Back to Journals » OncoTargets and Therapy » Volume 16
Recent Advances in the Management of Adverse Events Associated with Lorlatinib
Authors Liao D, Zhang J, Yan T, Chen S, Li W, Shangguan D, She Z
Received 21 June 2023
Accepted for publication 31 August 2023
Published 5 September 2023 Volume 2023:16 Pages 731—738
DOI https://doi.org/10.2147/OTT.S426989
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tohru Yamada
Dehua Liao,1 Jiwen Zhang,1,2 Ting Yan,1 Shanshan Chen,1 Wei Li,1 Dangang Shangguan,1,* Zhihua She3,*
1Department of Pharmacy, Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, People’s Republic of China; 2School of Pharmacy, University of South China, Hengyang, People’s Republic of China; 3Department of Medical Administration, Hunan Provincial Maternal and Child Health Care Hospital, Changsha, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhihua She, Department of Medical Administration, Hunan Provincial Maternal and Child Health Care Hospital, Changsha, People’s Republic of China, Email [email protected] Dangang Shangguan, Department of Pharmacy, Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, People’s Republic of China, Email [email protected]
Abstract: As a novel third-generation ALK tyrosine kinase inhibitor (TKI), lorlatinib has shown excellent systemic and intracranial activity in non-small cell lung cancer (NSCLC) patients who carry sensitizing ALK-activating mutations and progress on first- and second-generation TKIs. In comparison with other ALK-TKIs, lorlatinib has a unique safety profile for hyperlipidemia and central nervous system adverse events. Lorlatinib-induced adverse events are well tolerated, permanent discontinuations are rarely reported, and dose modifications and/or standard medical therapy are useful for the management of adverse events. Our present study reviews the safety profile of lorlatinib as well as the relevant management strategies. Our present study aims to provide a practical guide for the scientific management and application of lorlatinib.
Keywords: ALK, lorlatinib, adverse event, NSCLC, therapy management
Introduction
Lung cancer is one of the most common types of cancer and has the highest cancer-related mortality worldwide, with a 5-year survival rate of only 10 to 20%.1,2 Non-small cell lung cancer (NSCLC) accounts for 85% of all reported lung cancer cases.3 Based on pathologic classification, NSCLC is further classified into three major subtypes: adenocarcinoma, squamous carcinoma and large cell carcinoma.4 With the understanding of the molecular biology of NSCLC, numerous small molecular tyrosine kinase inhibitors (TKIs) have been successfully approved as targeted therapies in patients with oncogene-driven mutations, which are tremendous advances in NSCLC treatment.5,6 In comparison with traditional platinum-based chemotherapy, TKIs significantly improved overall survival (OS) and quality of life in NSCLC patients with oncogenic driver mutations.7
Epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements are the most common types of targetable driver mutations in NSCLC, which are generally mutually exclusive. Rearrangements of ALK are present in 5% of NSCLC patients, especially those with no or light smoking history, adenocarcinoma histology, younger age and female sex.8 The approval of TKIs has revolutionized the prognosis and management of ALK-positive NSCLC. Currently, there are three generations of ALK-TKIs have been approved for the first-line and later treatment of ALK-positive NSCLC, such as the first generation of crizotinib, the second generation of alectinib, brigatinib, ceritinib, and ensartinib, the third generation of lorlatinib. The efficacy and safety of these TKIs have been widely explored in large-scale, multicenter, randomized controlled trials.
