Back to Journals » International Journal of General Medicine » Volume 17
Recurrence Rate and Influencing Factors of Helicobacter Pylori Infection After Successful Eradication in Southern Coastal China
Authors Zhang D , Mao F, Huang S, Chen C, Li D , Zeng F , Bai F
Received 29 November 2023
Accepted for publication 10 March 2024
Published 19 March 2024 Volume 2024:17 Pages 1039—1046
DOI https://doi.org/10.2147/IJGM.S452348
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Hossam El-Din Shaaban
Daya Zhang,1,* Fengjiao Mao,1,* Shimei Huang,1,* Chen Chen,1 Da Li,1 Fan Zeng,1 Feihu Bai2,3
1Graduate School, Hainan Medical University, Haikou, People’s Republic of China; 2Department of Gastroenterology, The Second Affiliated Hospital of Hainan Medical University, Haikou, People’s Republic of China; 3The Gastroenterology Clinical Medical Center of Hainan Province, Haikou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Feihu Bai, Chief Physician and Professor of the Department of Gastroenterology, The Second Affiliated Hospital of Hainan Medical University, Yehai Avenue, #368, Longhua District, Haikou, Hainan Province, People’s Republic of China, Tel +86-18995181963, Fax +86-89866809168, Email [email protected]
Purpose: Recurrence rate of Helicobacter pylori (H. pylori) infection after successful eradication have gained attention. This study was to assess the recurrence rate of H. pylori infection after successful eradication in the southern coastal provinces of China and to analyze its factors.
Patients and Methods: 975 patients with upper gastrointestinal symptoms who were diagnosed with H. pylori infection using the 13C or 14C-urea breath test (UBT) underwent eradication treatment between August 2021 and December 2022. After eight to twelve weeks, repeat UBT was performed. Besides, 824 patients with successful eradication underwent a repeat UBT by completing questionnaires after a year. The 1-year recurrence rate was calculated, and the differences were analyzed based on baseline data, sociological characteristics, and lifestyle.
Results: A total of 734 patients completed the 1-year follow-up, out of which 26 (3.5%) patients experienced a recurrence of H. pylori infection. Exposure to other individuals infected with H. pylori (χ2=12.852, P< 0.001), poor hygiene conditions at dining out places (χ2=6.839, P=0.009), frequent dining out (χ2=24.315, P< 0.001), smoking (χ2=7.510, P=0.006), consumption of non-purified water (χ2=16.437, P< 0.001), consumption of pickled foods (χ2=5.682, P=0.017), irregular meal patterns (χ2=16.877, P< 0.001) and age (χ2=9.195, P=0.010) were significant factors for H. pylori infection recurrence. Exposure to other individuals infected with H. pylori, poor hygiene conditions at dining out places, consumption of non-purified water, frequent dining out and irregular meal patterns were independent risk factors (P=0.022, 0.016, 0.002, < 0.001, < 0.001; 95% CI 0.146– 0.861, 0.121– 0.806, 1.715– 10.845, 0.085– 0.521, 2.291– 14.556).
Conclusion: The one-year recurrence rate of H. pylori infection post-eradication in the southern coastal provinces of China is 3.5%. Contacting with infected individuals, poor hygiene in dining places, consumption of non-purified water, frequent dining out, and irregular meal patterns were identified as significant independent factors influencing H. pylori recurrence.
