Back to Journals » Infection and Drug Resistance » Volume 16
Recurrent Spinal Tuberculosis with HIV Infection After Surgery: A Rare Case of Recurrence and Drug Resistance
Received 1 September 2023
Accepted for publication 21 December 2023
Published 27 December 2023 Volume 2023:16 Pages 7827—7833
DOI https://doi.org/10.2147/IDR.S438184
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Kangpeng Li,* Bo Liu,* Qiang Zhang
National Center for Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiang Zhang, Email [email protected]
Abstract: Tuberculosis (TB) and acquired immunodeficiency syndrome (AIDS) are prevalent infectious diseases that continue to pose a significant global health burden. The co-infection of mycobacterium tuberculosis and human immunodeficiency virus (HIV) represents a substantial public health challenge, particularly in developing nations. In this study, we present an exceptional case of spinal tuberculosis complicated by HIV infection, which exhibited relapse post-surgery necessitating reoperation, along with the emergence of drug resistance. The first operation was lumbar lesion removal, decompression, internal fixation, and bone graft fusion assisted by lumbar discioscopy. The second operation was ultrasound-guided puncture and drainage of right psoas major abscess. The management of patients with HIV/TB co-infection demands specific considerations regarding medication regimens, surgical interventions, and nursing care. However, limited experience exists in treating such individuals, thus further research is imperative to enhance our understanding of HIV/TB co-infection.
Keywords: spinal tuberculosis, HIV, AIDS, recurrence, surgery
Introduction
Following infection with mycobacterium tuberculosis (MTB) in humans, it can be classified into two distinct states: latent tuberculosis infection (LTBI) and active tuberculosis (TB).1 HIV infection represents the most significant independent risk factor for LTBI progression to active TB, thereby augmenting the likelihood of reactivation of latent infection by 20-fold.2 Concurrently, TB also serves as a prevalent opportunistic infection and plays a crucial role in determining disease progression among patients with HIV.3 HIV/MTB co-infection represents a significant public health concern.4 The intricate interplay between HIV and MTB fosters the progression of their respective diseases, necessitating specific approaches for diagnosis and treatment.5 Spinal tuberculosis patients with concomitant HIV infection exhibit compromised immune status, accelerated disease advancement, and heightened susceptibility to multidrug-resistant tuberculosis (MDR-TB) as well as extensively drug-resistant tuberculosis (XDR-TB), often warranting surgical intervention.6 In this study, we present a rare case of postoperative recurrence of spinal tuberculosis in a patient living with HIV, leading to the need for secondary surgery. Notably, drug resistance has emerged during treatment. To our knowledge, this is the first reported case of postoperative recurrence of these two concurrent infections resulting in reoperation in the literature.
Case Presentation
A 59-year-old male patient had low back pain without obvious cause for nearly one year, which was aggravated during activities, accompanied by intermittent fever, night sweat, and fatigue. He presented to a local tuberculosis hospital with a suspicion of “lumbar tuberculosis” and was given anti-tuberculosis treatment: isoniazide 300 mg (po qd), rifampicin 450 mg (po qd), ethambutol 750 mg (po qd), streptomycin 750 mg (im qd). At this time, Mycobacterium susceptibility test showed sensitivity to all types of anti-TB drugs. The patient continued to have low back pain. For further treatment, he visited the orthopedics outpatient of our hospital, we considered lumbar tuberculosis, and recommended surgical treatment. This patient contracted HIV five years ago from homosexual intercourse. Early screening blood viral load is 129,800 copies/mL, then began to receive antiviral treatment (ART): lamivudine/tenofovir/efavirenz, drug regimen did not change in five years. The blood viral load dropped to undetectable levels 10 months after ART and remained at this level at annual viral load screening. CD4+T lymphocyte count fluctuated between 200–300 cells /ul.