As a novel, highly potent inhibitor of ALK, lorlatinib competitively binds to the adenosine triphosphate-binding pocket, blocking ALK-dependent oncogenic signaling (Figure 1). Previous studies have reported that lorlatinib exhibited anticancer effects in naïve ALK-positive NSCLC and those harboring mutations in ALK that conferred resistance to ALK inhibitors, including G1202R and I1171N mutations.9,10 A remarkable molecular structure feature of lorlatinib is that it is macrocyclic, which distinguishes it from other first- and second-generation ALK-TKIs.11 Furthermore, lorlatinib could effectively penetrate the blood-brain barrier in part by minimizing p-glycoprotein-1-mediated efflux, exhibiting highly effective treatment of those with CNS metastases.9,12 Hence, lorlatinib was approved as a subsequent line therapy for ALK-positive advanced NSCLC in 2018 and was approved for use in the first-line setting in later days.13,14
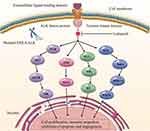 |
Figure 1 The anticancer mechanism of lorlatinib. |
As shown in previous clinical trials and real-world studies, treatment with lorlatinib is usually well tolerated, and the most common adverse events of lorlatinib include hypercholesterolemia, hypertriglyceridemia, edema and peripheral neuropathy.15,16 Grade 3 or 4 laboratory abnormalities of hypercholesterolemia, hypertriglyceridemia and hyperglycemia should also be noted, and treatment discontinuation due to adverse events was 7% with lorlatinib, as in the CROWN trial.14,17
This review summarizes the clinical safety of lorlatinib for ALK-positive NSCLC patients. The data are based on drug evaluation reports, clinical trials and real-world studies. It is important to regularly monitor common and special ADRs for oncologists and pharmacists. Early detection and intervention could help to minimize the consequences of ADR and maximize patient benefits. It is worth noting that ADR involves different organ systems, and multidisciplinary team management is crucial for the safe use of lorlatinib in ALK-positive NSCLC.
Hyperlipidemias
Hyperlipidemia is the most common ADR of lorlatinib in ALK-positive NSCLC, and hypercholesterolemia and hypertriglyceridemia are most commonly seen. Although they have a high incidence, hypercholesterolemia and hypertriglyceridemia are generally manageable and rarely result in temporary discontinuation or dose reduction.18 Pooled data from the Phase 1/2 study of 295 patients who received lorlatinib at the recommended dose of 100 mg QD. Among these patients, the incidence rates of hypercholesterolemia and hypertriglyceridemia were 96% and 90% respectively, and most of which were primarily grade 1 or 2 in severity. The median time to onset is 15 days of treatment, typically within the first few weeks.19 As shown in CROWN study, the higher incidences of grade 3–4 adverse events in the lorlatinib group were hypertriglyceridemia (23%) and hypercholesterolemia (19%).17 The results of a global Phase 2 trial of lorlatinib in ALK-positive advanced NSCLC showed that in Asian patients and non-Asian patients, the incidence rates of hypercholesterolemia were 83.3% and 83.9% respectively, while the incidence rates of hypertriglyceridemia were 75.9% and 57.1% respectively. Grade 3–4 hypercholesterolemia (15.7% Asian and 14.3% non-Asian patients) and hypertriglyceridemia (25.9% Asian and 12.4% non-Asian patients) were also reported in this study.20 In the subgroup analysis of the Chinese population and Japanese population, a similar tendency was observed. The rates of hypercholesterolemia and hypertriglyceridemia development were 92.7% and 90.8% respectively in the Chinese population.21 For the subgroup of the Japanese population, the incidence rates of hypercholesterolemia (79.5%) and hypertriglyceridemia (76.9%) are lower.22 An international real-world analysis of the safety of lorlatinib showed that the most common grade 1 to 2 treatment-related AEs recorded among all patients (n=95) were hypercholesterolemia (61%; Asians: 63% and non-Asians: 53%) and hypertriglyceridemia (43%; Asians: 43% and non-Asians: 42%), and grade 3 AEs included hypercholesterolemia (8%) and hypertriglyceridemia (4%).23
The management of hypercholesterolemia and hypertriglyceridemia is important for NSCLC patients who receive lorlatinib, especially for those with a history of cardiovascular disease. Monitoring of serum cholesterol and triglyceride levels by routine laboratory testing is necessary before and throughout treatment with lorlatinib. The intervention of lorlatinib hyperlipidemias is mainly based on different grades. When lipid levels are elevated after the administration of lorlatinib for a few days, lipid-lowering agents (such as statins) and lifestyle modifications are necessary.24 For some patients with elevated cholesterol (upper limit of normal, 300 mg/dL (upper limit of normal, 7.75 mmol/L)) and/or triglyceride levels (150–300 mg/dL (1.71–3.42 mmol/L)), coadministration of a preferred statin is recommended at the initial treatment.19,25 The choice and dosing of statins are mainly based on avoiding metabolizing enzymes CYP450 enzyme-induced drug interactions. As reported in previous studies and guidelines, pitavastatin, pravastatin, or rosuvastatin are recommended because of their low involvement with specific CYP450 enzymes that can interact with lorlatinib.25 In addition to statins, other lipid-lowering agents are also necessary for hyperlipidemia. Bauer et al19 have reported that at least one lipid-lowering agent is needed for most patients (81.0%) who received lorlatinib, and 22.1% and 30.8% required two or more agents for hypercholesterolemia and hypertriglyceridemia, respectively. When cholesterol levels are >500 mg/dL and/or triglyceride levels are >1000 mg/dL, dose interruptions of lorlatinib are recommended.19 When total cholesterol and TG decrease to levels below 400 mg/dL and 500 mg/dL, respectively, lorlatinib could be considered for rechallenge. It is worth noting that the lorlatinib dosage should be reduced to 75 mg once daily when severe hyperlipidemia recurs despite optimized lipid-lowering therapy.