Keywords: Helicobacter pylori, successful eradication, Southern Coastal Provinces of China, risk factors, 1-year follow-up
Introduction
Helicobacter pylori (H. pylori) is a microaerobic Gram-negative bacterium that colonizes the human stomach and duodenum. It is considered a pathogenic bacterium closely associated with the development of chronic gastritis, peptic ulcer, and gastric cancer.1–3 In fact, the International Agency for Research on Cancer (IARC) classified H. pylori as a definitive gastric cancer carcinogen in 1994.4 H. pylori infection is highly prevalent, with nearly 50% of the global population being infected in China, in particular, has a significantly high rate of H. pylori infection, with 46.7% of the population affected. Moreover, familial H. pylori infection is also common in China, with a prevalence of 71.2% and notable familial clustering.5,6 According to authoritative domestic and international guidelines, eradication therapy is recommended when H. pylori infection is detected unless contraindicating factors such as age (children under 14), severe heart, liver, or kidney insufficiency, or drug allergies/intolerances exist.7 However, despite eradication therapy, there is still a possibility of recurrence, leading to a reemergence of associated diseases and increased medical costs for subsequent treatment. Recurrence can occur through relapse or reinfection, with the former generally happening within 1 year after eradication and the latter referring to reinfection with a different strain after a longer period.8,9 Relapse is characterized by the return of H. pylori and related symptoms within a year of initial eradication treatment. It signifies that the bacterium was not completely eliminated and reactivates and multiplies after a latent period in the host.10,11 On the other hand, reinfection refers to a new infection with a different strain of H. pylori after the complete eradication of the original strain.11 To distinguish between relapse and reinfection, DNA fingerprinting can be employed, although its complexity and cost have limited its clinical implementation. Previous studies suggest that recurrence within one year of successful eradication is usually relapse, while recurrence after one year is more likely to be reinfection.11–14 Hu et al reported global annual rates of H. pylori recurrence, reinfection, and relapse as 4.3%, 3.1%, and 2.2%, respectively.15 It is important to note that published reinfection rates and associated factors vary considerably across different countries, regions, populations, sample sizes, socio-economic conditions, and investigation methods.10,15,16
Currently, there is a lack of large-scale studies on H. pylori recurrence in the coastal provinces of southern China.
Methods
Eradication Therapy and Follow-Up: Study Overview and Patient Demographics
From August 2021 to December 2022, our study included patients who had received successful H. pylori eradication therapy for the first time. The data for this study was partly from our previous study17 and partly from data collected in city and county outpatient clinics. The clinics included 21 different health service stations across five cities in Hainan province: Haikou, Sanya, Qionghai, Dongfang, and Wuzhishan. The inclusion criteria for this study were patients aged 14 years and older who had upper digestive tract symptoms and were diagnosed with H. pylori infection using either the 13C or 14C-UBT. The study was approved by the institutional ethics committee of the Second Hospital of Hainan Medical University (reference number: 2023-KJY-02), and all participants provided signed informed consent. For patients under 18 years of age, a parent or legal guardian provided the informed consent. The exclusion criteria included patients who had previously received eradication therapy, those who had used proton pump inhibitors, H2 receptor antagonists, bismuth, antibiotics, or other medications that could affect test results within the last four weeks, patients with a history of gastrointestinal tract cancer, gastric or esophageal surgery, Zollinger-Ellison syndrome, serious disorders of the liver, kidneys, cardiovascular, respiratory, hematological, neuropsychiatric, or endocrine system, individuals who had allergies to drugs used for treatment, pregnant or breastfeeding women, and patients with alcohol abuse or any other clinical condition that may increase the risk of treatment side effects.
A total of 975 H. pylori-infected patients received eradication therapy. The 13C-UBT or 14C-UBT was performed 8 to 12 weeks after therapy, and eradication was considered successful if the test result was negative. Out of these patients, a total of 824 succeeded in eradication and were followed up. From this group, 734 patients provided full information and were enrolled in the subsequent study, as shown in Figure 1. At one year after the eradication of H. pylori infection, patients were followed up using the 13C-UBT or 14C-UBT (Figure 1). We calculated the one-year eradication rates and compared the differences between recurrent patients and other patients in terms of basic data, sociological characteristics, lifestyle, and medical history.
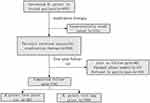 |
Figure 1 The Flow Chart of this Study. |
Comprehensive Data Collection Methods for H. pylori Survey
The questionnaire included gathering basic information such as the individuals’ level of education. It also collected data on various aspects of their lifestyle, including their source of drinking water and their meal patterns. Additionally, the survey aimed to identify any contact the participants may have had with other individuals infected with H. pylori. The survey process was conducted directly by the researcher or under the supervision of a highly trained investigator.
