Back to Journals » Breast Cancer: Targets and Therapy » Volume 9
Reduction in breast cancer susceptibility due to XbaI gene polymorphism of alpha estrogen receptor gene in Jordanians
Authors Atoum MF , Alzoughool F
Received 25 October 2016
Accepted for publication 16 December 2016
Published 24 January 2017 Volume 2017:9 Pages 45—49
DOI https://doi.org/10.2147/BCTT.S125652
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pranela Rameshwar
Manar Fayiz Atoum, Foad Alzoughool
Department of Medical Laboratory Sciences, Faculty of Allied Health, Hashemite University, Zarqa, Jordan
Abstract: Breast cancer is a global health concern among women worldwide. Estrogen receptor alpha (ERα) mediates diverse polymorphic effects in breast tissues that may relate to breast cancer susceptibility. The aim of this study was to evaluate the effect of −397 PvuII (T/C) and −351 XbaI (A/G) restriction fragment length polymorphism within intron 1 of ERα, and its effect on breast cancer susceptibility. A total of 156 women who were histopathologically diagnosed with breast cancer and 142 healthy Jordanian women were enrolled in this case–control study. Genomic DNA was extracted from whole peripheral blood, and the desired fragment was amplified using polymerase chain reaction followed by restriction digestion with PvuII and XbaI restriction enzymes. The results showed no significant association between PvuII polymorphism and breast cancer risk. However, a significant association was found between XbaI polymorphism and reduction in breast cancer risk within the “x” allele of heterozygotes (odds ratio [OR] 0.199, 95% confidence interval [CI] 0.09–0.044) and heterozygotes (OR 0.208, 95% CI 0.09–0.047). The combined analysis of PvuII and XbaI polymorphisms revealed a synergistic effect of Pp/Xx and pp/xx genotypes and a significant reduction in breast cancer risk with these genotypes. The results also showed no statistical differences among PvuII or XbaI polymorphisms based on stage, ER, progesterone receptor and expression of hormone receptor such as human epidermal growth factor receptor 2. This case–control study showed that XbaI polymorphism of alpha estrogen gene modified and reduced breast cancer susceptibility among Jordanians.
Keywords: breast cancer, gene polymorphism, XbaI
Introduction
Breast cancer is a serious life-threatening condition affecting women worldwide.1 It ranks second among various causes of cancer death in women.2 In Jordan, 42.9% of cancers diagnosed in women are breast cancers.3 Understanding the pathogenesis of breast cancer is important in the innovative therapies for breast cancer diagnosis and treatment. One of the most important risks of breast cancer is endogenous hormone level. The genes of estrogen receptor (ER) pathway may influence breast cancer risk by two possible mechanisms: 1) ER-mediated stimulation of breast cell proliferation with a concomitant enhanced rate of mutations and 2) metabolism of estradiol to genotoxic metabolites with a resulting increase in DNA mutations.4
Estrogen is a steroid hormone that is essential for the development of female secondary sexual characters.4 Under normal conditions, it affects the growth, differentiation and function of breast,5 uterus, vagina, ovary, testis, epididymis and prostate.6 However, under abnormal conditions, exposure to estrogen predisposes females to a risk of developing breast cancer. Current hormonal therapies have benefited breast cancer patients. However, their success is often limited to patients whose tumors express ERα, but entirely ineffective in ER-negative breast cancers.7
ERs are of special interest as their expression is always affected in malignant cancer cells.8 ERs are nuclear receptor proteins that have an estrogen-binding domain and a DNA-binding domain.9 There are two types of ERs: 1) ERα that is localized on 6q25.1 and associated with breast cancer and 2) ERβ that is located on chromosome 14q22-24 and functions as a tumor suppressor.10 Polymorphisms in the ER genes may determine the biosynthetic activity of circulating estrogen.
