Back to Journals » Neuropsychiatric Disease and Treatment » Volume 13
Reduced prefrontal activation during verbal fluency task in chronic insomnia disorder: a multichannel near-infrared spectroscopy study
Authors Sun JJ, Liu XM, Shen CY, Zhang XQ, Sun GX, Feng K, Xu B, Ren XJ, Ma XY, Liu PZ
Received 11 March 2017
Accepted for publication 31 May 2017
Published 30 June 2017 Volume 2017:13 Pages 1723—1731
DOI https://doi.org/10.2147/NDT.S136774
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Wai Kwong Tang
Jing-Jing Sun,1,2 Xiao-Min Liu,2 Chen-Yu Shen,2 Xiao-Qian Zhang,1,2 Gao-Xiang Sun,2 Kun Feng,2 Bo Xu,2 Xia-Jin Ren,1,2 Xiang-Yun Ma,1,2 Po-Zi Liu2
1Medical Center, Tsinghua University, Beijing, China, 2Department of Psychiatry, YuQuan Hospital, Tsinghua University, Beijing, China
Purpose: Daytime complaints such as memory and attention deficits and failure to accomplish daily tasks are common in insomnia patients. However, objective psychological tests to detect cognitive impairment are equivocal. Neural function associated with cognitive performance may explain the discrepancy. The aim of this study was to investigate the hemodynamic response patterns of patients with chronic insomnia disorder (CID) using the noninvasive and low-cost functional neuroimaging technique of multichannel near-infrared spectroscopy (NIRS) in order to identify changes of neural function associated with cognitive performance.
Patients and methods: Twenty-four CID patients and twenty-five healthy controls matched for age, right-hand dominance, educational level, and gender were examined during verbal fluency tasks (VFT) using NIRS. A covariance analysis was conducted to analyze differences of oxygenated hemoglobin (oxy-Hb) changes in prefrontal cortex (PFC) between the two groups and reduce the influence of the severity of depression. Pearson correlation coeffcients were calculated to examine the relationship between the oxy-Hb changes, with the severity of insomnia and depressive symptoms assessed by the Pittsburgh Sleep Quality Index (PSQI) and the Hamilton Rating Scale for Depression (HAMD).
Results: The number of words generated during the VFT in CID groups showed no statistical differences with healthy controls. CID patients showed hypoactivation in the PFC during the cognitive task. In addition, we found that the function of left orbitofrontal cortex (OFC) during the VFT was significantly negatively correlated with the PSQI scores and the function of right dorsolateral PFC (DLPFC) was significantly negatively correlated with the HAMD scores.
Conclusion: The present study detected dysfunctions in PFC in spite of intact performance which indicates the role of PFC in the neurophysiological underpinnings. Left OFC function is associated with insomnia symptoms and right DLPFC function is associated with depressive symptoms.
Keywords: chronic insomnia disorder, near-infrared spectroscopy, NIRS, prefrontal cortex, verbal fluency task, depressive symptoms
Introduction
Insomnia is a common complaint with a prevalence of ~4%–22% in the adult population worldwide.1–3 With social pressure increasing and population aging,4–6 the prevalence of insomnia has increased. Daytime complaints such as memory and attention deficits and failure to accomplish daily tasks must be present for the diagnosis of insomnia disorder7 and are the main reasons that insomniacs seek medical intervention.8 Cognitive dysfunction has been a prime criterion for insomnia diagnosis.7 Objective evidences of cognitive impairment in insomnia patients would be expected. A number of studies have examined neuropsychological tests including attention,9–12 memory,10,13–16 and executive function17,18 in insomniacs and found that they exhibit worse performance in reaction time and attention lapses.9,10 However, there are inconsistent studies that have reported no attention-related deficits.11,12 Intact performance of memory and executive function also have been reported in insomnia patients in spite of deficits in accessing semantic memory19 and verbal reasoning tasks.17 In other words, cognitive performance in insomnia patients does not seem to show consistent changes.8,18
There are several hypotheses that may explain these discrepant findings. First, the insomnia patients characteristically own high level of perfectionism20 that may mask their performance. Second, the prototypical neuropsychological tests are insensitive to such differences. Third, the changes of neural function associated with cognitive performance may explain this discrepancy between the subjective complaints and their objective performance. Evidence from functional magnetic resonance imaging (fMRI) using cognitive tasks supports the hypothesis that there are neural function changes in insomnia patients compared with individuals who have no problems with sleep.21–23 For example, the fMRI study showed decreased cerebral responses in the medial and inferior frontal