Statistical Analysis Methods for Investigating H. pylori Infection Recurrence
Data analysis was performed using SPSS 26.0 software. Continuous variables were presented as mean ± standard deviation (SD), and the comparison between groups was conducted using an independent sample t-test. Categorical variables were described as frequencies and percentages [n(%)], and chi-square tests or trend chi-square tests were utilized to compare the groups. Variables with a p-value < 0.05 in the univariate analysis were included in the multifactorial logistic regression analysis to investigate the potential relationship of H. pylori infection recurrence. The results were reported as odds ratios (OR) with 95% confidence intervals (CI). Statistical significance was established at a p-value less than 0.05.
Results
Demographic Characteristics of Participants in Follow-Up Study
A total of 734 patients, comprising 263 males and 471 females, with ages ranging from 14 to 70 years, completed the follow-up tests 1 year after eradication therapy and provided valid questionnaires (Table 1).
 |
Table 1 The General Factors of the Recurrence and Non-Recurrence Patients with H. pylori Infection |
H. pylori Infection Recurrence Rates Among Participants in Follow-Up Study
Among the 734 patients who completed the follow-up tests 1 year after eradication therapy, 26 individuals (3.5% of the total) experienced a recurrence of H. pylori infection.
Significant Risk Factors for H. pylori Infection Recurrence
The analysis of variance revealed several significant risk factors for H. pylori infection recurrence. Exposure to other H. pylori-infected individuals (χ2=12.852, P<0.001), poor hygiene condition of dining out places (χ2=6.839, P=0.009), frequency of dining out (χ2=24.315, P<0.001), smoking (χ2=7.510, P=0.006), consumption of non-purified water (χ2=16.437, P<0.001), consumption of pickles (χ2=5.682, P=0.017), irregular meals (χ2=16.877, P<0.001), and age (χ2=9.195, P=0.010) were identified as significant risk factors (Table 2). Moreover, the results indicated that exposure to other H. pylori-infected individuals, poor hygiene condition of dining out places, consumption of non-purified water, frequency of dining out, and irregular meals were independent influential factors for H. pylori recurrence (P=0.022, 0.016, 0.002, <0.001,<0.001, 95% CI 0.146–0.861, 0.121–0.806, 1.715–10.845, 0.085–0.521, 2.291–14.556) (Table 3).
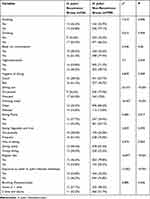 |
Table 2 Univariate Analysis of the Factors Affecting the Recurrence of H. pylori Infection |
 |
Table 3 Multivariate Analysis of Factors Affecting the Recurrence of H. pylori Infection |
Discussion
The effective eradication of H. pylori plays a crucial role in reducing the risk of gastric cancer.18 However, recurrence of H. pylori infection remains a major concern worldwide, particularly in underdeveloped regions with a high prevalence of H. pylori infection or gastric cancer.18
Various studies have indicated significant variation in the recurrence rates of H. pylori infection across different countries and regions. A meta-analysis conducted by Yan et al based on 77 studies and including 1226 patients (equivalent to 43,525 patient-years) found that the average annual recurrence rate of H. pylori infection globally was 2.82 ± 1.16%.19
Developed countries such as the United States, Germany, Norway, and Japan exhibit lower recurrence rates of H. pylori infection, ranging from 0.2% to 6.2%.15,20,21 Conversely, developing countries such as Peru, Turkey, Mexico, and India have higher recurrence rates, ranging from 2.2% to 73%.15,20,21 Niv et al observed a significantly higher recurrence rate of H. pylori infection in developing countries compared to developed countries (13.0% vs 2.7%) and found a negative correlation with the Human Development Index (HDI) proposed by Yan et al19,21 In our study, the 1-year recurrence rate was 3.2%, which is relatively high and could be attributed to factors such as Hainan’s socio-economic level, poor sanitary conditions, sample size, and regional differences. It’s important to note that our study was retrospective and lacked regular follow-up of patients, which may have influenced the accuracy of the recurrence rate statistics. Furthermore, some patients who had undergone successful radical treatment were not followed up due to lack of symptoms or insufficient attention, suggesting that the actual recurrence rate may be lower.
