, physiological adaptations of pregnancy should be considered when interpreting liver and renal function tests in a pregnant woman.
Keywords: reference interval, clinical chemistry, pregnant, non-pregnant
Background
The biological components of the human organism are exposed to variation caused by physiological phenomena, genetic differences, environmental factors and diseases.1 The significant difference in the reference interval (RI) of common renal function test (RFT) and liver function test (LFT) parameters among different countries and even population groups of the same country attributes the risk of unessential further investigations or default in the detection of the underlying disease or leads to wrong management of patient.2 Thus, it is the inevitable presumption for the need of establishing population-centered RI since interpretation of laboratory results requires a reasonable knowledge of the variation.3–5
In all clinical practice, RI is accepted to be the most widely used decision-making tool ranging from diagnosis of health disorders, evaluation of the toxicity of xenobiotic, disease staging to monitoring of treatment.1,6,7 The RI of many clinical laboratory tests is established by cutting off the threshold values within which the test results of a specified percentage (usually 95%) of apparently healthy individuals would fall.4
During pregnancy, a woman experiences physiological and hormonal changes that affect nearly every organ system in which kidney and liver are not an exception. Although it is a normal physiological phenomenon, it exhibits many biochemical changes ranging from change of electrolyte concentrations to further alteration in cortisol metabolism. As a result of these changes, many of the laboratory reference intervals of non-pregnant women are not appropriate for pregnant women. That is why clinical chemistry RI derived only from pregnant women are assumed to have an important role in passing medical decisions for pregnancy-related health disorders.7
Reference values established from samples of non-pregnant women are, therefore, not valuable for clinical decisions for pregnant women. Hence, establishing one’s own RI during pregnancy is monumental to isolate pathological conditions. In Ethiopia, no adequate numbers of community-based pregnancy-concerned RI studies have been established in clinical chemistry settings especially for pregnant women and non-pregnant women.8 Therefore, the aim of this study was to establish RIs for the common renal and liver function clinical chemistry parameters among apparently healthy pregnant and non-pregnant women in South Wollo zone, Amhara National Regional State, northeast Ethiopia.
Methods
Study Design, Period, and Area
A community-based cross-sectional study to determine RI for common renal and liver function clinical chemistry parameters on apparently healthy pregnant and non-pregnant women was conducted from April to June 2019 among healthy pregnant and non-pregnant women in South Wollo zone, Amhara National Regional State, Northeast Ethiopia. South Wollo Zone extends from 10.89°N and 38.99°E, having an area of 17,067.45 square kilometers.9
According to Federal Democratic Republic of Ethiopia Central Statistical Agency Population Projection in 2019 this zone would be expected to have a total population of 3,087132, of whom 1,528,769 are male and 1,558,363 female with 525,762 urban and 2,561,373 rural residents.
Amhara was the largest ethnic group reported in S Wollo (99.33%); all other ethnic groups made up 0.67% of the population. Amharic accounts the first spoken language (98.65%). The majority (70.89%) were Islamic religion followers while 28.8% of the population followed Ethiopian Orthodox Christianity.
Dessie is a city and a zone located in the Amhara region at a latitude and longitude of 11°8′N 39°38′E, north-eastern Ethiopia with an elevation between 2470 and 2550 meters above sea level. Kombolcha is a city and administration in north-central Ethiopia. It is located in the S Wollo zone of the Amhara Region. It has a latitude and longitude of 11°5′N 39°44′E with an elevation between 1842 and 1915 meters above sea level. Kalu is one of the woredas in the Amhara region of Ethiopia and part of the S Wollo zone. The altitude of this woreda ranges from 800 meters above sea level in the lowlands border. Legambo is another woreda in the S Wollo zone with an elevation of between 1500 to 3700 meters.9
Four study woredas (Dessie administration, Kombolcha administration, Legambo woreda and Kalu woreda) were selected in consideration of their density of residents and altitude. The study included were those individuals who were living at the aforementioned four areas and showed willingness to participate in the study.