On physical examination, the patient had a limp gait with tenderness and percussion in the spinous processes of lumbar 1–3 and paravertebral regions, without radiation to the lower limbs. The muscle strength of each muscle group of both lower limbs was grade 5. The active and passive movements of the lumbar spine were obviously limited. The active range of motion of the lumbar spine: forward flexion (10°), backward extension (20°), left flexion (10°), right flexion (20°), left rotation (10°), right rotation (20°). Bilateral straight leg raising test and strengthening test were negative, bilateral femoral nerve pull test was negative, bilateral knee tendon reflex and Achilles tendon reflex were normal, and bilateral Babinski sign was negative. Laboratory tests were as follows: C-reactive protein 11.9mg/L, erythrocyte sedimentation rate 38.00mm/h, CD4+T lymphocyte count 219 cells /ul, and albumin 33.0g/L.
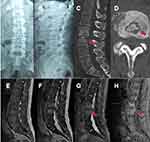
Preoperative lumbar X-ray showed uneven density and narrowing of the L2/3 intervertebral space (Figure 1A and B). Lumbar CT and enhanced MRI showed cavities and osteolytic destruction in L2/3 with multiple irregular sequestrum, the paravertebral abscess was large and prominent. Subsequently, the patient underwent lumbar focus debridement, decompression, internal fixation, and bone graft fusion under general anesthesia (Figure 1C–H). The operation was successful, anti-tuberculosis and anti-viral therapy was continued after the operation. The X-ray reexamination on the third day after operation showed that the internal fixation was firm and the position of the fusion cage was satisfactory. (Figure 2) He was discharged 20 days later.
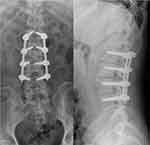 |
Figure 2 X-ray examination was performed on the third day after operation. The results showed that the internal fixation was firm and the position of the fusion cage was satisfactory. |
Four months after operation, the patient was found that the incision scar was swollen, with ulceration and pus (Figure 3), and was hospitalized again. Physical examination revealed a 15cm long longitudinal surgical scar in the middle of the waist, with a 1mm diameter tear at the lower end of it, yellowish green pus flowed out around the extrusion. The reexamination showed the formation of bilateral psoas abscess, central liquefaction necrosis, abnormal soft tissue density shadow under the lower back, considering inflammatory lesions, and local sinus formation. (Figure 4) After discussion with the general practitioner, the patient could be treated with ultrasound-guided puncture and drainage of pus (Figure 5), followed by surgical evacuation.
 |
Figure 3 A mass at the incision site developed and ruptured at 4 months after operation. |
 |
Figure 5 Ultrasound-guided right psoas major catheter drainage. |
All the operations were successful. After regular dressing change and observation of the incision, the wound healed smoothly and the patient was discharged. During the follow-up, it was learned that the patient was resistant to rifampin and isoniazid in the tuberculosis drug resistance test in the local hospital, and he was advised to change to oral rifapentine and prothionamide. So far, he has not relapsed and is living well.
Discussion
Due to the immune function defect, people living with HIV are more likely to suffer from TB after contact MTB, and TB will rapidly deteriorate and spread.7 In particular, patients who do not receive ART and have a very low CD4 count are very susceptible to extrapulmonary tuberculosis under the continuous attack of HIV on the immune system. Lymphoid tuberculosis, intestinal tuberculosis, bone tuberculosis, urinary system tuberculosis, tuberculous peritonitis, tuberculous meningitis are common extrapulmonary tuberculosis.8 However, HIV infection combined with spinal tuberculosis is relatively rare, especially the patient also underwent secondary surgery for spinal tuberculosis recurrence, and there is no relevant report in the world.