Several studies have indicated that smokers and alcohol drinkers have a higher risk of H. pylori infection. Interestingly, some studies have also suggested that alcohol consumption may actually reduce the rate of H. pylori infection. However, our study’s findings contradict these assertions, as we found no evidence that smoking and alcohol consumption increase the recurrence rate of H. pylori infection.22 It is well-established that the effectiveness of initial H. pylori treatment is negatively correlated with the recurrence rate of the infection. The higher the eradication rate of H. pylori achieved during treatment, the lower the chance of recurrence.23 However, our study encountered limitations in terms of the wide variation in the number of cases for each treatment regimen, as well as the lack of data on the eradication rate associated with each regimen. Consequently, further large-scale, prospective clinical follow-up studies are necessary to investigate the relationship between H. pylori eradication rates and recurrence rates for different treatment regimens.
An important finding of our investigation was the significant correlation between residential drinking water sources and H. pylori infection. This suggests that local drinking water contamination may be a factor contributing to H. pylori infection.24 Previous literature has reported that consuming non-purified water increases the risk of H. pylori infection. Additionally, consumption of vegetables contaminated with feces has also been identified as a risk factor for H. pylori infection.25
In our study, we discovered that individuals who frequently dined out under poor hygienic conditions had a higher likelihood of experiencing a recurrence of H. pylori infection. Previous research by XIA et al26 has shown that H. pylori infection primarily occurs through the digestive tract via fecal-oral transmission. Additionally, H. pylori can survive in various foods such as milk, vegetables, and meats, leading to the transmission of infections.27 Consequently, food contamination has been identified as a potential cause for the recurrence of H. pylori infections. Further studies by Lili Zhang28 on the population in Baoshan District, Shanghai, revealed that people who frequently dined out were more susceptible to H. pylori infection. Meirong Wang29 also found that sharing tableware while eating out increases the risk of cross-infection of H. pylori. Another study conducted by Li30 in Xi’an, which followed up with 422 patients who had received H. pylori eradication treatment, confirmed that poor hygiene in restaurants was a risk factor for H. pylori recurrence. From a sociological and public health perspective, it is crucial to improve people’s income and health awareness in order to prevent the recurrence of H. pylori infection.
A nationwide, large-scale, household-based cross-sectional survey conducted previously indicated that the overall household H. pylori infection rate ranged from 50.27% to 85.06%, with a mean infection rate of 71.21%, while the mean individual-based H. pylori infection rate was 40.66%.6 Family members who carry H. pylori bacteria and come into contact with infected family members may serve as the primary source of its transmission. Our study further revealed that exposure to other individuals infected with H. pylori was an independent, influential factor for the recurrence of H. pylori. This suggests that H. pylori can be transmitted from one family member to another through the gastrointestinal tract.15,31
Our study revealed that irregular meal patterns are significant risk factors for the recurrence of H. pylori infections. Skipping meals leads to low-grade inflammation, increased fat oxidation, and higher postprandial insulin concentrations, which are associated with the development of type 2 diabetes.32,33 In diabetic patients, dysfunction of glucose metabolism leads to immune dysfunction. This results in reduced adhesion and phagocytosis of granulocytes in the body, weakening the immune cell response to bacterial, viral, and chemical toxin invasions.34 Consequently, H. pylori is more likely to occur or recur in diabetic patients, and the degree of infection tends to be more severe compared to non-diabetic patients.34 One of the key factors that make diabetic patients more susceptible to H. pylori infections is delayed gastric emptying.35,36 Inflammation and immune responses in diabetic patients can impact the metabolism of peripheral nerves, leading to antrum pyloric motor incoordination and delayed gastric emptying, and in some cases, even gastroparesis caused by gastrointestinal motor neuropathy.35,36 These conditions weaken the ability of the gastric wall to resist various invasions, making it easier for H. pylori to settle and significantly increasing the infection rate of H. pylori in diabetic patients with gastroparesis.
