Back to Journals » International Journal of Women's Health » Volume 15
Recent Insights into Pregnancy and Lactation-Associated Osteoporosis (PLO)
Authors Scioscia MF, Zanchetta MB
Received 20 April 2023
Accepted for publication 11 July 2023
Published 2 August 2023 Volume 2023:15 Pages 1227—1238
DOI https://doi.org/10.2147/IJWH.S366254
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Maria Florencia Scioscia, Maria Belen Zanchetta
Instituto de Diagnóstico e Investigaciones Metabólicas (IDIM), Universidad del Salvador, Buenos Aires, ZC 1012, Argentina
Correspondence: Maria Belen Zanchetta, Email [email protected]
Abstract: During pregnancy and lactation, female physiology adapts to fulfill the fetal and neonatal calcium and phosphorus requirements. The physiological changes that take place during these periods do not affect maternal skeleton resistance to fracture in most of the cases. However, there is a small percentage of women that do experience fragility fractures during these times of life. Pregnancy and lactation-associated osteoporosis (PLO) is an infrequent condition defined by the occurrence of non-traumatic fractures – most frequently vertebral – during the third trimester of gestation and/or the first months of postpartum. Its physiopathology has not yet been completely elucidated. Several authors have reported that risk factors for secondary osteoporosis might be present in up to 80% of the cases of PLO patients. According to recent studies, genetic factors might also play a relevant role in PLO. Given its rarity, the available literature on this condition is limited. Most of the published data consist on case reports and case series articles. There are not any randomized controlled trials regarding this disorder. Although there is consensus about discontinuation of lactation and calcium and vitamin D supplementation as the first steps in the treatment of these patients, there is still controversy regarding the long-term and/or pharmacological management of this condition. Recent data on the use of teriparatide in this population looks promising. In this review, we aimed to revise and summarize current knowledge about the physiopathology and management of PLO.
Keywords: premenopausal, osteoporosis, pregnancy, lactation
Introduction
Bone Metabolism in Pregnancy and Lactation
The female body and its physiology are amazingly prepared to guarantee the perpetuation of humanity. Different hormonal axes interact to regulate the initiation of puberty, menstrual cycles, ovulation, pregnancy, and lactation, allowing the continuation of the species. Maternal physiology adapts perfectly to meet the nutritional demands of fetuses and neonates during pregnancy and lactation.1
Physiology During Pregnancy
By the end of pregnancy, the fetal skeleton contains 30 g calcium, 20 g phosphorus, and 0.8 g magnesium.2 As of week 12 of gestation, intestinal absorption of calcium doubles.2 This is the result of an upregulation of α-1-hydroxylase in the kidney and the subsequent increase in dihydroxycholecalciferol level – the active form of vitamin D – which in turn leads to better calcium absorption in the intestine.2 Through this mechanism, maternal calcium intake can meet the fetal calcium demand.2
Although the available data regarding bone mineral density during pregnancy are limited, it suggests that there is a positive balance of calcium by mid-term pregnancy. However, bone resorption increases during the third trimester of gestation.2 Results from several studies about bone mineral density (BMD) measured by Dual-energy X-ray absorptiometry (DXA) at the end of pregnancy are conflictive, with some showing a minimal decrease and others no changes at all.3–6 Holmberg-Marttila et al reported that bone loss at the lumbar spine during pregnancy was around 3%.7 Møller et al measured BMD by DXA in women before delivery and at 15 days postpartum and reported a decrease of 1.8 ± 0.5% at the lumbar spine, 3.2 ± 0.5% at the total hip and 2.4 ± 0.3% at the whole body.8 When viewed together, these studies suggest that most women have no change or a very modest decrease in BMD by full-term pregnancy.4–8
Physiology During Lactation
The neonate needs 200 mg calcium per day from milk during the first semester of lactation, and 120 mg during the following months.6 The central regulator of maternal bone metabolism during lactation is the “brain-breast-bone” circuit.2 Breast-suckling stimulates prolactin release. This inhibits hypothalamic GnRH (gonadotropin-releasing hormone), leading to the suppression of the gonadotropins and lowering levels of the ovarian sex steroids, estradiol and progesterone.2
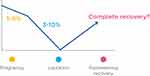
Additionally, the breast produces and releases PTHrP (parathyroid hormone–related peptide). The decrease in estrogen levels and the high circulating levels of PTHrp lead to an upregulation of RANKL (receptor activator of nuclear factor κB ligand), stimulating the development of osteoclasts and bone resorption. In this way, calcium is released from bone into the bloodstream so it can reach the breast ducts.6,7 Thus, the main mechanism by which female physiology meets the high calcium demands of the neonate appears to be a temporary demineralization of the skeleton.2,9 Figure 1 summarizes bone metabolism physiology during pregnancy and lactation.