regions during letter and category fluency tasks, although no task performance impairment was found in insomnia patients.23 Drummond et al22 showed diminished activation in the frontoparietal area and thalamus (nuclei with connections to cortical sites) during working memory tasks in primary patients. In addition, other functional neuroimaging studies used fMRI without cognitive task24–26 and positron emission tomography (PET)27,28 to evaluate insomnia patients also identified dysfunction in brain regions, including the prefrontal cortex (PFC). Li et al24 studied 55 patients with primary insomnia and 44 healthy controls using resting-state fMRI. Decreased brain activity in the left orbitofrontal cortex (OFC)/inferior frontal gyrus and middle frontal gyrus were identified in insomnia patients. In the most frequently cited neuroimaging study of insomnia patients, Nofzinger et al28 demonstrated reduced global cerebral glucose metabolism in the PFC while they were awake. These studies suggest that neural activity may be implicated in the pathophysiology of insomnia. On the basis of the results of previous studies, it would be reasonable to hypothesize that the insomnia patients exhibit dysfunction of PFC. The relationship between PFC function and insomnia-induced cognitive performance may be significant.
Near-infrared spectroscopy (NIRS) is a promising functional neuroimaging technique that can measure changes of oxygenated and deoxygenated hemoglobin concentrations (oxy-Hb and deoxy-Hb, respectively) induced by neural activity,29 which exhibit a strong correlation with the blood oxygenation level-dependent signals of fMRI.30 It has the advantages of (1) being noninvasive, (2) providing measurements in the natural environment, (3) having good time-resolution, and (4) being performed with different psychiatric conditions, such as major depression disorder (MDD),31 bipolar disorder,32 and schizophrenia.33,34 Recent studies using NIRS have suggested that left prefrontal hypoactivation is related to the sleep complaints that occur in MDD patients35 and that the self-rated sleep disturbances in MDD patients are associated with the prefrontal reactivity areas.36 However, to the best of our knowledge, studies using NIRS to investigate insomnia disorder are lacking.
Verbal fluency tasks (VFT) are psychological tests that have participants generate as many words as possible in limited time.37 The test is a valid index of verbal ability and executive control function because it demands not only verbal retrieval but also self-monitoring and inhibition of responses when time is up.38 It has been used to examine PFC activation in healthy controls and patients with mental disorders when combined with NIRS.32,39,40 Sleep loss can impair performance of this task.41 As a natural form of chronically disturbed sleep, it is reasonable to utilize VFT to find cognitive impairment in insomniacs.
It is known that depression levels in insomnia patients are usually higher than in people who sleep well.42 Moreover, previous studies have suggested that the severity of depression show a positive correlation with hemodynamic function in the PFC during a semantic categories verbal fluency.43 Noda et al35 using a letter version of VFT found that the severity of depression symptoms was negatively correlated with the activation of the right DLPFC. We cannot conclude that PFC dysfunction results from insomnia alone because depressive symptoms were rarely examined during these evaluations of cognitive performance.
In this study, NIRS was used to investigate the hemodynamic response patterns of patients with chronic insomnia disorder (CID) in the PFC during the VFT. We hypothesized that behavioral performance would be impaired and prefrontal activation would be decreased in CID during VFT using NIRS. In addition, this study investigated the relationship between the severity of insomnia symptoms, depressive symptoms, and oxy-Hb exchange during the VFT by NIRS separately.
Patients and methods
Subjects
Twenty-four patients with CID (13 males, 11 females) aged from 23 to 62 years were recruited from outpatients in YuQuan Hospital for this study. Twenty-five healthy controls were matched with the patients for age, right-hand dominance, educational level, and gender were also recruited from the local community. The controls were aged 21–59 years, and without sleep disorders. The CID subjects met the diagnostic criteria according to the International Classification of Sleep Disorders, 3rd version (American Academy of Sleep Medicine, 2014), with the score of Pittsburgh Sleep Quality Index (PSQI) >7.44
All subjects were evaluated with an unstructured clinical interview for the history of sleep and medical disorders, and with a structured interview (the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, version 5 administered by an experienced psychiatrist) to document life history of psychiatric disorders.