Reference Population
Clinical history centered questionnaire, physical examination and screening laboratory tests for infectious disease were used to selected eligible study participants. Apparently healthy volunteer pregnant and non-pregnant women between the ages of 15–60 years having normal body mass index of 18–25 kg/m2 in selected study area of S. Wollo zone, Amhara National Regional State, northeast, Ethiopia were included in the study population. Whereas women with hemoparasite infection, dehydrated women, those who received a blood transfusion within the previous three months, sera-positive women for human immunodeficiency virus (HIV), hepatitis C virus (HCV), hepatitis B virus (HBV) and syphilis were excluded. Moreover, women who had observable mental illness, strenuous physical exercise, working with hazardous chemicals (kerosene and acids), individuals that are taking dispensable or indispensable drugs, hypertensive individuals, chronic smokers and alcohol drinkers, history of chronic liver or kidney disease, malnourished (BMI < 17.5 kg/m2), known diabetes on oral therapy or insulin were excluded from the study.4
Sample Size and Sampling Method
According to NCCLS recommendation and for the ease of using conventional statistical methods, a minimum number of 120 study subjects by partition are required. However, in order to attain the minimum sample size requirement, the maximum possible numbers of subjects being excluded by clinical history, physical examination, laboratory screening test and outlier should be added. Therefore, a total of 42% total exclusion rate according to a cross sectional RI establishment study in northwest Ethiopia10 and about 10% outlier from similar study in Canada11 were added to 120. Finally, a total of 378 (from 189×2) reference sample group were screened in the study, of which 165 pregnant and 158 non-pregnant who met the inclusion criteria were selected. By taking altitude and residence difference into account and ease of accessibility of the areas, two study woredas from the lowlands and another two from the highlands were randomly selected from S Wollo zone. The determined sample size was allocated for each selected woredas proportionally to their population size. Households from each of the kebeles were addressed using the convenience sampling method. The available pregnant and non-pregnant women who showed willingness to participate were recruited and a maximum of one individual per partition per household was included in the study.
Data Collection and Laboratory Analysis
The objective of the study and detailed description of the questionnaire were discussed with data collectors and other concerned bodies before data collection. The lists of selected kebeles were distributed to the health extension workers. Then the purpose of the study was communicated to the participants and their willingness was confirmed. Volunteers with no easily identified prominent chronic and acute illness were scheduled to the nearby health institution.
In the health center participants were screened by obtaining medical history and symptom-directed physical examination by clinicians. Besides, anthropometric measurements like height, weight and blood pressure were recorded. Data like sociodemographic characteristics were collected from each participants using structured questionnaire via face-to-face interview. After completion of the interview, all eligible respondents were requested to give about 5 mL of venous blood, urine, and stool sample. Using the samples they provided, study participants were further screened for intestinal parasites using concentration technique, blood film examination for hemoparasite infection, urine analysis (dipstick, microscopy, and pregnancy), diabetes mellitus (using senso card). Screening tests for HIV, HBsAG, and HCV, Treponema palladium was done by rapid serological test kits. Biochemical analysis method for clinical chemistry parameters were bromocresol green-succinate buffer for albumin, IFCC modified without pyridoxal phosphate for ALT and AST, p-nitrophenyl phosphate diethanolamine for ALP, enzymatic-UV kinetic for UREA, biuret/endpoint for T protein and dizotized sulfanilic acid for bilirubin (direct and total) using clinical chemistry auto analyzer (A25 Bio system, Biosystems SA, Spain).