Central Nervous System (CNS) Adverse Events
A low propensity for P-glycoprotein 1-mediated efflux and a good brain penetration were observed for lorlatinib,16 and the higher accumulation of lorlatinib in the brain will always be accompanied by CNS AEs. Interestingly, CNS AEs are a distinct toxicity profile of lorlatinib when compared with other ALK-TKIs. Previous studies have reported that cognitive, mood, psychotic, and speech effects are the most common CNS AEs of lorlatinib. The CNS AEs were mainly reported within the first 2 months of lorlatinib administration, and dose modification is useful for alleviating CNS AEs. The patient’s life, both physically and mentally, will always be affected by CNS AEs, even grade 1 to 2. The frequency of CNS AEs in the Phase 3 CROWN study was 35%, while the frequency of second- or later-line AEs was 49% in B7461001, 53% in NCT01970865, and 60% in the MGH cohort.26,27 Previous studies have reported that the frequency of these adverse events was 39.7%, and 11.9% of patients experienced more than one type of CNS AE.19 Shaw et al28 also reported that CNS AEs occurred in 58 (39%) of 149 patients who received lorlatinib, and 34% were of grade 1–2 severity. The incidence rate and time were significantly different among CNS AEs of lorlatinib. The incidence rate of cognitive AEs was 23.1%, and the median time to onset was 53 days (range 1–423 days). The incidence rate of mood disorders was 21%, and the median time to onset was 43 days (range 1–452 days). The incidence rate of cognitive AEs was 9.5%, and the median time to onset was 42 days (range 1–404 days). Interestingly, previous studies have reported that the frequency of CNS AEs is significantly lower in the Asian population. Lu et al21 reported that the frequency of patients with CNS AEs (cluster terms of mood effects, cognitive effects, psychotic effects, and speech effects) was lower overall (6.4%) in the Chinese population when compared with the results of the global phase 2 study. Subgroup analysis from the global phase 2 trial NCT01970865 showed that cognitive effects and mood effects were lower in the Asian population.20
The management of lorlatinib-induced CNS AEs mainly depends on the grade of the adverse event. Dose interruptions should be considered in the presence of grade 1 CNS effects, and the same or lower dose of lorlatinib is considerable upon recovery to baseline. For grade 2 or 3 CNS AEs, a temporary discontinuation is needed until symptoms resolve to grade≤1, with resumption at a reduced dose. Severe events (grade 4) should be managed by permanent discontinuation of lorlatinib.19 For patients rechallenged with the same or lower dose of lorlatinib, the frequency of CNS AEs was 21.2% and 12.1% respectively. The severity of cognitive effects and mood effects were generally grade 1 or 2, with 6.5% of cases grade 3 and no grade 4 events reported. It is worth noting that the identification of lorlatinib-induced CNS AEs is important for the proper use of lorlatinib. Previous studies have reported that brain metastasis, CNS radiation, preexisting psychiatric illness, and certain neurotropic medications are risk factors for CNS AEs.29 Moreover, a mental baseline state assessment is important before initiating lorlatinib treatment, and mood and cognition should be monitored during treatment.
Peripheral Neuropathy
Peripheral neuropathy is one of the most common AEs associated with lorlatinib, and the frequency of these AEs was 47% in a previous phase 1/2 study, with a median time to onset of 77 days (range, 1–723).18,19 Within the cluster term, the peripheral neuropathy adverse events seen with lorlatinib included paresthesia, peripheral neuropathy, peripheral sensory neuropathy and muscular weakness. Tingling, numbness, and pain at night in the extremities were the main symptoms of peripheral neuropathy. The severity of peripheral neuropathy adverse events was mild for most patients, and dose modifications or interruption is useful for rescue.