None of the subjects had a history of mood disorders or other psychiatric diagnoses. Patients with other sleep disorders such as restless leg syndrome, narcolepsy, and obstructive sleep apnea were excluded. In addition, the participants were free of medications. The study was approved by the Clinical Trial Ethics Committee of YuQuan Hospital, and all the subjects provided informed consent in writing before the start of the study.
Clinical assessment
The severity of disturbances in sleep quality was evaluated with the PSQI by trained doctoral-level interviewers. All CID patients had PSQI scores >7. To assess the presence and severity of depressive symptoms on daytime impairment, the Hamilton Rating Scale for Depression (HAMD, 24-item)45 was assessed for the participants.
Activation task
The activation task was a semantic category version of the VFT, and changes in the relative concentrations of hemoglobin were measured during the VFT.39,40,46 The task consisted of a 30-s pre-task baseline, a 30-s VFT, and a 30-s post-task baseline. During the VFT, participants were required to describe verbally as many items as possible belonging to a given semantic categories (vegetables, family applications, four-footed animals, and fruits). Cognitive performance scores were determined by the number of correct words generated during the task by a participant. All the subjects participated in the task during the hours of 9:00 a.m. and 12:00 a.m.
NIRS measurements
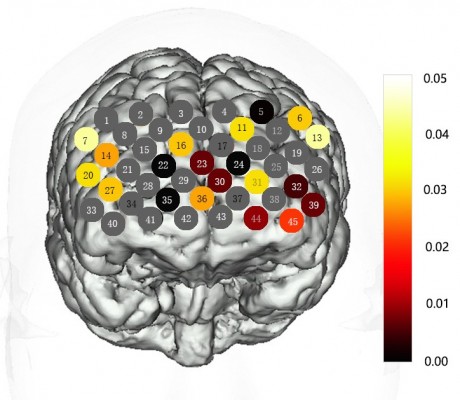
The relative concentration changes of oxy-Hb, deoxy-Hb, and total-Hb during the VFT were measured using a 45-channel NIRS system (FOIRE-3000, Shimadzu Corporation, Japan) based on the modified Beer–Lambert law, and 14 pairs of emitter and detector probes were placed on the frontal region of each participant with pairs of probes at a distance of 3.0 cm (Figure 1). The most inferior probes were positioned along the Fp1–Fp2 line according to the International 10–20 system of electroencephalogram electrode placement.47 The measurement point between each pair of emission-detector probes was defined as a “channel.”
  | Figure 1 The probe arrangement. |
Statistical analysis
For analyses of the demographic and clinical variables, Student’s t-test was performed to compare means between the CID patients and control groups, except the gender item (chi-squared test).
For the NIRS data, we calculated the value of the oxy-Hb increase, which was calculated by subtracting the mean oxy-Hb of the pre-task period from the mean oxy-Hb of the task period. We then used a covariance analysis to analyze the difference of the oxy-Hb increase between the groups to reduce the influence of the severity of depression. Oxy-Hb was selected as statistical analyses for the reason that oxy-Hb change was considered as the most directly reflected changes of regional cerebral blood flow.48,49
For the CID group, Pearson correlation coefficients were calculated to examine the relationship between the mean oxy-Hb changes and the scores of HAMD, PSQI for each channel. Statistical calculations were performed using the Statistical Package for the Social Sciences for Windows (SPSS version 22.0; IBM Corp., Armonk, NY, USA). For multiple comparison analyses of the 45-channel NIRS data, the false discovery rate (FDR) procedure was adopted to ensure that no more than 5% false positives were observed on average (FDR corrected).50
Results
Demographic characteristics
The demographic characteristics of participants are presented in Table 1. Age, gender, and education level were not different between normal controls and CID patients, but the scores of HAMD and PSQI were significantly different between the two groups. As expected, the CID group had significant higher scores of HAMD and PSQI than the control group.