Nearly over half of all types of breast cancers overexpress ERα and ~70% of these respond to anti-estrogen.11 Elevated levels of ERα in benign breast epithelium indicate an increased risk of breast cancer.12 ERα status is essential in making decisions about endocrine therapy with anti-estrogen since ERα (+) status correlates with improved prognosis and better overall survival.9
ERα is regarded as one of the significant prognostic factors for breast, ovarian and lung cancers,13 miscarriage and endometriosis. Single nucleotide polymorphisms were associated with increased risk of cancers.14 The most characterized single nucleotide polymorphisms of ERα located in the first intron are the c454-397T>C- and c454-351A>G-site polymorphisms. These polymorphisms are 397 and 351 base pairs upstream of exon 2 and are detected by restriction enzymes PvuII or XbaI, respectively.15 These polymorphisms were correlated with breast cancer incidence.16 PvuII altered protein expression by interfering with ERα mRNA splicing, but the mechanism of action of XbaI is still unknown.
Genetic studies on cancer are scarce in Jordan and only few studies have screened gene polymorphism in breast cancer.17–20 To our knowledge, no studies have determined the role of ERα PvuII and XbaI polymorphisms in breast cancer susceptibility among Jordanians. Therefore, the aim of this study was to determine the frequency of ERα PvuII and XbaI genotypes and the possible involvement of these polymorphisms in breast cancer susceptibility among Jordanian women with breast cancer.
Materials and methods
This study enrolled 156 women who were histologically and clinically diagnosed with breast cancer and 142 age-matched controls with no previous family history of any cancer from Al-Basheer Hospital (2013–2015). International review board approval was obtained from the Hashemite University international review board committee and signed consent forms were obtained from all patients. All clinical samples were staged according to Atoum et al.21
Genomic DNA was extracted from all blood samples using Wizard extraction kit (Promega, Madison, WI, USA) and was stored at −80°C. Polymerase chain reaction was carried out to amplify intron 1–exon 2 region of ERα gene in three steps with annealing at 60°C in a thermal cycler (Bio-Rad, Munich, Germany) in 25 μL reaction volume. Each reaction mixture contained 10 pmol of each primer, 3 mM of MgCl2, 100 nM of each deoxynucleotide triphosphate, 1 unit of Taq DNA polymerase and 100 ng of genomic DNA. Primer sequences are as follows: forward primer: 5′ AGG GTT ATG TGG CAA TGA CG 3′; reverse primer: 5′ CCT GCA CCA GAA TAT GTT ACC T 3′. The amplified fragment of 1374 base pairs was then digested with PvuII and XbaI. The presence of PvuII restriction site on both alleles (pp) was identified by two fragments (850, 450), while the presence of XbaI on both alleles (xx) was identified by two fragments (900, 400). Digested fragments were then electrophoresed on 1.5% agarose gel with ethidium bromide.
Statistical analysis
Statistical analysis was carried out using SPSS software. The distribution of genotypes in cancer patients and controls was evaluated by the Chi-square test. Odds ratio (OR) and 95% confidence interval (CI) were used to estimate the relative risk. A p-value <0.05 was considered statistically significant.
Results
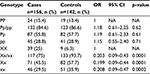
Genotypic distribution of ERα PvuII and XbaI is shown in Table 1. With reference to PP genotype, no significant associations were found between the variations of breast cancer risk and P alleles in homozygotes or heterozygotes. There was no statistical difference in the prevalence of P allele between cases (84.6%) and controls (86.6%). Nearly 15.4% of the patients and 13.4% of the controls were homozygous for P allele. About 55.8% of the patients and 57.7% of the controls were heterozygous for this allele. No significant association was found between breast cancer risk and P allele in heterozygotes (OR 1.19, 95% CI 0.61–2.33) or homozygotes (OR 1.15, 95% CI 0.55–2.40). A statistical difference was observed in the prevalence of X allele between cases (75%) and controls (93.7%). XbaI polymorphism in x allele was more prevalent among controls (35.9%) compared to patients with breast cancer (29.5%), (OR 0.208, 95% CI 0.09–0.47).
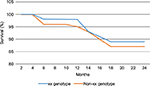  | Figure 1 Kaplan–Meier survival plot showing differences in survival probability among xx breast cancer genotype compared to non-xx genotype. |
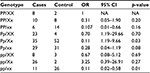
Combined analysis of PvuII and XbaI genotypes revealed a synergistic effect of Pp/Xx (OR 0.11, 95% CI 1.19–9.66, p = 0.03) and pp/xx (OR 0.11, 95% CI 0.02–0.58, p = 0.01) as carriers of these genotypes showed reduced risk of breast cancer (Table 2).
  | Table 2 Breast cancer risk in relation to PvuII and XbaI combination genotypes Note: Significant p-value <0.05. Abbreviations: CI, confidence interval; OR, odds ratio; NA, not available. |
Table 3 shows the histological subtypes of different PvuII and XbaI genotypes among women with breast cancer. No statistical difference was found within either PvuII or XbaI genotypes (p = 0.83, 0.81, respectively) among different breast cancer stages.