Our study has several limitations that need to be acknowledged. Firstly, it is important to note that this study was retrospective in nature, which means that there may have been inconsistencies in follow-up and the timing of eradication treatment. Additionally, some patients tested positive for H. pylori even after receiving multiple medications and undergoing multiple follow-up reviews throughout the 1-year follow-up period. This may have introduced some bias into our results. Secondly, the number of cases of H. pylori recurrence was relatively small, resulting in limited representation and potentially affecting the generalizability of our findings. Thirdly, we failed to consider certain important risk factors in our study, such as antibiotic resistance, hand hygiene practices before meals and after using the restroom, the specific regimen used for eradication treatment, and whether the patients were hospitalized after undergoing eradication therapy.
In conclusion, our study found that the recurrence rate of H. pylori in Hainan, China, was comparable to that observed in developing countries. We identified several independent risk factors for H. pylori recurrence, including exposure to other individuals infected with H. pylori, poor hygiene conditions at dining establishments, consumption of non-purified water, frequent dining out, and irregular meal patterns. Given these findings, regular surveillance of high-risk groups for H. pylori infection is necessary.
Abbreviations
H. pylori, Helicobacter pylori; 14C-UBT, Carbon-14 urea breath test; SD, standard deviation; CI, confidence interval; ORs, odds ratios.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The protocol was approved by the institutional ethics committee of the Second Hospital of Hainan Medical University (2023-KJY-02) and performed per Helsinki’s Declaration. All participants provided written informed consent for data collection and storage.
Author Contributions
All authors made a significant contribution to the work reported in terms of the conception, study design, execution, acquisition of data, analysis and interpretation. They took part in drafting, revising, or reviewing the article; gave final approval of the final manuscript to be published; agreed on the journal to which the article was submitted; and agreed to be accountable for all aspects of the work.
Funding
This work was supported by Hainan Province Clinical Medical Center (No. 2021818), The specific research fund of The Innovation Platform for Academicians of Hainan Province (YSPTZX202313), Hainan Provincial Health Industry Research Project (22A200078), Hainan Provincial Postgraduate Innovation Research Project (Qhyb2022-133) and National Clinical Key Speciality Capacity Building Project (202330).
Disclosure
The authors declare that they have no competing interests.
References
1. Zeng M, Mao XH, Li JX, et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomized, double-blind, placebo-controlled, Phase 3 trial. Lancet. 2015;386(10002):1457–1464. doi:10.1016/S0140-6736(15)60310-5
2. Usui Y, Taniyama Y, Endo M, et al. Helicobacter pylori, homologous-recombination genes, and gastric cancer. N Engl J Med. 2023;388(13):1181–1190. doi:10.1056/NEJMoa2211807
3. Ralser A, Dietl A, Jarosch S, et al. Helicobacter pylori promotes colorectal carcinogenesis by deregulating intestinal immunity and inducing a mucus-degrading microbiota signature. Gut. 2023;72(7):1258–1270. doi:10.1136/gutjnl-2022-328075
4. Zhang C, Yamada N, Wu YL, et al. Helicobacter pylori infection, glandular atrophy and intestinal metaplasia in superficial gastritis, gastric erosion, erosive gastritis, gastric ulcer and early gastric cancer. World J Gastroenterol. 2005;11(6):791–796. doi:10.3748/wjg.v11.i6.791
5. Ding SZ, Du YQ, Lu H, et al. Chinese consensus report on family-based Helicobacter pylori infection control and management (2021 Edition). Gut. 2022;71(2):238–253. doi:10.1136/gutjnl-2021-325630
6. Zhou XZ, Lyu NH, Zhu HY, et al. Large-scale, national, family-based epidemiological study on Helicobacter pylori infection in China: the time to change practice for related disease prevention. Gut. 2023;72(5):855–869. doi:10.1136/gutjnl-2022-328965