The management of peripheral neuropathy is mainly based on its severity. For grade 1 and 2 events, reducing the dose of lorlatinib is the best choice. For grade ≥3 peripheral neuropathy, treatment should be temporarily discontinued and enforced until reduced to grade ≤2 or returned to baseline. In addition, a reduced lorlatinib dose is needed for rechallenged patients. For patients with severe symptoms, some medical therapy is needed. Vitamin B1 and vitamin B6 and medications for pain associated with peripheral neuropathy are useful for relieving symptoms. For carpal tunnel syndrome, the use of a night splint has been found to provide improvement in some patients. It is pivotal for monitoring the symptoms of peripheral neuropathy at baseline and follow-up consultations.
Edema
Edema is a common adverse event of ALK-TKIs, especially for lorlatinib. Previous studies have reported that the frequency of edema was 51.2%, with a median time to onset of edema of 42 days (range, 1–232).16 Edema-induced dose interruptions (5.8%) and reductions (6.1%) were most common in clinical practice. Solomon et al15 reported that the frequency of edema was lower in the Chinese population when compared with the lorlatinib phase 2 global study (26.6% vs 43%). 48.8% of patients experienced grade ≤2 adverse events, which indicated that most adverse events were mild in severity.
Compression stockings, leg elevations, increased exercise, and reduced salt intake were recommended for mild-to-moderate edema. Diuretics are also necessary for some conditions, such as signs of pulmonary edema or disruption to quality of life. For some instances, if edema persists or worsens, a reduction or interruption in lorlatinib dose is needed until resolution or return to baseline. Rechallenge should be initiated at a reduced dose.19
Bodyweight Gain
As a common adverse event, 24% of patients experienced weight gain after administration of lorlatinib. Fortunately, most body weight gain was grade 1 or 2 in severity,19 and these adverse event-induced dose interruptions or reductions were not common in clinical practice. The appearance of body weight gain typically occurred within 2 months of initiation of lorlatinib, with a median time to onset of 64 days (range 1–519 days). The administration of lorlatinib is always accompanied by increasing appetite, and body weight gain may be linked to increased food intake. The management of bodyweight increase is easy, and mainly focuses on implementing weight reduction activities such as diet and moderate exercise. Lifestyle modifications are preferred over lorlatinib dose reductions.
Heart Toxicity
With the wide use of TKIs, lorlatinib-induced heart toxicity, such as QTc prolongation and atrioventricular blocks, has attracted increasing attention. A previous study enrolled healthy volunteers who were administered lorlatinib, and the results showed that PR prolongation was observed for some cases but without atrioventricular blocks. For lorlatinib in 295 advanced NSCLC patients, the frequencies of grade 1 and grade 3 atrioventricular blocks were 0.7% and 0.3% respectively. The incidence of QTc prolongation was higher (6.4%) when compared with atrioventricular blocks, most of which were grade 2 or less, and temporary interruption was also observed for one patient with grade 3 QTc prolongation.30 Hence, an electrocardiogram evaluation before initiating lorlatinib therapy is meaningful. It is also suggested that electrocardiogram evaluation should be repeated every four months during treatment or whenever justified by heart toxicity symptoms.
Other AEs
Except for the abovementioned common AEs, other uncommon AEs should also be noted in clinical practice. Similar to other ALK-TKIs, interstitial lung disease (ILD) or pneumonitis was also observed for lorlatinib in advanced NSCLC, and the incidence of any grade ILD was 0.6%. Like hypercholesterolemia and hypertriglyceridemia, hyperglycemia was another metabolic phenotype adverse event of lorlatinib. Bauer et al19 reported that the frequency of lorlatinib-induced hyperglycemia was 2.2% in a phase 2 study: four out of 275 patients were grade 1–2 (1.5%), and two out of 275 patients were in grade 3 (0.7%). In addition, lorlatinib induced rare AEs, such as proteinuria, visual and auditory hallucinations, pulmonary arterial hypertension and renal impairment. The reported adverse events related to lorlatinib treatment are summarized in Table 1.