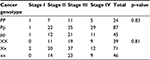  | Table 3 Breast cancer patient stages among PvuII and XbaI genotypes Note: Significant p-value <0.05. |
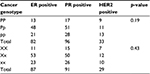
No statistical differences were found among PvuII or XbaI polymorphisms based on ER, progesterone receptor and expression of hormone receptor such as human epidermal growth factor receptor 2 (HER2) (Table 4).
Discussion
This case–control study was conducted to analyze the association between PvuII and XbaI polymorphisms and breast cancer risk among Jordanian females. No association between the variation of breast cancer risk and pp genotype was found compared to PP genotype, but a significant association was found between reduced breast cancer risk and xx genotype compared to XX genotype. Also no association was found between the variation of breast cancer risk and PvuII or XbaI genotypes based on breast cancer stages, presence of ER, progesterone receptor and expression of hormone receptor HER2 among breast cancer females. The results of this study are in contrast with those of a Chinese study that analyzed PvuII and XbaI for breast cancer risk. A significant association was found between Pp and pp genotypes and increased breast cancer risk, whereas no significant association was found between Xx and xx genotypes and increased breast cancer risk.22 The results of this study are also in contrast with the results of another Chinese study that was conducted among another Chinese group23; the results showed no effect of PvuII and XbaI polymorphisms on breast cancer risk. This study showed a significant association between reduced breast cancer risk and xx genotype, in contrast to the results of a study on Pakistani breast cancer patients,24 and similar to the results of a study on Portuguese population where xx genotype was associated with decreased breast cancer risk.16 This contradiction may be due to the large and different biological processes where alpha estrogens and their receptors are involved. The possible mechanisms by which PvuII and XbaI polymorphisms affect breast cancer are still not known. The position of PvuII and XbaI polymorphisms in the intron near the promoter may contain regulatory sequences like enhancers that affect the expression of alpha receptor gene. Intronic enhancers act as regulatory sequences that control transcriptional regulation.25 Nowadays, ERα has been signaled as a possible marker for many diseases, including different cancers and immunological diseases.
Conclusion
The results of this case–control study showed a significant reduction in breast cancer risk in women carrying xx genotype. So further studies are needed with larger sample size among different populations to clarify how ERα and XbaI may modify an individual’s susceptibility to breast cancer risk.
Acknowledgment
We acknowledge the Hashemite University for the financial support to this project and the workers at Al-Basher Hospitals for their help in sample collection.
Disclosure
The authors report no conflicts of interest in this work.
References
Breast Cancer Facts & Figures [webpage on the Internet]. American Cancer Society. Available from: http://www.cancer.org/research/cancer-facts-statistics/breast-cancer-facts-figures.html. Accessed January 19, 2017. | ||
http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key-statistics. Accessed December 1, 2016. | ||
Jordanian Ministry of Health, 2008. Available from: http://www.breastcancer.org. Accessed December 1, 2016. | ||
Santen RJ, Yue W, Wang JP. Estrogen metabolites and breast cancer. Steroids. 2015;99(Pt A):61–66. | ||
Lluch A, Eroles P, Perez-Fidalgo JA. Emerging EGFR antagonists for breast cancer. Expert Opin Emerg Drugs. 2014;19(2):165–181. | ||
Shao R, Shi J, Liu H, et al. Epithelial-to-mesenchymal transition and estrogen receptor α mediated epithelial dedifferentiation mark the development of benign prostatic hyperplasia. Prostate. 2014;74(9):970–982. | ||