Task performance
During the VFT, the number of words generated showed no statistical differences between the two groups. As for the four items (vegetables, family applications, four-footed animals, and fruits), none of them showed statistical differences between the two groups (Table 1).
Group comparisons of NIRS activation
None of these channels exhibited significant interactions between HAMD levels and groups (P>0.05). As shown in Figure 2, the CID exhibited a significantly lower oxy-Hb activation than the healthy controls in 20 channels (Ch-5–7, 11, 13, 14, 16, 20, 22–24, 27, 30–32, 35, 36, 39, 44, 45; FDR-corrected P<0.05) during the VFT, which were distributed predominantly in the bilateral ventrolateral PFC (VLPFC), OFC, and dorsolateral PFC (DLPFC). P-values of the 45 channels in CID patients compared with healthy controls during VFT are shown in Figure 2.
Correlation of NIRS activation and clinical assessment
In CID groups, a significant negative correlation occurred between the oxy-Hb changes and the PSQI scores in three channels (Ch-31, 37, and 43; r: −0.666, −0.618, and −0.578; FDR-corrected P<0.05), which were located approximately in the left OFC region (Figure 3). Meanwhile, the oxy-Hb changes during the tasks showed a negative correlation with HAMD scores in two channels (Ch-14 and 21; r: −0.672 and −0.575; FDR-corrected P<0.05) that were located approximately in the right DLPFC region (Figure 4).
Discussion
This research explored activation patterns in the cerebral cortex using NIRS during cognitive task in CID patients relative to matched controls, and we also explored the relationships between oxy-Hb changes and symptoms of insomnia and depression. To the best of our knowledge, this is the first study to evaluate functional NIRS signal differences combined with a VFT in CID patients.
Prefrontal activation and cognitive task performance
The present results demonstrated significantly reduced oxy-Hb activation in the PFC, mainly in the bilateral VLPFC, OFC, and DLPFC during VFT in CID patients compared to individuals with good sleep patterns. This result is consistent with previous imaging studies conducted with PET,27 single-photon emission computed tomography (SPECT),51 fMRI,23 and structural neuroimaging.52 Nofzinger et al28 studied insomnia patients with PET and demonstrated reduced global cerebral glucose metabolism in the PFC during waking hours. A previous SPECT51 study found that, compared with individuals who slept well, insomnia patients displayed significant decreases in blood flow in the frontal lobes during sleep. Altena et al23 using the letter and category fluency task examined 21 chronic insomnia patients measured by fMRI and showed significantly decreased cerebral responses in the medial and inferior frontal regions in insomnia patients, and that the activation of the cortex was increased after effective treatment, although no group difference in task performance was found. Dai et al26 found lower brain activity in bilateral frontal lobe in patients with insomnia using resting-state fMRI studies. Joo et al53 found a significantly smaller volume of gray matter in multiple brain regions, including the bilateral DLPFCs (bilateral superior, middle, and inferior frontal gyri and left orbitofrontal gyri) and medial frontal gyrus compared with healthy controls.
It should be noted that all of these brain imaging techniques including NIRS cannot measure absolute oxy-Hb concentration between individuals, but can measure changes of oxy-Hb concentration within an individual. As a result, four reasons may account for the prefrontal hypoactivation during the VFT: (1) lower absolute brain activation levels during task performance, (2) higher activation levels during the pre-task baseline period, (3) a combination of lower levels of activation of both states, or (4) higher levels of activation of both states. Prefrontal hypoactivation in the CID patients suggests that the prefrontal areas failed to obtain a corresponding increased blood supply to compensate for the consumed oxygen, which is crucial for proper neuronal activity, or that the hypoactivation was the result of prolonged hyperactivity according to the hyperarousal hypothesis.54 This hypothesis predicates that the CID group does not relax and the minds of these insomniacs are overactive. This state may lead to failure to reduce activation in the frontal areas, to the extent that occurs in the healthy control group during the resting state. Prefrontal hypoactivation may suggest reduced deactivation during the resting state. This inability to deactivate in insomniacs without challenged cognitive performance tasks may account for their major daytime feelings of fatigue, and lead to their reduced attention and memory function during many ordinary everyday tasks.55
VLPFC is the end area of the ventral pathway that brings stimuli’s information,56 and orbitofrontal is involved in monitoring the intensity of thermal stimuli.57 DLPFC can maintain the target information to the priority in bottom–up and top–down attention to avoid interference with the target irrelevant information.58 The dysfunction of this area reduce the filtering of stimuli’s information leading the insomniac cannot sleep with the irrelevant information.