Data Quality Assurance
The anticipated good quality of study participants was addressed through exhaustively organized questionnaire, appropriate history taking, physical examination, and laboratory examinations to recruit healthy individuals. Standard operating procedures (SOPs) were followed for sample collection, processing, storage and handling of the sample. Internal quality control was done for each parameter by using two quality control levels. The laboratory strived to comply with the principles of good clinical laboratory practice protocols.12 Between-run and within-run precision for the common RFT and LFT analytes was done using 20 measurements made both on the same and separate days, respectively using normal control samples. The result was compared with precision limits claimed in the analyte reagent insert kits. As clearly depicted in Table 1, the majority of the clinical chemistry tests were within the precision limits indicated by the reagent manufacturer, which basically augments the reliability of the reference values documented in this study.
 |
Table 1 Methods Used (Traceability) and Analytical Precisions for Selected Assays |
The control sample results were interpreted using Westgard multirule algorithm. The laboratory was under regular control of the Ethiopian public health institute. Furthermore, all the laboratory staff received equipment and procedure (protocol) training from highly trained personnel. The two quality control levels (pathological/abnormal and nonpathological/normal) were run on daily basis till it falls within the acceptable ranges before testing samples
Data Analysis and Interpretation
The data was revised and checked for completeness manually and entered to EPI Info version 3.5.3 (CDC, USA) statistical software and then transferred to Statistical Package for Social Sciences SPSS version 20 (IBM Corporation, Armonk, NY, USA) software for analysis. The data was partitioned into two groups: pregnant women and non-pregnant women. The actual exclusion of those outliers was done by the quartile method.13 After all outliers were excluded, descriptive statistics were employed to determine the mean, median and 95% range of each analytes. Reference intervals were calculated in accordance with CLSI/IFCC guideline using nonparametric methods. Kolmogorov–Smirnov (KS) test was used for all test results of each partition to check its normality in SPSS data analysis software. All the observed data of each analyte was found to be not normally distributed. The presence of significant differences between pregnant and non-pregnant women was evaluated using the Mann–Whitney U test and P≤0.05 claimed the presence of statistically significance difference.
Operational Definition
Apparently Healthy Pregnant Women
Individuals with age ≥15 years without disease based on clinical sign and symptom plus laboratory investigations and positive urine human chorionic gonadotropin hormone test result.
Apparently Healthy Non-Pregnant Women
Individuals with age ≥15 years having negative urine human chorionic gonadotropin test result, without disease based on clinical sign and symptom plus laboratory investigations.
Common Liver Function Clinical Chemistry Parameters
Albumin, total protein, AST, ALT, ALP, bilirubin (direct and total).
Common Renal Function Clinical Chemistry Parameters
Urea and creatinine.
Results
Screening Result
A total of 378 apparently healthy adult women (189 pregnant and 189 non-pregnant) consented and were screened to establish the RI of common liver and renal function clinical chemistry parameters from S Wollo zone, northeast Ethiopia. Of these participants a total of 24 (12.6%) pregnant women were excluded as a result of positive laboratory test results for HIV, HBV, HCV, and syphilis in a magnitude of 6 (3.1%), 5 (2.6%), 2 (1.05%) and 3 (1.5%), respectively and 8 (4.2%) by clinical history, physical examination and others while 31 (16.4%) non-pregnant were excluded as a result of positive laboratory test results for HIV, HBV, HCV, and syphilis in a magnitude of 6 (3.1%), 5 (2.6%), 1 (0.5%) and 4 (2.1%), respectively and 15 (7.9%) by clinical history, physical examination and others. Those who were positive for HIV, HBV, HCV, syphilis, blood parasites, and other disease conditions were referred to the nearby governmental health care institutions and were not included in the study. A total of 165 pregnant and 158 non-pregnant participants who met the eligibility criteria were enrolled and became a reference sample group in this study.
Sociodemographic Characteristics
The mean and median age of all the study participants at the study entry period was 27.2 (SD: 6.6) and 27 (interquartile range (IQR: 23–30) years, respectively. The mean age and mean gestational age of pregnant were 27.4 (SD: 4.4) years and 19.7 (SD: 7.7) weeks, respectively. The mean weight, mean height and mean body mass index (BMI) for pregnant women were 62 (SD: 4.3) kg, 1.63 (SD: 0.05) meter and 23.3 (SD: 1.7) kg/m2, respectively whereas 59 (SD: 3.9) kg, 1.62 (SD: 0.06) meter and 22.4 (SD:1.5) kg/m2, respectively for non-pregnant women. Around one-quarter of the participants 86 (26.6%) and 73 (22.6%) were single and had at least completed high school, respectively (Table 2).