Regarding BMD, there is a decrease of 3 to 10% during the first 2 to 6 months of lactation, predominantly in the trabecular compartment – mainly lumbar spine, and a lower loss in the cortical compartment – at the hip.6,8 The average rate of bone loss in lactation ranges from 1 to 3% per month. After the cessation of lactation, a substantial increase in bone mass and mineralization takes place to reverse the loss that occurred during breastfeeding.6,8 Data from many available studies suggest that loss of bone density is completely reversed between 6 and 12 months after discontinuing lactation in most women (see Figure 2).10–12 The underlying mechanisms of this recovery are not well known. Recently, data obtained from mice have suggested that osteoblasts are potently stimulated to produce osteoid and that calcitriol and/or a calcium-enriched diet are needed for this immature bone to become mineralized.13–15
Pregnancy and lactation are major challenging periods for maternal bone metabolism. However, the female skeleton is well prepared to face these periods successfully, and resistance does not seem to be affected in the long term.2,11,16,17 According to most epidemiologic trials in premenopausal and postmenopausal women, pregnancy and breastfeeding do not adversely affect peak bone mass (PBM), bone mineral density, or future risk of hip fractures.2,10,16,17
Osteoporosis During Pregnancy and Lactation
Pregnancy- and lactation-associated osteoporosis (PLO) is a rare condition in which women suffer fragility fractures, most commonly vertebral, during the third trimester of pregnancy or early postpartum. It was first described by Nordin and Roper in 1955.1 The incidence is calculated to be around 0.4 per 100000 women.2 This condition usually affects primiparous women in their fourth decade of life.2 Severe back pain is the most frequent form of presentation and it is often interpreted as a typical pregnancy or postpartum symptom. Thus, this condition is usually misdiagnosed.18 Less frequently, patients might suffer hip fractures. Although PLO is infrequent, it has a significant impact on the life of women of childbearing age who might experience lifelong consequences such as chronic pain and irreversible static disorders of the spine.19–21
The pathophysiology of this disorder is still not well known. According to most studies performed in women with PLO, risk factors are present in up to 80–85%.22 These factors include: inadequate calcium intake and/or absorption; low peak bone mass; genetic factors – such as inactivating mutations in low-density lipoprotein receptor-related protein 5 (LRP5)-; vitamin D insufficiency; inadequate high release of PTHrP; diverse conditions that present with low estrogen levels such as anorexia nervosa, oligomenorrhea and premature ovarian failure; low BMI; prolonged bedrest; smoking; hypercalciuria; and pharmacotherapy that may induce bone loss such as heparin, systemic glucocorticoids, GnRH analogues, and anticonvulsants23,24 (Table 1). We studied a group of seven women with PLO and only two of them had major risk factors for osteoporosis (high doses of glucocorticoids and kidney stones with hypercalciuria).25 In the rest, it was our impression that the severity of risk factors (low calcium intake, family history of osteoporosis, smoking) was not enough to account for the occurrence of vertebral fractures in these premenopausal women. We also observed a trend towards a lower BMI in our patients with PLO, who had a mean BMI of 20.9 ± 1.9 kg/m2 vs 22.3 ± 2.6 kg/m2 in controls, as other authors have already described.25–27 In a recent study, Cohen et al assessed women with PLO using transiliac bone biopsy and found both lower mineral apposition and bone formation rates in comparison with premenopausal osteoporotic women.28 They also reported that serum bone turnover markers and micro computed tomography findings showed that women with PLO had low bone formation without decrease in the number of osteoblasts, which might suggest an underlying defect in the function of these cells.28 In the last few years, Butscheidt et al have observed the presence of genetic variants of LRP5, Wnt, Col1A1 and Col1A2 in patients with PLO, which suggests that they may play an important role in this condition, even predisposing to more severe presentations.29,30 As there is no indication of BMD assessment in healthy premenopausal women, most patients are not aware of having low bone mass before getting pregnant. We hypothesize that PLO is caused by a prior condition that prevents the skeleton from successfully undergoing the challenge of pregnancy and lactation.2
 |
Table 1 Risk Factors and/or Conditions for Pregnancy and Lactation Associated Osteoporosis |
The available international literature on this condition is limited. Most of the published data consist on case reports and case series articles. No randomized controlled trials have been performed on this matter. There is expert consensus about discontinuation of lactation and calcium and vitamin D supplementation as the first steps in the treatment of these of this disorder, but there is still controversy regarding the long-term management and the indication of pharmacological treatment in these patients.31–36 According to recent data, on the use of teriparatide in this population looks promising.2,31,35,36
In this review, we revise and summarize the highlights of current knowledge on pregnancy and lactation-associated osteoporosis.
How Can We Assess Bone Metabolism and Density in Patients with PLO?
Bone Metabolism
Laboratory tests in these patients should include a complete blood count and metabolic panel, as well as bone metabolism parameters and other assessments to rule out any cause of secondary osteoporosis.1 Table 2 shows the main tests to be performed in these patients.