In our study, although CID patients reported daytime feelings and complaints that are a defining feature, they did not show deficits on task performance compared to controls. Hypoactivation of these areas without cognitive impairment may suggest that neural processing of the intact task was different among the groups. According to the hyperarousal theory, which posits that individuals can rally cognitive resources with compensatory mechanisms to achieve cognitive performance,59 we speculate that other brain regions are individually or differentially recruited to allowed the insomniacs to perform equally well on short duration, challenging cognitive performance tasks. Hypoactivation of prefrontal areas may be a characteristic of CID patients in spite of the similar task performance of individuals who sleep well.
Association between NIRS signals and clinical symptoms
To our knowledge, abnormal prefrontal function is associated with many psychiatric disorders, including depression,31 bipolar disorder,32 and schizophrenia.33,34 In this study, although secondary insomnia caused by mood disorder was excluded, the HAMD scores of the CID group were significantly higher than those of the controls, which is consistent with previous studies.42 This paper shows the clinical value of NIRS data in identification of different symptoms by exploring the relationship between prefrontal function and the severity of insomnia symptoms and depressive symptoms.
Our correlational analyses showed an association between left OFC areas and insomnia symptoms and right DLPFC areas with depressive symptoms. As for insomnia symptoms, the negative correlation with oxy-Hb changes in the OFC areas activated by VFT in Ch-31, 37, and 43 may represent the neural vulnerability in CIDs. The oxy-Hb changes in the left OFC tend to increase gradually with declining sleep quality. These associations suggest that the function of the left OFC has a relationship with insomnia symptoms. Further studies are needed to clarify this relationship.
A previous study35 showed that the right frontopolar and orbitofrontal areas are related to the score of “insomnia early,” one of the items of HAMD. The item represents the state of insomnia. Another study36 evaluated insomnia symptoms with PSQI, for persistent changes of the sleep quality over 1 month, and demonstrated that the left prefrontal region is affected. Our study found that the left OFC is related to the severity of sleep complaints represented by PSQI. It may suggest that long-term disturbances of sleep quality mainly affect the left side of the prefrontal region, which is responsible for verbal function.
Individual differences in GM density in left inferior OFC were also correlated negatively with early morning awakening.60 Atrophy in the OFC was associated with sleep quality in a longitudinal analysis.61 Overall, the converging evidence highlights the importance of the OFC for insomnia symptoms. However, recent research results of fMRI and structural neuroimaging studies are not consistent with our results. They do not have a relationship with the PFC25 or exhibit different areas associated with insomnia symptoms.24,26,62
The following reasons may account for inconsistency of these studies. First, different instruments were applied in the different studies, including MRI and NIRS. MRI can detect subcortical signals, whereas NIRS has limited spatial resolution and depth sensitivity. Second, the different methodologies may affect the discrepancies in results. In this study, we utilized VFT, whereas previous studies observed correlations under the no-task state.24–26,62 Finally, the diversity of clinical manifestations of insomnia patients and duration of insomnia symptoms may contribute to the discrepancy.24,35,36
The depressive symptoms in this study were negatively correlated with oxy-Hb changes in Ch-14 and 21, near the region of the right DLPFC. This result was in-line with previous studies where subjects were MDD patients35 and schizophrenia with depressive symptoms.63 The right side of the brain is responsible for regulating emotions64 and the DLPFC is involved in cognition. The oxy-Hb changes in right DLPFC may become significant gradually with the increase in the severity of depressive symptoms. From a clinical viewpoint, the DLPFC plays an important role in assessment with depressive symptoms. Therefore, the results suggest that the function of the right DLPFC is associated with depressive symptoms. However, depression is a process of dynamic changes and can be thought of as common symptoms of various diseases. Whether the right DLPFC change is a common feature for depressive symptoms will need to be confirmed by further research.