7. Malfertheiner P, Megraud F, Rokkas T, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut;2022. gutjnl-2022–327745. doi:10.1136/gutjnl-2022-327745
8. Zhang YY, Xia HH, Zhuang ZH, et al. Review article: ‘true’ re-infection of Helicobacter pylori after successful eradication–worldwide annual rates, risk factors and clinical implications. Aliment Pharmacol Ther. 2009;29(2):145–160. doi:10.1111/j.1365-2036.2008.03873.x
9. Sjomina O, Pavlova J, Niv Y, Leja M. Epidemiology of Helicobacter pylori infection. Helicobacter. 2018;23(Suppl 1):e12514. doi:10.1111/hel.12514
10. Zhou LY, Song ZQ, Xue Y, et al. Recurrence of Helicobacter pylori infection and the affecting factors: a follow-up study. J Digest Dis. 2017;18(1):47–55. doi:10.1111/1751-2980.12440
11. Raymond J, Thiberge JM, Dauga C. Diagnosis of Helicobacter pylori recurrence: relapse or reinfection? Usefulness of molecular tools. Scand J Gastroenterol. 2016;51(6):672–678. doi:10.3109/00365521.2015.1132338
12. Burucoa C, Lhomme V, Fauchere JL. Performance criteria of DNA fingerprinting methods for typing of Helicobacter pylori isolates: experimental results and meta-analysis. J Clin Microbiol. 1999;37(12):4071–4080. doi:10.1128/JCM.37.12.4071-4080.1999
13. Silva FM, Navarro-Rodriguez T, Barbuti RC, et al. Helicobacter pylori reinfection in Brazilian patients with peptic ulcer disease: a 5-year follow-up. Helicobacter. 2010;15(1):46–52. doi:10.1111/j.1523-5378.2009.00734.x
14. Mansour-Ghanaei F, Taefeh N, Joukar F, et al. Recurrence of Helicobacter pylori infection 1 year after successful eradication: a prospective study in Northern Iran. Med Sci Monit. 2010;16(3):CR144–CR148.
15. Hu Y, Wan JH, Li XY, et al. Systematic review with meta-analysis: the global recurrence rate of Helicobacter pylori. Aliment Pharmacol Ther. 2017;46(9):773–779. doi:10.1111/apt.14319
16. Xue Y, Zhou LY, Lu HP, et al. Recurrence of Helicobacter pylori infection: incidence and influential factors. Chin Med J. 2019;132(7):765–771. doi:10.1097/CM9.0000000000000146
17. Chen RX, Zhang DY, Zhang X, et al. A survey on Helicobacter pylori infection rate in Hainan Province and analysis of related risk factors. BMC Gastroenterol. 2023;23(1):338. doi:10.1186/s12876-023-02973-3
18. Suzuki H, Mori H. World trends for H. pylori eradication therapy and gastric cancer prevention strategy by H. pylori test-and-treat. J Gastroenterol. 2018;53(3):354–361. doi:10.1007/s00535-017-1407-1
19. Yan TL, Hu QD, Zhang Q, et al. National rates of Helicobacter pylori recurrence are significantly and inversely correlated with human development index. Aliment Pharmacol Ther. 2013;37(10):963–968. doi:10.1111/apt.12293
20. Morgan DR, Torres J, Sexton R, et al. Risk of recurrent Helicobacter pylori infection 1 year after initial eradication therapy in 7 Latin American communities. JAMA. 2013;309(6):578–586. doi:10.1001/jama.2013.311
21. Corral JE, Mera R, Dye CW, et al. Helicobacter pylori recurrence after eradication in Latin America: implications for gastric cancer prevention. World J Gastrointest Oncol. 2017;9(4):184–193. doi:10.4251/wjgo.v9.i4.184
22. Ozaydin N, Turkyilmaz SA, Cali S. Prevalence and risk factors of Helicobacter pylori in Turkey: a nationally-representative, cross-sectional, screening with the 13 C-Urea breath test. BMC Public Health. 2013;13(1):1215. doi:10.1186/1471-2458-13-1215
23. Kim SY, Hyun JJ, Jung SW, et al. Helicobacter pylori Recurrence after First-and Second-Line Eradication Therapy in Korea: the Problem of Recrudescence or Reinfection. Helicobacter. 2014;19(3):202–206. doi:10.1111/hel.12117
24. Basílio ILD, Catão MFC, Carvalho JDDS, Freire-Neto FP, Ferreira LC, Jerônimo SMB. Risk factors of Helicobacter pylori infection in an urban community in Northeast Brazil and the relationship between the infection and gastric diseases. Rev Soc Bras Med Trop. 2018;51(2):183–189. doi:10.1590/0037-8682-0412-2016
25. Niu H, Su B. Current status of epidemiological study of Helicobacter pylori infection in Inner Mongolia. Mod Digest Inter Diagnos Treat. 2019;24(2):216–218.