Spinal tuberculosis can occur at any age, more common in young adults over 25 years old and children under 15 years old. It mostly occurs in the thoracolumbar spine.9 If spinal tuberculosis is not treated in time, it will cause spinal cord compression and lead to paraplegia, which will greatly affect the quality of life of patients. Anti-tuberculosis drug therapy is the fundamental treatment of spinal tuberculosis.10 On the basis of paying attention to rest and strengthening nutrition, chemotherapy should run through the whole process of treatment.11 At the same time, the principle of “Early”, “Regular”, “Whole course”, “Combined” and “Appropriate amount” should be followed.12 Due to the special pathology of spinal tuberculosis, the blood supply of the lesion is poor and the drug concentration in the bone tissue is low.13 At present, the standard chemotherapy regimen is used for the treatment of spinal tuberculosis - 3SHRE/15HRE or 6SHRE/12HRE.14 However, HIV co-infection with TB makes the selection of drugs more challenging: 1) There are interactions between anti-HIV drugs and anti-TB drugs, such as the interaction between rifampicin and protease inhibitors and non-nucleoside antiviral drugs, which will reduce the effective plasma concentration of the latter, so rifampicin should be replaced by ribbutin. 2) Concomitant antiviral and anti-TB therapy may reduce treatment compliance and increase adverse drug reactions. 3) The concomitant treatment of two diseases increases the drug burden of patients.15 In addition, the emergence of MDR-TB and XDR-TB is a wake-up call that the standard empirical treatment regimen is not appropriate for drug-resistant TB, as in this case, the patient developed resistance to rifampin and isoniazid.16 We recommend that drug susceptibility testing be performed as soon as possible in patients with spinal tuberculosis. Doctors select second-line drugs and formulate individualized chemotherapy regimens according to the results of drug sensitivity test. Early detection and effective treatment are the key to the treatment of drug-resistant tuberculosis.
The postoperative recurrence of spinal tuberculosis should be avoided by clinicians.17 The risk factors of recurrence included poor nutritional status, no standardized anti-tuberculosis treatment before and after operation, incomplete debridement of lesions during operation, no early detection and treatment of postoperative effusion, poor spinal stability after operation, and no timely detection of drug resistance.18 The experience in the diagnosis and treatment of recurrent spinal tuberculosis emphasizes that the lesion should be removed under a relatively wide field of vision, and various angles of curettage, pressure irrigation or negative pressure aspiration should be used to remove the lesion as far as possible. We do not recommend removing a large amount of bone for complete removal of the lesion. If the vertebra is only partially destroyed, some sclerotic bone can be preserved. At the same time, the tolerance and compliance of patients taking anti-tuberculosis drugs should be evaluated on a monthly basis. Regular anti-tuberculosis drug chemotherapy is the main line of the whole treatment of spinal tuberculosis.
To avoid TB infection in HIV patients and increase the disease burden, TB screening should be carried out in key populations in time: ① All newly diagnosed HIV/AIDS patients should receive TB screening; ② AIDS patients with CD4 cell count < 200/μL; ③ HIV/AIDS patients with recurrent/ongoing TB should be screened for TB annually.19 Screening methods including whether there is any related clinical symptoms: fever, cough, hemoptysis, night sweats, chest pain, swollen lymph nodes, fatigue, difficulty in breathing, etc; If there are related symptoms, sputum smear, sputum culture, imaging examination, enlarged lymph node biopsy should be performed to exclude the possibility of active tuberculosis, and to identify the presence of non-tuberculous mycobacterial infection.20
There are also limitations in this case. Since our hospital did not have the conditions to carry out Mycobacterium susceptibility test at that time, the drug resistance information of this patient was only from the examination in other hospitals, including the first visit and follow-up. It is also alarming that samples taken during the first operation were not tested for drug susceptibility. We do not know the exact time of development of resistance, which is not conducive to disease surveillance. In fact, after each discharge, we remind the patient to get tested once a month at a local hospital that can do Mycobacterium susceptibility test. Unfortunately, the patient did not follow through.
In summary, HIV co-infection with spinal tuberculosis is uncommon, but it can be extremely harmful if poorly treated. The treatment of these patients has unique requirements in many aspects such as surgery, medicine and nursing. In the future, standardized anti-tuberculosis drug treatment, adequate antiviral therapy, surgical treatment and enhanced recovery after surgery (ERAS) concept are indispensable in the face of this type of patients with co-infection.