 |
Table 2 Laboratory Assessments in Women with PLO |
Imaging
X-Rays and Magnetic Resonance Imaging to Confirm Fractures
Women with PLO suffer fractures that occur, most frequently, between the last trimester of pregnancy and the first 3–4 months postpartum.37,38 When vertebral fractures occur during advanced pregnancy; the dorsal or lumbar pain might be wrongfully attributed to the increase of weight and the lumbar lordosis typical of this period. Therefore, the diagnosis is most likely made retrospectively, once the baby is born.39
In breastfeeding women, front and profile X-rays of the dorsal and lumbar spine or hip might be the first and fastest approach to the diagnosis of PLO.31 Magnetic Resonance Imaging (MRI) of dorsal and lumbar spine or hip without contrast is useful to observe the presence or absence of edema. The presence of edema is identified as hypointense areas in T1 and hyperintense areas in T2.31 After a vertebral fracture occurs, edema might persist for up to six months, approximately. Thus, the presence of edema in MRI indicates the acute and/or subacute condition of the fractures.31 On another note, the characteristics of the fracture are helpful to suspect a non-osteoporotic etiology in case the vertebral posterior wall or the pedicle is compromised; these signs are seen in secondary malignant lesions, very infrequent in this population of patients.40,41
DXA Scan to Assess Bone Mineral Density
Areal bone mineral density of lumbar spine and hip is generally assessed by DXA.22,26,27 Many authors have evaluated patients with PLO using DXA scans and noticed the greater compromise of lumbar spine versus the hip.22,26,27,42 In 2005, Sarli et al studied eight patients with PLO and the reported media T score was −2.85 ± 0.09 and −2.33 ± 0.81 at lumbar spine and femoral neck, respectively.27 O’Sullivan et al evaluated 11 patients with PLO and they also observed a decrease in BMD measured by DXA -media T score −2.8 at the lumbar spine and −2.0 at femoral neck-.26 More recently, Laroche et al studied a cohort of 52 patients with PLO and reported that the average T-score was - 3.4 at the lumbar spine and −2.0 at the hip.22 Similarly, in our cohort of seven women with PLO, we observed that the media for Z score was −3.2 ± 0.7 at LS and −2.0 ± 0.9 at FN.25 In comparison with healthy lactating women, BMD of our patients was decreased by 32% and 26% in LS and CF, respectively.25 In conclusion, although BMD prior to pregnancy is usually unknown due to lack of indication, when it is assessed after these patients suffer a fracture, it is usually low, even in comparison with healthy women who are undergoing the same physiological process.25
Bone Microarchitecture to Understand Pathophysiology
High Resolution peripheral Quantitative Computed Tomography (HR-pQCT) is a noninvasive tool that evaluates bone microarchitecture by scanning the non-dominant distal tibia and distal radius.43 The scan obtains 110 slices with a resolution of 82 μm, building a 3-dimensional representation of the bone.43 High resolution enables the assessment of trabecular and cortical bone separately, and the measurement of volumetric bone density and microarchitecture parameters, such as trabecular number and thickness.43 Globally, it is being used to help understand different pathologies and therapeutic actions of bone targeted agents.43 Also, it can help to identify which patients with osteopenia have a higher risk of fracture.43
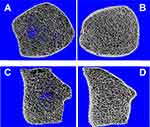
Bjørnerem et al evaluated healthy lactating women using HR-pQCT. They observed that the trabecular compartment was significantly affected, with lower trabecular density and number as well as greater trabecular separation.44 These findings confirm that the trabecular bone is the main responsible for the skeleton response to the extra calcium demand during lactation.25
We recently evaluated bone microarchitecture using HR-pQCT in our group of seven patients with PLO and compared them with a control group of eight healthy lactating women.25 We observed deterioration of bone microarchitecture in women with PLO in comparison with the control group, who were also going through a physiologically intense bone resorption process.25 The trabecular compartment was the most affected.25 It showed significantly lower trabecular density, number, and thickness, with subsequent greater trabecular separation and network heterogeneity.25 With regard to the cortical compartment, its density and thickness were also deteriorated, but to a lesser extent25 (see Figure 3). The reason why the two compartments are affected in a different way in women with PLO seems to be that the trabecular bone is the most metabolically active one, because it is closest to the bone marrow and therefore intimately related to blood vessels. It is specially designed to rapidly liberate calcium to maintain normal calcium serum levels.45 As for the cortical compartment, it is harder and more compact than the trabecular bone.43 Also, it is not so close to the vessels and it is less metabolically active.43 So, it is not as quickly affected with the increase of bone turnover.43 The fact that PLO patients presented with severe bone microarchitecture impairment compared to control women who were also undergoing a profound process of trabecular bone resorption due to lactation is in line with the hypothesis that bone quality might have been deteriorated before gestation in most of our patients and consequently, they were not able to fulfill the increased calcium requirements successfully.25 Other authors also found results in the same line.46 It is worth noting that although not always available, HR-pQCT is a useful noninvasive tool to assess bone microarchitecture, giving us a new vision into the pathophysiology of PLO.25
Management of Patients with PLO
Possible management includes non-pharmacological and pharmacological options. Due to lack of randomized controlled trials, there is still controversy regarding pharmacological treatment in this special population.2,26,31,35,36 In this context, the treatment decision must be made according to clinical judgement after considering the potential risks and benefits of each treatment.2,47 It is important to remember that these young women's skeletons are capable of experiencing spontaneous recovery of bone mineral density and strength through the following years.2,47
Non-Pharmacological Treatment
As a first approach, calcium and vitamin D should be optimized.2,47 Of course, if any cause of secondary osteoporosis is identified, it should be treated first (see Table 1).47 These patients must have a calcium intake between 1000 and 1500 mg/day, whereas 25 OH-dihydroxycholecalciferol levels should be ≥30 ng/dl (75 nmol/l).1,48
Calcium requirements can be achieved through a daily dairy products intake.49 For example, a cup of milk contains 250–300 mg of calcium, fortified yogurt contains 500 mg and one serving of cheese (50 g) contains 200–350 mg of calcium. If the patient cannot achieve the required intake of calcium through a daily diet, supplementation with calcium tablets is also available. It is important to explain to the patients that calcium should be taken in different servings of a maximum of 500 mg each throughout the day to maximize its absorption.50
In general, vitamin D can be obtained mostly by sun exposure. However, if the recently fractured PLO patient presents with lower than 30 ng/mL levels, she should be supplemented immediately with at least 1000 to 2000 UI per day (depending on baseline levels) to rapidly reach the desired target.50,51
These patients can experience a significant spontaneous increase in BMD of about 6 to 12% at LS at 6–12 months after weaning. Similar significant improvement has been reported at the hip.22,26,52 Consequently, in order to decrease the high bone resorption that takes place during lactation, women with PLO should be advised to discontinue breastfeeding as early as possible.