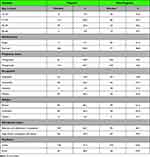 |
Table 2 Sociodemographic Characteristics of Study Participants from S Wollo Zone, Northeast Ethiopia, 2019 |
Reference Intervals for Commonly Performed Liver and Renal Function Clinical Chemistry Tests
As shown in Table 3, the study showed the presence of statistically significant differences between pregnant and non-pregnant women in the majority of commonly performed liver and renal function clinical chemistry parameters except for AST, ALT, bilirubin (direct), and bilirubin (total) where pregnant women had significantly lower values than non-pregnant in the majority of the analytes.
Ninety-five Percent Confidence Intervals for the Established Lower and Upper Reference Limits
The 95%CI for lower and upper reference limits of established RIs in clinical chemistry parameters were calculated and presented in Table 4.
Discussion
The study assessed the comparability of the current RI with studies from other parts of Ethiopia, Africa, USA, and China. The results obtained from this study revealed that the reference values for most analytes varied from the fixed reference values on the manufaturer's package to be used for clinical management in the study area both for pregnant and non-pregnant women. Generally, as shown in Table 5, no consistent pattern was seen among the various studies.
 |
Table 5 Comparison of Common RFT and LFT RIs of Healthy Non-pregnant Women in S. Wollo Zone, Amhara National Regional State, Northeast Ethiopia with Manufacturer RIs and Other Similar Studies |
Relatively higher and wider RI for albumin, total protein, ALT and AST was observed in studies conducted in northwest Ethiopia,10 southwest Ethiopia,14 and middle belt of Ghana,16 Zimbabwe,17 south and eastern Ethiopia,6 USA,18 and Hong Kong China19 than the current study in non-pregnant women. In non-pregnant women the lower reference limit of T. protein was comparable with related studies done in northwest Ethiopia,10 Amhara region,15 middle belts of Ghana,16 southern and eastern Africa,6 USA,18 and manufacturer, but slightly lower than studies in southwest Ethiopia,14 Zimbabwe,17 and Hong Kong China.19
The lower reference limit of bilirubin (direct) of non-pregnant women in this study was comparable with similar bilirubin RIs study conducted in Gojjam zone northwest Ethiopia,10 other sites in Amhara national regional state,15 southern and eastern Africa,6 USA,18 China,19 and reagent insert kit claiming RI, but slightly lower than study conducted in the middle belt of Ghana16 which could be due to different analytical methods used in Ghana for bilirubin analysis (3–5, dichlorophenyl-diazonium-tetraforoborate). Moreover, the lower and upper reference limit of this study for AST, ALT and ALP was slightly higher than and comparable in the order given with manufacturer's declared limit whereas slightly lower than RI studies done in most of the aforementioned countries. This near similarity was probably due to similar IFCC methods used by all laboratories in the aforementioned countries.
In spite of a matching value of lower and upper reference limit for creatinine in this study was observed with related studies done in the Amhara region15 and Hong Kong China,19 a slightly higher lower reference limit was observed than similar studies conducted in northwest Ethiopia,10 southwest Ethiopia,14 and USA18 and a slightly lower upper reference limit than studies in southwest Ethiopia,14 south and eastern Africa,6 the middle belt of Ghana,16 USA,18 and manufacturer stated reference limit. Factors like demographic variation, ethnic and genetic difference, nutritional behaviors, culture, lifestyle and seasonal differences might be entities which contribute to such relatively inconsistent values of clinical laboratory RIs among apparently healthy women across population of the same country and among different nations.