Ethical Statement and Informed Consent
The Ethics Committee of the Beijing Ditan Hospital of Capital Medical University approved the study. The patient provided written informed consent for publication of these case reports and accompanying images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study did not receive any funding.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Rahlwes KC, Dias BRS, Campos PC, et al. Pathogenicity and virulence of Mycobacterium tuberculosis. Virulence. 2023;14(1):2150449. doi:10.1080/21505594.2022.2150449
2. Peters JS, Andrews JR, Hatherill M, et al. Advances in the understanding of Mycobacterium tuberculosis transmission in HIV-endemic settings. Lancet Infect Dis. 2019;19(3):e65–e76. doi:10.1016/S1473-3099(18)30477-8
3. Yang Q, Han J, Shen J, et al. Diagnosis and treatment of tuberculosis in adults with HIV. Medicine. 2022;101(35):e30405. doi:10.1097/MD.0000000000030405
4. Liu M, Li W, Qiao W, et al. Knowledge domain and emerging trends in HIV-MTB co-infection from 2017 to 2022: a scientometric analysis based on VOSviewer and CiteSpace. Front Public Health. 2023;11:1044426. doi:10.3389/fpubh.2023.1044426
5. Azevedo-Pereira JM, Pires D, Calado M, et al. HIV/Mtb co-infection: from the amplification of disease pathogenesis to an “emerging syndemic”. Microorganisms. 2023;11(4):853. doi:10.3390/microorganisms11040853
6. Dookie N, Ngema SL, Perumal R, et al. The changing paradigm of drug-resistant tuberculosis treatment: successes, pitfalls, and future perspectives. Clin Microbiol Rev. 2022;35(4):e0018019. doi:10.1128/cmr.00180-19
7. Rewari BB, Kumar A, Mandal PP, et al. HIV TB coinfection - perspectives from India. Expert Rev Respir Med. 2021;15(7):911–930. doi:10.1080/17476348.2021.1921577
8. Rodriguez-Takeuchi SY, Renjifo ME, Medina FJ. Extrapulmonary tuberculosis: pathophysiology and imaging findings. Radiographics. 2019;39(7):2023–2037. doi:10.1148/rg.2019190109
9. Jain AK, Rajasekaran S, Jaggi KR, et al. Tuberculosis of the spine. J Bone Joint Surg Am. 2020;102(7):617–628. doi:10.2106/JBJS.19.00001
10. Suárez I, Fünger SM, Kröger S, et al. The diagnosis and treatment of tuberculosis. Dtsch Arztebl Int. 2019;116(43):729–735. doi:10.3238/arztebl.2019.0729
11. Furin J, Cox H, Pai M. Tuberculosis. Lancet. 2019;393(10181):1642–1656. doi:10.1016/S0140-6736(19)30308-3
12. Kestler B, Tyler SK. Latent tuberculosis testing through the ages: the search for a sleeping killer. Am J Physiol Lung Cell Mol Physiol. 2022;322(3):L412–L419. doi:10.1152/ajplung.00217.2021
13. Liebenberg D, Gordhan BG, Kana BD. Drug resistant tuberculosis: implications for transmission, diagnosis, and disease management. Front Cell Infect Microbiol. 2022;12:943545. doi:10.3389/fcimb.2022.943545
14. Donald P, Kaufmann S, Thee S, et al. Pathogenesis of tuberculosis: the 1930 Lübeck disaster revisited. Eur Respir Rev. 2022;31(164):220046. doi:10.1183/16000617.0046-2022
15. Bell LCK, Noursadeghi M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat Rev Microbiol. 2018;16(2):80–90. doi:10.1038/nrmicro.2017.128
16. Migliori GB, Tiberi S, Zumla A, et al. MDR/XDR-TB management of patients and contacts: challenges facing the new decade. The 2020 clinical update by the global tuberculosis network. Int J Infect Dis. 2020;92S:S15–S25. doi:10.1016/j.ijid.2020.01.042
17. Wang B, Kong L, Zhu Z, et al. Recurrent complex spinal tuberculosis accompanied by sinus tract formation: causes of recurrence and clinical treatments. Sci Rep. 2018;8(1):6933. doi:10.1038/s41598-018-25142-z
18. Lin Y, Lin H, Xiao L, et al. Tuberculosis recurrence over a 7-year follow-up period in successfully treated patients in a routine program setting in China: a prospective longitudinal study. Int J Infect Dis. 2021;110:403–409. doi:10.1016/j.ijid.2021.07.057
19. Jin J. Screening for latent tuberculosis. JAMA. 2023;329(17):1526. doi:10.1001/jama.2023.6495
20. Ketai L. Tuberculosis and the prospects for lung cancer screening worldwide. Radiology. 2020;296(1):189–190. doi:10.1148/radiol.2020200696
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.