Other considerations include adequate analgesia for painful vertebral fractures and physical therapy. Besides, when fractures consolidate, after 3 to 6 months, these patients should be encouraged to perform reasonable, but not excessive, weight-bearing and physical resistance activity to help maintain bone and muscle mass and mobility.1
Pharmacological Treatment
Most experts still suggest that pharmacological treatment should be reserved for the most severe cases with multiple vertebral fractures, persistent disabling pain, or those patients who do not experience a satisfactory recovery in BMD after weaning and adequate calcium and vitamin D supplementation.2,53
Patients with PLO are different from postmenopausal women, who are likely to continue losing bone mass over time. These premenopausal women are going to have spontaneous recovery after weaning, and will most likely maintain their skeleton BMD since they will have adequate circulating estrogen levels. This argument and the possibility of future pregnancies are the main issues evaluated by those experts who recommend conservative treatment as the first option.2
The available evidence on pharmacological treatment in this population is limited. The great majority of data comes from case report series and retrospective studies where patients were treated with bisphosphonates, denosumab or teriparatide.22,26,52,54–56 These osteoactive treatments should be prescribed and monitored by physicians specialized in bone metabolism.2,47
Bisphosphonates
O’ Sullivan et al retrospectively evaluated a cohort of eleven women with PLO who were followed up during a period of 20 years.26 Ten of them had had vertebral fractures, while one presented with hip and bilateral wrist fractures. At the time of fracture, mean age was 30 ± 4.4 years.26 All of them were advised to stop breastfeeding and received calcium and vitamin D supplementation. Nine out of eleven patients received treatment with bisphosphonates –alendronate, pamidronate and/or zoledronate.26 After a median duration of treatment of 24 months, they observed a substantial increase of 23% in BMD in the patients who had received bisphosphonates.26 The patients who did not receive early bisphosphonates therapy also had a smaller but still significant increase of about 11% in LS BMD.26 However, this study has limitations: it was a retrospective analysis, and only 2 out of the 11 patients had not received pharmacological treatment.26
In Laroche’s large case series of 52 women with PLO, bisphosphonates improved BMD recovery, with a mean annual gain of 10% versus 6.6% in those who did not receive any specific pharmacological treatment.22 Other authors have also reported that bisphosphonates can reduce bone turnover markers and improve pain and physical functioning by reducing inflammation in the affected area.37,55 However, we should remember that bisphosphonates are retained in the skeleton for several years, and they can also cross the placenta.57 Although the literature has not found unexplained congenital malformations in the offspring of women who had previously received bisphosphonates, there are still safety concerns regarding the use of these drugs in women of childbearing potential.58–60
Denosumab
There are very few reports on the use of denosumab in patients with PLO. In 2016, we reported two cases of PLO treated with denosumab.56 The patients had suffered vertebral fractures during early postpartum and were treated with denosumab 60 mg subcutaneous every 6 months.56 After one year of treatment, we observed an increase of 14% in LS BMD, a significant improvement in trabecular microarchitecture assessed by HRpQCT and a clear remission of pain.56
More recently, Ijuin et al published the case of one patient with PLO who received teriparatide 56.5 mcg s.c. weekly for 6 months and was switched to denosumab 60 mg s.c. every 6 months. After 6 months of treatment with denosumab, BMD increased by 16.5% and 3.9% at LS and FN, respectively, and the patient reported that pain had subsided.54
Available literature on the use of denosumab in patients with PLO is limited to the two papers mentioned above. Considering that denosumab is an antiresorptive drug that is not retained in the human body for more than 6 months, more data is needed before it can be considered as an option in the therapeutic approach to these patients. There is no information regarding whether the gain in BMD is maintained after the discontinuation in this population or if they need to receive bisphosphonates as postmenopausal women do.54,56
Teriparatide
The earliest publications about teriparatide in women with PLO were case reports or series that informed a significant improvement in back pain and mobility within the first two months of treatment.61 The increase in BMD ranged from 24.4 to 36% at LS and 12 to 13.4% at the hip after 13 to 18 months of treatment.61
More recently, Lampropoulou-Adamidou compared a group of women with PLO treated with teriparatide versus a control group treated only with calcium and vitamin D supplementation.52 Women treated with teriparatide showed an increase in lumbar spine BMD of 21 ± 12% vs 6 ± 5% in controls at 12 months and 33 ± 13% vs 12 ± 4% at 24 months, respectively.52 Lee et al published a retrospective study of a cohort of 64 women with PLO.62 Forty-three of them were treated with teriparatide for 12 months (media) and followed up for 3 years. After teriparatide, 13 women received antiresorptive treatment (TPDT-ART), while 20 of them did not receive any anticatabolic drug (TPDT-no ART). The authors observed that BMD at lumbar spine increased at 1, 2, and 3 years from baseline in both the TPTD-ART (14%, 22%, and 24%, respectively) and the TPTD-no ART (17%, 24%, and 23%, respectively) groups, without significant differences between them.62 Similar results were observed at the hip.62 They concluded that BMD gain by teriparatide administration in premenopausal women with PLO can be maintained without sequential antiresorptive treatment.62 This is in line with previous reports that suggested that the presence of estrogens might avoid the bone loss described in postmenopausal women after stopping teriparatide.46
As we mentioned before, in many cases, low peak bone mass and impaired osteoblast activity may play an important role in the pathogenesis of PLO.28
Considering this, teriparatide appears as an attractive option for those patients who might need pharmacological treatment due to severe presentation or inadequate response to conservative treatment.