In Table 6, the proportion of out-of-range values (OOR %) segregated by pregnancy status is displayed based on the company based RIs which is being utilized by the Amhara public health institute Dessie branch laboratory. Accordingly, large OOR values were detected especially for pregnant women for the RIs of albumin, urea, T. protein and bilirubin (direct).
 |
Table 6 OOR (N) and OOR (%) of Current RI Study as Compared to APHIDB Used RI (Adopted from Leaflet) |
This study showed the presence of significant difference for the majority of common renal and liver function clinical chemistry parameter values by pregnancy status that agreed with other similar studies in Africa. The majority of common LFT and RFT clinical chemistry parameter values were lower among pregnant than non-pregnant women except for AST, ALT, bilirubin (direct and total). The finding of significantly lower albumin values in pregnant than non-pregnant women in this study was slightly lower than currently used by the laboratory as the laboratory directly adopted RI given for non-pregnant women to use it as a reference frame. The lower reference interval for non-pregnant women compared to pregnant could be probably due to alteration of plasma fluid distribution and hemodilution with that of high demand of albumin by the growing fetus. This lower albumin RI values agreed with results from related studies in Kenya,20 India,21 France,22 and north central Nigeria.23 This decrease in serum albumin concentration could be attributed to pregnancy-related plasma expansion with hemodilution while increased ALP activity with pregnancy could be ascribed to production of placental isoenzyme and fetal bone marrow development.24
According to a study and finding of significant and progressive decrease in the levels of urea and creatinine in all trimester in Northern central Nigeria23 as was support by this study, which demonstrated lower urea and creatinine values though not partitioned by trimester. These higher and lower values were different across studies that could be the result of demographic and racial differences beside the variability of analytical methods, equipment types and reagents being used. Moreover, variations in such clinical chemistry parameters results could be mainly due to the effects of progesterone and estrogen that are produced largely by ovary and placenta to allow the fetus to grow, but at the same time attributed to the increased in cardiac output, renal plasma flow and glomerular filtration rate during pregnancy which in turn increases urea and creatinine excretion in pregnant than non-pregnant women.24
Generally, the current study was relatively close to the company derived values which are available for the non-pregnant women only. Lower limit of urea is lower than the company value (8 vs 15 mg/dL) though higher than Zimbabwe17 (3.9 gmmg/dL) and Ghana16 (5.4 gmmg/dL). Bilirubin direct and total upper limit values were almost two times lower when compared to the company value but close to the RI from Zimbabwe17 and USA18 studies (total bilirubin for example, 0.2–1.11 for the current study vs 0.3–1.0 mg/dL). The upper limit of total protein was comparable to the company value and the other studies (except a study from Amhara region15 and Zimbabwe).17
The proportion of %OOR values for commonly evaluated LFT and RFT; ALT, AST, bilirubin (direct) bilirubin (total), urea and creatinine during screening or enrollment and safety monitoring of participants in the clinical trial studies was up to 6.6%, 4.2%, 23.6%, 9.6, 69%, and 30.9%, respectively for pregnant and 1.8%, 2.5%, 29%, 4.8%, 53%, and 7.5%, respectively for non-pregnant women based on currently deserved insert kit reference interval as compared to result of this study. Moreover, higher %OOR values were also observed in albumin (60%), total protein (35%) and bilirubin (direct) (23.6%) for pregnant and albumin (24%), total protein (3.7%) and bilirubin (direct)(29%) for non-pregnant women. This means that based on insert kit claimed RIs applied in the study area, up to 60%, for example for total protein, in pregnant women that could be of potential study participants would have been declared as having abnormal results or if enrolled, would be reported as having adverse events (AEs). The theme is on how such significant numbers of eligible study participants would wrongly either have been affirmed as abnormal results or admitted participants would be reported as having adverse events. These all favor an implication in need of establishing local population-based RIs for their valid uses in medical care setups.
Conclusion
There was significant variation for the majority of common liver and renal function biochemistry parameters between pregnant and non-pregnant women. Reference intervals established from samples of non-pregnant women are not necessarily applicable for passing medical decision for pregnant. Thus, it will be paramount important to use special reference interval specific to pregnancy. Therefore, biochemical parameters reference intervals established could be of valuable for diagnosis, treatment, and follow-ups, clinical trial studies for pregnant and non-pregnant in the region and, also for other stakeholders in need of it. However, in every region of different population must establish their own reference ranges in accordance with the characteristics of their population.