Limitations
This study has several limitations that should be clarified. First, NIRS is an instrument to detect the relative concentration of oxy-Hb during the task period relative to pre-task period and does not provide an absolute quantitative result. Second, sleep disorders other than CID may have affected the result due to the absence of polysomnography and insomnia severity index.65 Therefore, although a thorough sleep and psychiatric history performed by psychiatrists with fellowship training (P-ZL and KF) to minimize the effect was performed, they could not exclude all other diagnoses for sleep disorders. Finally, measuring the oxy-Hb changes during sleep may offer more information for understanding the pathogenic mechanisms.
Conclusion
Using NIRS, this study detected dysfunction in the PFC, which indicates the role of the PFC in the neurophysiological underpinnings in CID patients. Moreover, we confirmed that the function in the left OFC was associated with the severity of sleep complaints in patients and the function in the right DLPFC was associated with the severity of depressive symptoms. These findings may represent a novel insight into the pathophysiology of CID patients, and the NIRS imaging tool could be useful to detect the neural basis and associated symptoms.
Acknowledgments
The authors thank all the participants in this study. This study was supported by the Independent Scientific Research Program of Tsinghua University (grant number 548105001).
Disclosure
The authors report no conflicts of interest in this work.
References
Fernandez-Mendoza J, Vgontzas AN, Bixler EO, et al. Clinical and polysomnographic predictors of the natural history of poor sleep in the general population. Sleep. 2012;35(5):689–697. | ||
Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International statistical classification of diseases and related health problems, tenth revision; and research diagnostic criteria/international classification of sleep disorders, second edition criteria: results from the America Insomnia survey. Biol Psychiatry. 2011;69(6):592–600. | ||
Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. | ||
Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129–1141. | ||
Sateia MJ, Nowell PD. Insomnia. Lancet. 2004;364(9449):1959–1973. | ||
Van Someren E. Circadian and sleep disturbances in the elderly. Exp Gerontol. 2000;35(9):1229–1237. | ||
Medicine AAoS. International classification of sleep disorders–third edition (ICSD-3). AASM Resource Library; 2014. | ||
Shekleton JA, Rogers NL, Rajaratnam SM. Searching for the daytime impairments of primary insomnia. Sleep Med Rev. 2010;14(1):47–60. | ||
Schneider C, Fulda S, Schulz H. Daytime variation in performance and tiredness/sleepiness ratings in patients with insomnia, narcolepsy, sleep apnea and normal controls. J Sleep Res. 2004;13(4):373–383. | ||
Vignola A, Lamoureux C, Bastien CH, Morin CM. Effects of chronic insomnia and use of benzodiazepines on daytime performance in older adults. J Gerontol. 2000;55(1):54–62. | ||
Fulda S, Schulz H. Cognitive dysfunction in sleep disorders. Sleep Med Rev. 2001;5(6):423–445. | ||
Backhaus J, Junghanns K, Born J, Hohaus K, Faasch F, Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: influence of sleep architecture and nocturnal cortisol release. Biol Psychiatry. 2006;60(12):1324–1330. | ||
Bonnet MH, Arand DL. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18(7):581–588. | ||
Varkevisser M, Kerkhof GA. Chronic insomnia and performance in a 24-h constant routine study. J Sleep Res. 2005;14(1):49–59. | ||
Uttl B. Measurement of individual differences: lessons from memory assessment in research and clinical practice. Psychol Sci. 2005;16(6):460–467. | ||
Uttl B, Graf P, Richter LK. Verbal paired associates tests limits on validity and reliability. Arch Clin Neuropsychol. 2002;17(6):567–581. | ||
Schneider-Helmert D. Twenty-four-hour sleep-wake function and personality patterns in chronic insomniacs and healthy controls. Sleep. 1987;10(5):452–462. | ||