Romosozumab
In 2022, Kaneuchi et al published the first report of a patient with PLO treated with romosozumab.63 The patient had suffered multiple vertebral fractures. She was treated with teriparatide injection for 4 months, but the treatment was discontinued after the appearance of new vertebral fractures.63 She was started on romosozumab and completed 12 months of treatment. After the romosozumab treatment, her BMD was increased from the baseline by 23.6% at L1–L4, 6.2% at the femoral neck, and 11.2% at the total hip without any new fractures.63 The authors suggest that romosozumab might as well be a therapeutic option to improve BMD and reduce the risk of fracture in patients with PLO.63
Long Term Follow-Up
The occurrence of new fractures during subsequent pregnancies is very infrequent, although a few cases have been published. Laroche reported new fractures in two out of seven women with PLO who got pregnant again (follow-up period was up to 36 months).22 Kyvernitakis et al reported a follow-up of 6 years in 20 women with PLO who had a new pregnancy and found that 20% suffered a pregnancy-associated fracture.64 The study showed that those women who had more than one fracture at baseline were at a higher risk of suffering a subsequent fracture64 The great majority of these patients do not suffer new fragility fractures in the long term.64
However, there is a small percentage of women, especially those who have several and/or major risk factors for osteoporosis, that may experience new fractures in vertebrae, tibia, radius, sacrum and/or humerus.22,64
In our series of patients with follow-up periods ranging from 5 to 10 years, two suffered new fractures in the long term: one of them had a wrist fracture following a fall from her standing height and the other had a fibula fracture after a 40 cm height fall (data not published).
Long-term follow-up of these patients is crucial to monitor recovery and counsel them regarding adequate calcium and vitamin D supplementation and a healthy lifestyle in order to avoid potential new fragility fractures.
Conclusion
In this paper, we have summarized current knowledge about pregnancy and lactation-associated osteoporosis.
Although very rare, PLO represents a major factor of physical and emotional distress. It is crucial to increase awareness of the major role of calcium and vitamin D within the medical community and patients during this highly demanding period, especially in women with high-risk factors such as low calcium and vitamin D levels and low BMI.
PLO patients might have impaired bone mass and microarchitecture before pregnancy, and that is the reason why their skeletons are incapable of meeting the gestation and lactation requirements successfully. A spontaneous increase in bone mass and strength is expected to occur in most women after discontinuing lactation, even in those who fractured. Women with PLO should be advised to stop breastfeeding and receive adequate calcium and vitamin D supplementation. Other factors that may cause bone loss should be corrected to optimize skeletal health for the long term and in advance of a future pregnancy. Pharmacological therapy is also an option but further studies are necessary to determine when it should be used in these patients, as well as its duration.
In recent years, observational studies and case reports have helped us better understand PLO. Many issues remain unanswered in terms of its causes and management, though. Most of these women seem to show a good evolution, with improvements in pain and mobility and an increase in BMD over time. Yet, it is essential for clinicians to bear in mind that pregnant women should receive adequate amounts of vitamin D and calcium and that fragility fractures can occur during pregnancy and lactation. Being aware of this possibility would facilitate a prompt diagnosis, which is crucial for earlier treatment if there is an underlying metabolic bone disorder. We still need to determine the best approach to the treatment of women with PLO and consequently, further research on this condition is required.