 |
Table 1 The Reported Adverse Events Related to Lorlatinib Treatment |
Drug-Drug Interactions
Lorlatinib is mainly metabolized by cytochrome P450 (CYP)3A4 in the liver; to a smaller extent, CYP2C8, CYP2C19, CYP3A5, and lorlatinib are moderate inducers of CYP3A at steady state. Hence, drug-drug interactions with lorlatinib should be considered when concomitant administration of supportive therapy. Lorlatinib plasma concentrations may be significantly affected by concomitant administration with strong CYP3A4/5 inducers or inhibitors. Hence, these concomitant administrations are contraindicated or avoided. When a strong CYP3A4/5 inhibitor or inducer must be prescribed, the lorlatinib dosage should be moderated according to the guidelines, preferably based on the plasma concentration. Li et al have reported that lorlatinib could be coadministered with moderate CYP3A inducers, such as modafinil.31 Moreover, lorlatinib is a moderate CYP3A4/5 inducer, and concomitant CYP3A4/5 substrates should be noted. As an oral dosage, lorlatinib exposure is associated with the administration, distribution, metabolism and excretion. Besides the interaction of metabolism enzymes, the effect of transporters such as P-glycoprotein should also be considered, as lorlatinib is a moderate inducer of P-glycoprotein. Previous studies have reported that no dose adjustments are recommended for patients with mild hepatic impairment or mild-to-moderate renal impairment.32 In addition to drug-drug interaction, drug-food interactions are also pivotal for oral dosage. Xu et al reported that lorlatinib can be administered regardless of food intake.33
Acquired Resistance to Lorlatinib
Although it has significant clinical activity, acquired resistance to lorlatinib inevitably occurs. The mechanism of acquired resistance has been widely reported, often due to the development of compound ALK mutations and bypass signaling and changes in cell types.6,34 Yoda et al35 reported that ALK C1156Y/L1198F and G1202R/L1196M were the most common compound mutations for lorlatinib resistance. In addition, ALK I1171S/G1269A, L1196M/D1203N, G1202R/F1174L, G1202R/L1196M and D1203N/I1171N mutations are other potential resistance mechanism that have been reported.36,37 Bypass signaling and changes in cell types are other mechanism of off-target resistance to lorlatinib. Previous studies37,38 have reported that the activation of EGFR, MET, KIT, KRAS or AXL is the most common bypass signaling pathway, and transformations to small cells and epithelial-mesenchymal transition were also reported in previous studies. As the risk for drug toxicities would be increased by increasing lorlatinib dosing, hence, dose expansion is not recommended to overcome lorlatinib-induced acquired resistance. Previous studies39,40 have reported that two fourth-generation ALK-TKIs (TPX-0131 and NVL-655) exhibited good inhibition of acquired double “compound” ALK mutations in preclinical studies, and Phase I–II studies are ongoing.
Conclusion
As an oral, reversible, highly selective third-generation ALK TKI, lorlatinib is an effective therapy method for ALK-positive NSCLC. Different from other ALK-TKIs, lorlatinib is effective for those resistance mutations acquired from the first- and second-generation ALK TKIs, and its strong intracranial activity is also noteworthy. Most lorlatinib-induced adverse reactions are similar to those of other ALK-TKIs, and lorlatinib induced AEs are also generally well tolerated. However, lorlatinib-induced particular toxicity profiles, such as hypercholesterolemia, and hypertriglyceridemia are worth noting, as these AEs may be rather challenging for cancer-treating. Furthermore, drug interactions should also be considered when concurrently administrating of lorlatinib and other supportive therapies.