Abbreviations
AAU, Addis Ababa University; AB, antibody; AE, adverse event; AG, antigen; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; APHIDB, Amhara Public Health Institute Dessie Branch; AST, aspartate aminotransferase; BIL, bilirubin; BMI, body mass index; BUN, blood urea nitrogen; CI, confidence interval; CLSI, Clinical Laboratory Standards Institute; CREA, creatinine; DADIS, Division of AIDS; DBIL, direct bilirubin; DRHRL, Dessie Regional Health Research Laboratory; ELISA, enzyme linked immunosorbent assay; ETB, Ethiopian Birr; HBV, hepatitis B virus; HCG, human chorionic gonadotropin; HCV, hepatitis C virus; HGB, hemoglobin; HBsAG, hepatitis B surface antigen; HCT, hematocrit; HIV, human immunodeficiency virus; IFCC, International Federation for Clinical Chemistry; IU, international unit; kg, kilogram; L, liter; LFT, liver function test; m, meter; NCCLS, National Committee for Clinical Laboratory standard; OOR, out-of-range; RFT, renal function test; SD, standard deviation; SPSS, Statistical Package for Social Sciences; S Wollo, South Wollo; TP, total protein; UA, uric acid; VCT, voluntary counseling and testing; VDRL, Venereal Disease Research Laboratory.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical consideration
The study was conducted following the ethical requirements established in the Declaration of Helsinki of 1964 and its sixth revision of 2089.25 It was conducted after ethical approval was obtained from Research and Ethics Review Board of the Addis Ababa University. Informed written consent was obtained from each study participant above 18 years old while their parents informed written consent was obtained for participants under 18 years of age before the actual data collection. Individual’s positive for the screened infections and other disease conditions had been linked to nearby government health institution for further diagnosis and treatment accordingly. Information obtained at any point of the study was kept confidential.
Acknowledgments
The authors would like to extend their appreciation to Addis Ababa University to give us this chance of doing this thesis,26 Dessie Regional Health Research Laboratory staffs for their help in the processing and analyzing of laboratory tests and. Our sincere respect is also forwarded for study participants for their willingness, health extension workers for their contribution in the data collection. Finally, we would like to pass our special affection for study participants for their willingness, our parents and to the many people, in many locations, who so generously contributed to the work presented in this study.
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Huma T, Waheed U. The need to establish reference ranges. J Pub Health Bio Sci. 2013;2(2):188–190.
2. Solberg HE. Approved recommendation (1986) on the theory of reference values. Part 1. The concept of reference values. Clinica Chimica Acta. 1987;165(1):111–118. doi:10.1016/0009-8981(87)90224-5
3. Murphy EA. The normal, and the perils of the sylleptic argument. Perspect Biol Med. 1972;15(4):566–582. doi:10.1353/pbm.1972.0003
4. NCCLS. How to Define and Determine Reference Intervals in the Clinical Laboratory; Approved Guideline.
5. Ceriotti F, Hinzmann R, Panteghini M. Reference intervals: the way forward. Ann Clin Biochem. 2009;46(1):8–17. doi:10.1258/acb.2008.008170
6. Karita E, Ketter N, Price MA, et al.. CLSI-derived hematology and biochemistry reference intervals for healthy adults in eastern and Southern Africa. PloS one. 2009;4(2):e4401. doi:10.1371/journal.pone.0004401
7. Kibaya RS, Bautista CT, Sawe FK, et al.. Reference ranges for the clinical laboratory derived from a rural population in Kericho, Kenya. PloS one. 2008;3(10):e3327. doi:10.1371/journal.pone.0003327
8. Saathoff E, Schneider P, Kleinfeldt V, et al.. Laboratory reference values for healthy adults from southern Tanzania. Trop Med Int Health. 2008;13(5):612–625. doi:10.1111/j.1365-3156.2008.02047.x
9. Ethiopian demography and health (internet source). Amhara landforms, climate and economy; 2008. Available from: http://www.ethiodemographyandhealth.org/Amhara.html.