Orff H, Drummond SP, Nowakowski S, Perils ML. Discrepancy between subjective symptomatology and objective neuropsychological performance in insomnia. Sleep. 2007;30(9):1205. | ||
Mendelson WB, Garnett D, Gillin JC, Weingartner H. The experience of insomnia and daytime and nighttime functioning. Psychiatry Res. 1984;12(3):235–250. | ||
Vincent NK, Walker JR. Perfectionism and chronic insomnia. J Psychosom Res. 2000;49(5):349–354. | ||
Li Y, Liu L, Wang E, et al. Abnormal neural network of primary insomnia: evidence from spatial working memory task fMRI. Eur Neurol. 2016;75(1–2):48–57. | ||
Drummond SP, Walker M, Almklov E, Campos M, Anderson DE, Straus LD. Neural correlates of working memory performance in primary insomnia. Sleep. 2013;36(9):1307–1316. | ||
Altena E, Van Der Werf YD, Sanz-Arigita EJ, et al. Prefrontal hypoactivation and recovery in insomnia. Sleep. 2008;31(9):1271–1276. | ||
Li C, Ma X, Dong M, et al. Abnormal spontaneous regional brain activity in primary insomnia: a resting-state functional magnetic resonance imaging study. Neuropsychiatr Dis Treat. 2016;12:1371–1378. | ||
Wang T, Li S, Jiang G, et al. Regional homogeneity changes in patients with primary insomnia. Eur Radiol. 2016;26(5):1292–1300. | ||
Dai XJ, Peng DC, Gong HH, et al. Altered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2014;10:2163–2175. | ||
Hasler BP, Germain A, Nofzinger EA, et al. Chronotype and diurnal patterns of positive affect and affective neural circuitry in primary insomnia. J Sleep Res. 2012;21(5):515–526. | ||
Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161(11):2126–2128. | ||
Boas DA, Dale AM, Franceschini MA. Diffuse optical imaging of brain activation: approaches to optimizing image sensitivity, resolution, and accuracy. NeuroImage. 2004;23(Suppl 1):S275–S288. | ||
Sato H, Yahata N, Funane T, et al. A NIRS-fMRI investigation of prefrontal cortex activity during a working memory task. NeuroImage. 2013;83:158–173. | ||
Pu S, Matsumura H, Yamada T, et al. Reduced frontopolar activation during verbal fluency task associated with poor social functioning in late-onset major depression: multi-channel near-infrared spectroscopy study. Psychiatry Clin Neurosci. 2008;62(6):728–737. | ||
Kameyama M, Fukuda M, Yamagishi Y, et al. Frontal lobe function in bipolar disorder: a multichannel near-infrared spectroscopy study. NeuroImage. 2006;29(1):172–184. | ||
Ehlis AC, Herrmann MJ, Plichta MM, Fallgatter AJ. Cortical activation during two verbal fluency tasks in schizophrenic patients and healthy controls as assessed by multi-channel near-infrared spectroscopy. Psychiatry Res. 2007;156(1):1–13. | ||
Suto T, Fukuda M, Ito M, Uehara T, Mikuni M. Multichannel near-infrared spectroscopy in depression and schizophrenia: cognitive brain activation study. Biol Psychiatry. 2004;55(5):501–511. | ||
Noda T, Yoshida S, Matsuda T, et al. Frontal and right temporal activations correlate negatively with depression severity during verbal fluency task: a multi-channel near-infrared spectroscopy study. J Psychiatr Res. 2012;46(7):905–912. | ||
Nishida M, Kikuchi S, Matsumoto K, Yamauchi Y, Saito H, Suda S. Sleep complaints are associated with reduced left prefrontal activation during a verbal fluency task in patients with major depression: a multi-channel near-infrared spectroscopy study. J Affect Dis. 2017;207:102–109. | ||
Matsuo K, Watanabe A, Onodera Y, Kato N, Kato T. Prefrontal hemodynamic response to verbal-fluency task and hyperventilation in bipolar disorder measured by multi-channel near-infrared spectroscopy. J Affect Disord. 2004;82(1):85–92. | ||
Fisk JE, Sharp CA. Age-related impairment in executive functioning: updating, inhibition, shifting, and access. J Clin Exp Neuropsychol. 2004;26(7):874–890. | ||
Kahlaoui K, Di Sante G, Barbeau J, et al. Contribution of NIRS to the study of prefrontal cortex for verbal fluency in aging. Brain Language. 2012;121(2):164–173. | ||