Funding
This study was supported by Hunan Pharmaceutical Association Foundation (No: 2020YXH001), Hunan Provincial Natural Science Foundation of China (No: 2021JJ70025), and Climbing Plan of Hunan Cancer Hospital (No: 2021NSFC-A003).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5(3):288. doi:10.21037/tlcr.2016.06.07
4. Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol. 2013;31(8):992–1001. doi:10.1200/JCO.2012.46.9270
5. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. doi:10.1038/nature25183
6. Yun KM, Bazhenova LA. Update on lorlatinib: role in reducing the risk of disease progression in ALK-positive NSCLC. Cancer Manag Res. 2022;843–850. doi:10.2147/CMAR.S283199
7. Britschgi C, Addeo A, Rechsteiner M, et al. Real-world treatment patterns and survival outcome in advanced anaplastic lymphoma kinase (ALK) rearranged non-small-cell lung cancer patients. Front Oncol. 2020;10:1299. doi:10.3389/fonc.2020.01299
8. Golding B, Luu A, Jones R, Viloria-Petit AM. The function and therapeutic targeting of anaplastic lymphoma kinase (ALK) in non-small cell lung cancer (NSCLC). Mol Cancer. 2018;17(1):1–15. doi:10.1186/s12943-018-0810-4
9. El Darsa H, Abdel-Rahman O, Sangha R. Pharmacological and clinical properties of lorlatinib in the treatment of ALK-rearranged advanced non-small cell lung cancer. Expert Opin Pharmacother. 2020;21(13):1547–1554. doi:10.1080/14656566.2020.1774552
10. Choo JR-E, Soo RA. Lorlatinib for the treatment of ALK-positive metastatic non-small cell lung cancer. Expert Rev Anticancer Ther. 2020;20(4):233–240. doi:10.1080/14737140.2020.1744438
11. Johnson TW, Richardson PF, Bailey S, et al. Discovery of (10 R)-7-Amino-12-fluoro-2, 10, 16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8, 4-(metheno) pyrazolo [4,3-h][2, 5, 11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57(11):4720–4744. doi:10.1021/jm500261q
12. Page S, Milner-Watts C, Perna M, et al. Systemic treatment of brain metastases in non-small cell lung cancer. Eur J Cancer. 2020;132:187–198. doi:10.1016/j.ejca.2020.03.006
13. Syed YY. Lorlatinib: first global approval. Drugs. 2019;79:93–98. doi:10.1007/s40265-018-1041-0
14. Shaw AT, Bauer TM, de Marinis F, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018–2029. doi:10.1056/NEJMoa2027187
15. Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19(12):1654–1667. doi:10.1016/S1470-2045(18)30649-1
16. Nagasaka M, Ge Y, Sukari A, Kukreja G, Ou S-HI. A user’s guide to lorlatinib. Crit Rev Oncol Hematol. 2020;151:102969. doi:10.1016/j.critrevonc.2020.102969
17. Solomon BJ, Bauer TM, Mok TS, et al. Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir Med. 2023;11(4):354–366. doi:10.1016/S2213-2600(22)00437-4
18. Reed M, Rosales A-LS, Chioda MD, Parker L, Devgan G, Kettle J. Consensus recommendations for management and counseling of adverse events associated with lorlatinib: a guide for healthcare practitioners. Adv Ther. 2020;37:3019–3030. doi:10.1007/s12325-020-01365-3
19. Bauer TM, Felip E, Solomon BJ, et al. Clinical management of adverse events associated with lorlatinib. Oncologist. 2019;24(8):1103–1110. doi:10.1634/theoncologist.2018-0380
20. Soo RA, Tan EH, Hayashi H, et al. Efficacy and safety of lorlatinib in Asian and non-Asian patients with ALK-positive advanced non-small cell lung cancer: subgroup analysis of a global phase 2 trial. Lung Cancer. 2022;169:67–76. doi:10.1016/j.lungcan.2022.05.012
21. Lu S, Zhou Q, Liu X, et al. Lorlatinib for previously treated ALK-positive advanced NSCLC: primary efficacy and safety from a phase 2 study in People’s Republic of China. J Thorac Oncol. 2022;17(6):816–826. doi:10.1016/j.jtho.2022.02.014
22. Seto T, Hayashi H, Satouchi M, et al. Lorlatinib in previously treated anaplastic lymphoma kinase‐rearranged non–small cell lung cancer: Japanese subgroup analysis of a global study. Cancer Sci. 2020;111(10):3726–3738. doi:10.1111/cas.14576