26. Xia Y, Meng G, Zhang Q, et al. Dietary patterns are associated with Helicobacter pylori Infection in Chinese adults: a cross-sectional study. Scirep. 2016;6:32334.
27. Zamani M, Vahedi A, Maghdouri Z, et al. Role of food in environmental transmission of Helicobacter pylori. Caspian Intern Med. 2017;8(3):146–152.
28. Zhang L, Li N, Zou H, Cai Y, Wang B, Zhu L. Analysis of the current status of Helicobacter pylori infection and its influencing factors in the physical examination and outpatient population in Baoshan area, Shanghai. Gastroenterology. 2019;24(06):326–330.
29. Wang M, Lin Y, Xu X, Wang L, Lin J, Lin X. Analysis of the distribution of body type and susceptibility factors in patients with Helicobacter pylori infection in traditional Chinese medicine. Chin Folk Therapy. 2019;24:57–60.
30. Li X, Feng Y, Zhang D, Ren X, He S, Ren M. Study on recurrence of Helicobacter pylori infection and its influencing factors. J Xi’an Jiaotong Univ. 2019;40(06):933–936.
31. Bruce MG, Bruden DL, Morris JM, et al. Reinfection after successful eradication of Helicobacter pylori in three different populations in Alaska. Epidemiol Infect. 2015;143(6):1236–1246. doi:10.1017/S0950268814001770
32. Nas A, Mirza N, Hägele F, et al. Impact of breakfast skipping compared with dinner skipping on regulation of energy balance and metabolic risk. Am J Clin Nutr. 2017;105(6):1351–1361. doi:10.3945/ajcn.116.151332
33. Jakubowicz D, Wainstein J, Ahren B, Landau Z, Bar-Dayan Y, Froy O. Fasting until noon triggers increased postprandial hyperglycemia and impaired insulin response after lunch and dinner in individuals with type 2 diabetes: a randomized clinical trial. Diabetes Care. 2015;38(10):1820–1826. doi:10.2337/dc15-0761
34. Kofteridis DP, Giourgouli G, Plataki MN, et al. Community-acquired pneumonia in elderly adults with type 2 diabetes mellitus. J Am Geriatr Soc. 2016;64(3):649–651. doi:10.1111/jgs.14011
35. Sahoo OS, Mitra R, Bhattacharjee A, Kar S, Mukherjee O. Is diabetes mellitus a predisposing factor for helicobacter pylori infections? Curr Diab Rep. 2023;23(8):195–205. doi:10.1007/s11892-023-01511-5
36. Zhou X, Zhang C, Wu J, Zhang G. Association between Helicobacter pylori infection and diabetes mellitus: a meta-analysis of observational studies. Diabet Res Clin Pract. 2013;99(2):200–208. doi:10.1016/j.diabres.2012.11.012
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