Takahashi T, Takikawa Y, Kawagoe R, Shibuya S, Iwano T, Kitazawa S. Influence of skin blood flow on near-infrared spectroscopy signals measured on the forehead during a verbal fluency task. NeuroImage. 2011;57(3):991–1002. | ||
Horne JA. Sleep loss and “divergent” thinking ability. Sleep Med Rev. 1988;11(6):528–536. | ||
Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: a focus on insomnia. Sleep Med Rev. 2010;14(4):227–238. | ||
Liu X, Sun G, Zhang X, et al. Relationship between the prefrontal function and the severity of the emotional symptoms during a verbal fluency task in patients with major depressive disorder: a multi-channel NIRS study. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:114–121. | ||
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. | ||
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56. | ||
Schudlo LC, Chau T. Towards a ternary NIRS-BCI: single-trial classification of verbal fluency task, Stroop task and unconstrained rest. J Neural Eng. 2015;12(6):066008. | ||
Okamoto M, Dan H, Sakamoto K, et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. NeuroImage. 2004;21(1):99–111. | ||
Hori H, Ozeki Y, Terada S, Kunugi H. Functional near-infrared spectroscopy reveals altered hemispheric laterality in relation to schizotypy during verbal fluency task. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1944–1951. | ||
Kono T, Matsuo K, Tsunashima K, et al. Multiple-time replicability of near-infrared spectroscopy recording during prefrontal activation task in healthy men. Neurosci Res. 2007;57(4):504–512. | ||
Singh AK, Dan I. Exploring the false discovery rate in multichannel NIRS. NeuroImage. 2006;33(2):542–549. | ||
Smith M, Perlis ML, Chengazi VU, et al. Neuroimaging of NREM sleep in primary insomnia: a Tc-99-HMPAO single photon emission computed tomography study. Sleep Med Rev. 2002;25(3):325. | ||
Altena E, Vrenken H, Van Der Werf YD, van den Heuvel OA, Van Someren EJ. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010;67(2):182–185. | ||
Joo EY, Noh HJ, Kim JS, et al. Brain gray matter deficits in patients with chronic primary insomnia. Sleep. 2013;36(7):999–1007. | ||
Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14(1):9–15. | ||
Lovato N, Lack L, Wright H, Cant M, Humphreys J. Working memory performance of older adults with insomnia. J Sleep Res. 2013;22(3):251–257. | ||
Lee TG, Blumenfeld RS, D’Esposito M. Disruption of dorsolateral but not ventrolateral prefrontal cortex improves unconscious perceptual memories. J Neurosci. 2013;33(32):13233–13237. | ||
Rolls ET, Grabenhorst F, Parris BA. Warm pleasant feelings in the brain. NeuroImage. 2008;41(4):1504–1513. | ||
Valet M, Gündel H, Sprenger T, et al. Patients with pain disorder show gray-matter loss in pain-processing structures: a voxel-based morphometric study. Psychosom Med. 2009;71(1):49. | ||
Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31. | ||
Stoffers D, Moens S, Benjamins J, et al. Orbitofrontal gray matter relates to early morning awakening: a neural correlate of insomnia complaints? Front Neurol. 2012;3:105. | ||
Sexton CE, Storsve AB, Walhovd KB, Johansenberg H, Fjell AM. Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology. 2014;83(11):967. | ||
Liu CH, Liu CZ, Zhang J, et al. Reduced spontaneous neuronal activity in the insular cortex and thalamus in healthy adults with insomnia symptoms. Brain Res. 2016;1648(Pt A):317–324. | ||
Pu S, Nakagome K, Miura A, Iwata M, Nagata I, Kaneko K. Associations between depressive symptoms and fronto-temporal activities during a verbal fluency task in patients with schizophrenia. Sci Rep. 2016;6:30685. | ||
Heller AS, Johnstone T, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Increased prefrontal cortex activity during negative emotion regulation as a predictor of depression symptom severity trajectory over 6 months. JAMA Psychiatry. 2013;70(11):1181–1189. | ||
Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.