23. Zhu VW, Lin Y-T, Kim D-W, et al. An international real-world analysis of the efficacy and safety of lorlatinib through early or expanded access programs in patients with tyrosine kinase inhibitor–refractory ALK-positive or ROS1-positive NSCLC. J Thorac Oncol. 2020;15(9):1484–1496. doi:10.1016/j.jtho.2020.04.019
24. Lee P-H, Chen K-C, Hsu K-H, et al. Real-world efficacy and safety of lorlatinib in treating advanced ALK-positive non–small cell lung cancer patients. Anticancer Drugs. 2021;32(10):1099–1104. doi:10.1097/CAD.0000000000001107
25. Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid‐lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80(6):565–581. doi:10.1016/j.clpt.2006.09.003
26. Solomon BJ, Bauer TM, Ignatius Ou S-H, et al. Post hoc analysis of lorlatinib intracranial efficacy and safety in patients with ALK-positive advanced non–small-cell lung cancer from the Phase III CROWN Study. J Clin Oncol. 2022;40(31):3593–3602. doi:10.1200/JCO.21.02278
27. Dagogo-Jack I, Abbattista A, Murphy JF, et al. Factors associated with developing neurocognitive adverse events in patients receiving lorlatinib after progression on other targeted therapies. J Thorac Oncol. 2023;18(1):67–78. doi:10.1016/j.jtho.2022.09.219
28. Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18(12):1590–1599. doi:10.1016/S1470-2045(17)30680-0
29. Jia K, Ren S. Neurocognitive adverse events of lorlatinib: on the way to precise prediction? J Thorac Oncol. 2023;18(1):26–28. doi:10.1016/j.jtho.2022.11.003
30. Topol EJ, Califf RM. Textbook of Cardiovascular Medicine. Lippincott Williams & Wilkins; 2007.
31. Li J, Pithavala YK, Gong J, LaBadie RR, Mfopou JK, Chen J. The effect of modafinil on the safety and pharmacokinetics of Lorlatinib: a Phase I study in healthy participants. Clin Pharmacokinet. 2021;60:1303–1312. doi:10.1007/s40262-021-01026-w
32. Xtandi E. EPAR—Product Information. European Medicines Agency; 2018.
33. Xu H, O’Gorman MT, Nepal S, James LP, Ginman K, Pithavala YK. Phase 1 Study evaluating the effects of the proton pump inhibitor rabeprazole and food on the pharmacokinetics of lorlatinib in healthy participants. Clin Pharmacol Drug Dev. 2021;10(11):1395–1404. doi:10.1002/cpdd.1000
34. Riudavets M, Planchard D. An update on lorlatinib: a novel first line treatment for ALK-positive advanced lung cancer. Expert Opin Pharmacother. 2023;24(3):291–299. doi:10.1080/14656566.2022.2161880
35. Yoda S, Lin JJ, Lawrence MS, et al. Sequential ALK inhibitors can select for lorlatinib-resistant compound ALK mutations in ALK-positive lung cancer. Cancer Discov. 2018;8(6):714–729. doi:10.1158/2159-8290.CD-17-1256
36. Dagogo-Jack I, Rooney M, Lin JJ, et al. Treatment with next-generation ALK inhibitors fuels plasma ALK mutation diversity. Clin Cancer Res. 2019;25(22):6662–6670. doi:10.1158/1078-0432.CCR-19-1436
37. Recondo G, Mezquita L, Facchinetti F, et al. Diverse resistance mechanisms to the third-generation ALK inhibitor lorlatinib in ALK-rearranged lung cancer. Clin Cancer Res. 2020;26(1):242–255. doi:10.1158/1078-0432.CCR-19-1104
38. Mizuta H, Okada K, Araki M, et al. Gilteritinib overcomes lorlatinib resistance in ALK-rearranged cancer. Nat Commun. 2021;12(1):1261. doi:10.1038/s41467-021-21396-w
39. Murray BW, Zhai D, Deng W, et al. TPX-0131, a potent CNS-penetrant, next-generation inhibitor of wild-type ALK and ALK-resistant mutations. Mol Cancer Ther. 2021;20(9):1499–1507. doi:10.1158/1535-7163.MCT-21-0221
40. Pelish HE, Tangpeerachaikul A, Kohl NE, Porter JR, Shair MD, Horan JC. Abstract 1468: NUV-655 (NVL-655) is a selective, brain-penetrant ALK inhibitor with antitumor activity against the lorlatinib-resistant G1202R/L1196M compound mutation. Cancer Res. 2021;81(13_Supplement):1468. doi:10.1158/1538-7445.AM2021-1468
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