Disclosure
Dr Maria Belen Zanchetta reports personal fees from Amgen and Adium, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Kovacs CS. Calcium and bone metabolism disorders during pregnancy and lactation. Endocrinol Metab Clin North Am. 2011;40(4):795–826. doi:10.1016/j.ecl.2011.08.002
2. Kovacs CS. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol Rev. 2016;96(2):449–547. doi:10.1152/physrev.00027.2015
3. Gallacher SJ, Fraser WD, Owens OJ, et al. Changes in calciotrophic hormones and biochemical markers of bone turnover in normal human pregnancy. Eur J Endocrinol. 1994;131(4):369–374. doi:10.1530/eje.0.1310369
4. Karlsson C, Obrant KJ, Karlsson M. Pregnancy and lactation confer reversible bone loss in humans. Osteoporos Int. 2001;12(10):828–834. doi:10.1007/s001980170033
5. Laskey MA, Prentice A. Bone mineral changes during and after lactation. Obstet Gynecol. 1999;94(4):608–615. doi:10.1016/s0029-7844(99)00369-5
6. Sohlstrom A, Forsum E. Changes in adipose tissue volume and distribution during reproduction in Swedish women as assessed by magnetic resonance imaging. Am J Clin Nutr. 1995;61(2):287–295. doi:10.1093/ajcn/61.2.287
7. Holmberg-Marttila D, Sievanen H, Tuimala R. Changes in bone mineral density during pregnancy and postpartum: prospective data on five women. Osteoporos Int. 1999;10(1):41–46. doi:10.1007/s001980050192
8. Moller UK, Við Streym S, Mosekilde L, et al. Changes in bone mineral density and body composition during pregnancy and postpartum. A controlled cohort study. Osteoporos Int. 2012;23(4):1213–1223. doi:10.1007/s00198-011-1654-6
9. Cross NA, Hillman LS, Allen SH, et al. Changes in bone mineral density and markers of bone remodeling during lactation and postweaning in women consuming high amounts of calcium. J Bone Miner Res. 1995;10(9):1312–1320. doi:10.1002/jbmr.5650100907
10. Sowers M. Pregnancy and lactation as risk factors for subsequent bone loss and osteoporosis. J Bone Miner Res. 1996;11(8):1052–1060. doi:10.1002/jbmr.5650110803
11. Sowers M, Randolph J, Shapiro B, et al. A prospective study of bone density and pregnancy after an extended period of lactation with bone loss. Obstet Gynecol. 1995;85(2):285–289. doi:10.1016/0029-7844(94)00351-D
12. Chan SM, Nelson EAS, Leung SSF, et al. Bone mineral density and calcium metabolism of Hong Kong Chinese postpartum women--a 1-y longitudinal study. Eur J Clin Nutr. 2005;59(7):868–876. doi:10.1038/sj.ejcn.1602148
13. Fudge NJ, Kovacs CS. Pregnancy up-regulates intestinal calcium absorption and skeletal mineralization independently of the vitamin D receptor. Endocrinology. 2010;151(3):886–895. doi:10.1210/en.2009-1010
14. Gillies BR, Ryan BA, Tonkin BA, et al. Absence of calcitriol causes increased lactational bone loss and lower milk calcium but does not impair post-lactation bone recovery in Cyp27b1 null mice. J Bone Miner Res. 2018;33(1):16–26. doi:10.1002/jbmr.3217
15. Ryan BA, McGregor NE, Kirby BJ, et al. Calcitriol-dependent and -independent regulation of intestinal calcium absorption, osteoblast function, and skeletal mineralization during lactation and recovery in mice. J Bone Miner Res. 2022;37(12):2483–2497. doi:10.1002/jbmr.4712
16. Cooke-Hubley S, Gao Z, Mugford G, et al. Parity and lactation are not associated with incident fragility fractures or radiographic vertebral fractures over 16 years of follow-up: Canadian Multicentre Osteoporosis Study (CaMos). Arch Osteoporos. 2019;14(1):49. doi:10.1007/s11657-019-0601-6
17. Jimenez-Arreola J, Aguilera Barreiro Mde L. Lactancia materna como factor preventivo para la osteoporosis en mujeres adultas [Breast feeding as preventive factor for osteoporosis in adult women]. Nutr Hosp. 2015;32(6):2600–2605. Spanish. doi:10.3305/nh.2015.32.6.9047
18. Pola E, Colangelo D, Nasto LA, et al. Pregnancy-associated osteoporosis (PAO) with multiple vertebral fragility fractures: diagnosis and treatment in a young primigravid woman. J Biol Regul Homeost Agents. 2016;30(4 Suppl 1):153–158.
19. Ozturk C, Atamaz FC, Akkurt H, et al. Pregnancy-associated osteoporosis presenting severe vertebral fractures. J Obstet Gynaecol Res. 2014;40(1):288–292. doi:10.1111/jog.12157
20. Saraux A, Bourgeais F, Ehrhart A, et al. Ostéoporose de la grossesse: 4 observations [Osteoporosis during pregnancy. 4 cases]. Rev Rhum Ed Fr. 1993;60(9):596–600. French.