Reeder-Hayes KE, Carey LA, Sikov WM. Clinical trials in triple negative breast cancer. Breast Dis. 2010;32(1–2):123–136. | ||
Izadi P, Mehrdad N, Foruzandeh F, Reza NM. Association of poor prognosis subtypes of breast cancer with estrogen receptor alpha methylation in Iranian women. Asian Pac J Cancer Prev. 2012;13(8):4113–4117. | ||
Heger Z, Rodrigo MA, Krizkova S, et al. Identification of estrogen receptor proteins in breast cancer cells using matrix-assisted laser desorption/ionization time of flight mass spectrometry (Review). Oncol Lett. 2014;7(5):1341–1344. | ||
Nakamura Y, Felizola SJ, Kurotaki Y, et al. Cyclin D1 (CCND1) expression is involved in estrogen receptor beta (ERβ) in human prostate cancer. Prostate. 2013;73(6):590–595. | ||
Santos-Martínez N, Díaz L, Ordaz-Rosado D, et al. Calcitriol restores antiestrogen responsiveness in estrogen receptor negative breast cancer cells: a potential new therapeutic approach. BMC Cancer. 2014;14:230. | ||
Ali S, Coombes RC. Estrogen receptor alpha in human breast cancer: occurrence and significance. J Mammary Gland Biol Neoplasia. 2000;5(3):271–281. | ||
Kuo LC, Cheng LC, Lin CJ, Li LA. Dioxin and estrogen signaling in lung adenocarcinoma cells with different aryl hydrocarbon receptor/estrogen receptor α phenotypes. Am J Respir Cell Mol Biol. 2013;49(6):1064–1073. | ||
Darabi H, Czene K, Wedrén S, et al. Genetic variation in the androgen estrogen conversion pathway in relation to breast cancer prognosticators. Breast Cancer Res Treat. 2011;127(2):503–509. | ||
Araújo KL, Rezende LC, Souza LC, et al. Prevalence of estrogen receptor alpha PvuII(c454-397T>C) and XbaI (c454A>G) polymorphisms in a population of Brazilian women. Braz Arch Biol Technol. 2011;54(6). | ||
Ramalhinho AC, Marques J, Fonseca-Moutinho JA, Breitenfeld L. Genetic polymorphims of estrogen receptor alpha -397 PvuII(T>C) and -351 XbaI (A>G) in a portuguese population: prevalence and relation with breast cancer susceptibility. Mol Biol Rep. 2013;40(8):5093–5103. | ||
Atoum MF, Tanashat RQ, Mahmoud SA. Negative association of the HLA-DQB1*02 allele with breast cancer development among Jordanians. Asian Pac J Cancer Prev. 2013;14(11):7007–7010. | ||
Atoum MF, Tchoporyan MN. Association between circulating vitamin D, the Taq1 vitamin D receptor gene polymorphism and colorectal cancer risk among Jordanians. Asian Pac J Cancer Prev. 2014;15(17):7337–7341. | ||
Atoum MF, AlKateeb D, AlHaj Mahmoud SA. The Fok1 vitamin D receptor gene polymorphism and 25(OH) D serum levels and prostate cancer among Jordanian men. Asian Pac J Cancer Prev. 2015;16(6):2227–2230. | ||
Atoum MF. ACC interleukin-10 gene promoter haplotype as a breast cancer risk factor predictor among Jordanian females. Onco Targets Ther. 2016;9:3353–3357. | ||
Atoum MF, Hourani HM, Shoter A, Al-Raheem SN, Al Muhrib TK. TNM staging and classification (familial and nonfamilial) of breast cancer in Jordanian females. Indian J Cancer. 2010;47(2):194–198. | ||
Cai Q, Shu XO, Jin F, et al. Genetic polymorphisms in the estrogen receptor alpha gene and risk of breast cancer: results from the Shanghai breast cancer study. Cancer Epidemiol Biomarkers Prev. 2003;12(9):853–859. | ||
Shen Y, Li DK, Wu J, Zhang Z, Gao E. Joint effects of the CYP1A1 MspI, ERalpha PvuII, and ERalpha XbaI polymorphisms on the risk of breast cancer: results from a population-based case-control study in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2006;15(2):342–347. | ||
Javed S, Ali M, Sadia S, et al. Combined effect of menopause age and genotype on occurrence of breast cancer risk in Pakistani population. Maturitas. 2011;69(4):377–382. | ||
Park SG, Hannenhalli S, Choi SS. Conservation in first introns is positively associated with the number of exons within genes and the presence of regulatory epigenetic signals. BMC Genomics. 2014;15:526. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.