10. Mekonnen Z, Amuamuta A, Mulu W, et al.. Clinical chemistry reference intervals of healthy adult populations in Gojjam Zones of Amhara National Regional State, Northwest Ethiopia. PloS one. 2017;12(9):e0184665. doi:10.1371/journal.pone.0184665
11. Adeli K, Higgins V, Nieuwesteeg M, et al.. Biochemical marker reference values across pediatric, adult, and geriatric ages: establishment of robust pediatric and adult reference intervals on the basis of the Canadian Health Measures Survey. Clin Chem. 2015;61(8):1049–1062. doi:10.1373/clinchem.2015.240515
12. Ezzelle J, Rodriguez-Chavez IR, Darden JM, et al.. Guidelines on good clinical laboratory practice: bridging operations between research and clinical research laboratories. J Pharm Biomed Anal. 2008;46(1):18–29. doi:10.1016/j.jpba.2007.10.010
13. Chromiński K, Tkacz M. Comparison of outlier detection methods in biomedical data. J Med Inform Tech. 2010;16:89–94.
14. Woldemichael K, Haileamlak A, Muluneh AT, et al.. Biochemical Profile at Gilgel Gibe Field Research Center, Southwest Ethiopia. Ethiop J Health Sci. 2012;22(4):50–60.
15. Abebe M, Melku M, Enawgaw B, et al.. Reference intervals of routine clinical chemistry parameters among apparently healthy young adults in Amhara National Regional State, Ethiopia. PloS one. 2018;13(8):e0201782. doi:10.1371/journal.pone.0201782
16. Dosoo DK, Kayan K, Adu-Gyasi D, et al.. Haematological and biochemical reference values for healthy adults in the middle belt of Ghana. PloS one. 2012;7(4):e36308. doi:10.1371/journal.pone.0036308
17. Samaneka WP, Mandozana G, Tinago W, et al.. Adult hematology and clinical chemistry laboratory reference ranges in a Zimbabwean population. PloS one. 2016;11(11):e0165821. doi:10.1371/journal.pone.0165821
18. Kratz A, Ferraro M, Sluss PM, Lewandrowski KB. Normal reference laboratory values. N Engl J Med. 2004;351(15):1548–1563. doi:10.1056/NEJMcpc049016
19. Lo YC, Armbruster DA. Reference intervals of common clinical chemistry analytes for adults in Hong Kong. EJIFCC. 2012;23(1):5.
20. Mutua DN, Mwaniki Njagi EN, Orinda G. Liver function tests in normal pregnant women. J Liver. 2018;7:228. doi:10.4172/2167-0889.1000228
21. Gohel MG, Joshi AG, Anand JS, Makadia JS, Kamariya CP. Evaluation of changes in liver function test in first, second and third trimester of normal pregnancy. Int J Reprod Contracep Obstet Gynecol. 2013;2(4):616–620. doi:10.5455/2320-1770.ijrcog20131225
22. Bacq Y, Zarka O, Brechot J, et al.. Liver function tests in normal pregnancy: a prospective study of 103 pregnant women and 103 matched controls. Hepatology. 1996;23(5):1030–1034. doi:10.1002/hep.510230514
23. Patricia OO, Christiana BA, Raphael OJ. Evaluation of changes in renal functions of pregnant women attending ante-natal clinic in Vom Plateau State, North-Central Nigeria. Arch Appl Sci Res. 2013;5:111–116.
24. Soma-Pillay P, Catherine NP, Tolppanen H, Mebazaa A, Tolppanen H, Mebazaa A. Physiological changes in pregnancy. Cardiovasc J Afr. 2016;27(2):89. doi:10.5830/CVJA-2016-021
25. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
26. Assen MM. Establishment of reference intervals for the common renal and liver function clinical chemistry parameters among apparently healthy pregnant and non-pregnant women in South Wollo zone, Amhara National Regional State, northeast Ethiopia MSc thesis: Addis Ababa university; 2019. Available from: http://213.55.95.56/browse?type=author&value=Assen%2C+Miftah+Mohammed.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.