21. Sarikaya S, Özdolap Ş, Açıkgöz G, et al. Pregnancy-associated osteoporosis with vertebral fractures and scoliosis. Joint Bone Spine. 2004;71(1):84–85. doi:10.1016/j.jbspin.2003.05.003
22. Laroche M, Talibart M, Cormier C, et al. Pregnancy-related fractures: a retrospective study of a French cohort of 52 patients and review of the literature. Osteoporos Int. 2017;28(11):3135–3142. doi:10.1007/s00198-017-4165-2
23. Ryan BA, Kovacs CS. The puzzle of lactational bone physiology: osteocytes masquerade as osteoclasts and osteoblasts. J Clin Invest. 2019;129(8):3041–3044. doi:10.1172/JCI130640
24. Di Gregorio S, Danilowicz K, Rubin Z, et al. Osteoporosis with vertebral fractures associated with pregnancy and lactation. Nutrition. 2000;16(11–12):1052–1055. doi:10.1016/S0899-9007(00)00430-5
25. Scioscia MF, Vidal M, Sarli M, et al. Severe bone microarchitecture impairment in women with pregnancy and lactation-associated osteoporosis. J Endocr Soc. 2021;5(5):031. doi:10.1210/jendso/bvab031
26. O’Sullivan SM, Grey AB, Singh R, et al. Bisphosphonates in pregnancy and lactation-associated osteoporosis. Osteoporos Int. 2006;17(7):1008–1012. doi:10.1007/s00198-006-0112-3
27. Sarli M, Hakim C, Rey P, et al. Osteoporosis del embarazo y la lactancia [Osteoporosis during pregnancy and lactation. Report of eight cases]. Medicina. 2005;65(6):489–494. Spanish.
28. Cohen A, Kamanda‐Kosseh M, Dempster DW, et al. Women With Pregnancy and Lactation-Associated Osteoporosis (PLO) have low bone remodeling rates at the tissue level. J Bone Miner Res. 2019;34(9):1552–1561. doi:10.1002/jbmr.3750
29. Butscheidt S, Delsmann A, Rolvien T, et al. Mutational analysis uncovers monogenic bone disorders in women with pregnancy-associated osteoporosis: three novel mutations in LRP5, COL1A1, and COL1A2. Osteoporos Int. 2018;29(7):1643–1651. doi:10.1007/s00198-018-4499-4
30. Butscheidt S, Tsourdi E, Rolvien T, et al. Relevant genetic variants are common in women with pregnancy and lactation-associated osteoporosis (PLO) and predispose to more severe clinical manifestations. Bone. 2021;147:115911. doi:10.1016/j.bone.2021.115911
31. Hardcastle SA. ”Pregnancy and Lactation Associated Osteoporosis”. Calcif Tissue Int. 2022;110(5):531–545. doi:10.1007/s00223-021-00815-6
32. Jia P, Wang R, Yuan J, et al. A case of pregnancy and lactation-associated osteoporosis and a review of the literature. Arch Osteoporos. 2020;15(1):94. doi:10.1007/s11657-020-00768-7
33. Lujano-Negrete AY, Rodríguez-Ruiz MC, Skinner-Taylor CM, et al. Bone metabolism and osteoporosis during pregnancy and lactation. Arch Osteoporos. 2022;17(1):36. doi:10.1007/s11657-022-01077-x
34. Qian Y, Wang L, Yu L, et al. Pregnancy- and lactation-associated osteoporosis with vertebral fractures: a systematic review. BMC Musculoskelet Disord. 2021;22(1):926. doi:10.1186/s12891-021-04776-7
35. Yang B, Kong L, Hao D. Challenges in the management of pregnancy and lactation associated osteoporosis: literature review and retrospective study. Curr Stem Cell Res Ther. 2021;16(6):688–694. doi:10.2174/1574888X15999200729162502
36. Aytar MH, Ozcan-Eksi EE, Eksi MS, et al. Management of pregnancy-and lactation-related osteoporosis: case series. Turk Neurosurg. 2022;32(2):323–329. doi:10.5137/1019-5149.JTN.36058-21.1
37. Grizzo FM, da Silva Martins J, Pinheiro MM, et al. Pregnancy and lactation-associated osteoporosis: bone histomorphometric analysis and response to treatment with zoledronic acid. Calcif Tissue Int. 2015;97(4):421–425. doi:10.1007/s00223-015-0028-z
38. Hadji P, Boekhoff J, Hahn M, et al. Pregnancy-associated osteoporosis: a case-control study. Osteoporos Int. 2017;28(4):1393–1399. doi:10.1007/s00198-016-3897-8
39. Terzi R, Terzi H, Özer T, et al. A rare cause of postpartum low back pain: pregnancy- and lactation-associated osteoporosis. Biomed Res Int. 2014;2014:287832. doi:10.1155/2014/287832
40. Yildiz AE, Özbalcı AB, Ergen FB, et al. Pregnancy- and lactation-associated vertebral compression fractures: MRI prevalence and characteristics. Osteoporos Int. 2021;32(5):981–989. doi:10.1007/s00198-020-05754-w
41. Pongpornsup S, Wajanawichakorn P, Danchaivijitr N. Benign versus malignant compression fracture: a diagnostic accuracy of magnetic resonance imaging. J Med Assoc Thai. 2009;92(1):64–72.
42. Hardcastle SA, Yahya F, Bhalla AK. Pregnancy-associated osteoporosis: a UK case series and literature review. Osteoporos Int. 2019;30(5):939–948. doi:10.1007/s00198-019-04842-w
43. Zanchetta MB, Costa F, Longobardi V, et al. Significant bone microarchitecture impairment in premenopausal women with active celiac disease. Bone. 2015;76:149–157. doi:10.1016/j.bone.2015.03.005
44. Bjornerem A, Ghasem-Zadeh A, Wang X, et al. Irreversible deterioration of cortical and trabecular microstructure associated with breastfeeding. J Bone Miner Res. 2017;32(4):681–687. doi:10.1002/jbmr.3018
45. Frost HM. Defining osteopenias and osteoporoses: another view (with insights from a new paradigm). Bone. 1997;20(5):385–391. doi:10.1016/S8756-3282(97)00019-7
46. Cohen A, Stein EM, Recker RR, et al. Teriparatide for idiopathic osteoporosis in premenopausal women: a pilot study. J Clin Endocrinol Metab. 2013;98(5):1971–1981. doi:10.1210/jc.2013-1172
47. Kovacs CS, Ralston SH. Presentation and management of osteoporosis presenting in association with pregnancy or lactation. Osteoporos Int. 2015;26(9):2223–2241. doi:10.1007/s00198-015-3149-3
48. Mousa A, Naqash A, Lim S. Macronutrient and micronutrient intake during pregnancy: an overview of recent evidence. Nutrients. 2019;11(2):443. doi:10.3390/nu11020443
49. Ortega RM, Ortega AI, Sánchez JM, et al. Valor nutricional de los lácteos y consumo diario aconsejado [Nutritional value of dairy products and recommended daily consumption]. Nutr Hosp. 2019;36(Spec No3):25–29. Spanish.
50. Reid IR, Bolland MJ. Calcium and/or Vitamin D Supplementation for the Prevention of Fragility Fractures: who Needs It? Nutrients. 2020;12(4):1011. doi:10.3390/nu12041011
51. Pludowski P, Takacs I, Boyanov M, et al. Clinical practice in the prevention, diagnosis and treatment of vitamin D deficiency: a central and eastern European expert consensus statement. Nutrients. 2022;14(7):1483. doi:10.3390/nu14071483
52. Lampropoulou-Adamidou K, Trovas G, Triantafyllopoulos IK, et al. Teriparatide treatment in patients with pregnancy- and lactation-associated osteoporosis. Calcif Tissue Int. 2021;109(5):554–562. doi:10.1007/s00223-021-00871-y
53. Tuna F, Akleylek C, Özdemir H, et al. Risk factors, fractures, and management of pregnancy-associated osteoporosis: a retrospective study of 14 Turkish patients. Gynecol Endocrinol. 2020;36(3):238–242. doi:10.1080/09513590.2019.1648417
54. Ijuin A, Yoshikata H, Asano R, et al. Teriparatide and denosumab treatment for pregnancy and lactation-associated osteoporosis with multiple vertebral fractures: a case study. Taiwan J Obstet Gynecol. 2017;56(6):863–866. doi:10.1016/j.tjog.2017.10.028
55. Li LJ, Zhang J, Gao P, et al. Clinical characteristics and bisphosphonates treatment of rare pregnancy- and lactation-associated osteoporosis. Clin Rheumatol. 2018;37(11):3141–3150. doi:10.1007/s10067-018-4185-0
56. Sanchez A, Zanchetta MB, Danilowicz K. Two cases of pregnancy- and lactation- associated osteoporosis successfully treated with denosumab. Clin Cases Miner Bone Metab. 2016;13(3):244–246. doi:10.11138/ccmbm/2016.13.3.244
57. Papapoulos SE, Cremers SC. Prolonged bisphosphonate release after treatment in children. N Engl J Med. 2007;356(10):1075–1076. doi:10.1056/NEJMc062792
58. Losada I, Sartori L, Di Gianantonio E, et al. Bisphosphonates in patients with autoimmune rheumatic diseases: can they be used in women of childbearing age? Autoimmun Rev. 2010;9(8):547–552. doi:10.1016/j.autrev.2010.03.002
59. Ornoy A, Wajnberg R, Diav-Citrin O. The outcome of pregnancy following pre-pregnancy or early pregnancy alendronate treatment. Reprod Toxicol. 2006;22(4):578–579. doi:10.1016/j.reprotox.2006.05.009
60. Stathopoulos IP, Liakou C, Katsalira A, et al. The use of bisphosphonates in women prior to or during pregnancy and lactation. Hormones. 2011;10(4):280–291. doi:10.14310/horm.2002.1319
61. Lampropoulou-Adamidou K, Trovas G, Stathopoulos I, et al. Case report: teriparatide treatment in a case of severe pregnancy -and lactation- associated osteoporosis. Hormones. 2012;11(4):495–500. doi:10.14310/horm.2002.1383
62. Lee S, Hong N, Kim KJ, et al. Bone density after teriparatide discontinuation with or without antiresorptive therapy in pregnancy- and lactation-associated osteoporosis. Calcif Tissue Int. 2021;109(5):544–553. doi:10.1007/s00223-021-00869-6
63. Kaneuchi Y, Iwabuchi M, Hakozaki M, et al. Pregnancy and lactation-associated osteoporosis successfully treated with romosozumab: a case report. Medicina. 2022;59(1). doi:10.3390/medicina59010019
64. Kyvernitakis I, Reuter TC, Hellmeyer L, et al. Subsequent fracture risk of women with pregnancy and lactation-associated osteoporosis after a median of 6 years of follow-up. Osteoporos Int. 2018;29(1):135–142. doi:10.1007/s00198-017-4239-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.