Back to Journals » Pragmatic and Observational Research » Volume 11
Real-World Safety and Effectiveness of Tadalafil in Patients with Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: A Japanese Post-Marketing Surveillance Study
Authors Yamazaki H, Tsujimoto N, Koyanagi M, Katoh MC , Tajima K, Komori M
Received 18 November 2019
Accepted for publication 13 March 2020
Published 4 May 2020 Volume 2020:11 Pages 45—54
DOI https://doi.org/10.2147/POR.S237821
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor David Price
Hiroyoshi Yamazaki,1 Naoto Tsujimoto,1 Momoha Koyanagi,1 Megumi C Katoh,1 Koyuki Tajima,2 Mika Komori1
1Medicines Development Unit Japan and Medical Affairs, Eli Lilly Japan K.K., Kobe, Japan; 2Post Marketing Surveillance Clinical Research Department, Nippon Shinyaku Co., Ltd., Kyoto, Japan
Correspondence: Hiroyoshi Yamazaki
Medicines Development Unit Japan and Medical Affairs, Eli Lilly Japan K.K., Lilly Plaza One Bldg., 5-1-28, Isogamidori, Chuo-ku, Kobe 651-0086, Japan
Tel +81 78 242 4391
Fax +81 78 242 9299
Email [email protected]
Objective: To evaluate the long-term safety and effectiveness of tadalafil in Japanese men with lower urinary tract symptoms secondary to benign prostatic hyperplasia in real-world clinical practice; and to investigate the safety profile in patients aged ≥ 75 years.
Patients and Methods: This was a prospective, non-interventional, multicenter, post-marketing surveillance study in which Japanese patients with lower urinary tract symptoms secondary to benign prostatic hyperplasia were observed for up to 18 months after initiating tadalafil treatment. The real-world safety and effectiveness outcomes were assessed at baseline and at 1, 3, 6, 12, and 18 months post-treatment or the last day of treatment.
Results: Most patients received tadalafil 5 mg per day throughout the observation period. Among 1393 patients analyzed for safety, the overall incidence of adverse drug reactions was 8.3%. These adverse drug reactions were generally consistent with the known safety profile of tadalafil and no new safety risks were identified in long-term use. There was no statistical difference in the frequency of adverse drug reactions between patients aged < 75 and ≥ 75 years. The mean change in total International Prostate Symptom Score (IPSS) and IPSS-quality of life subscore was significantly improved at each timepoint. At 18 months, IPSS had improved by 5.0 points (P < 0.001) and IPSS-quality of life subscore had improved by 1.5 points (P < 0.001). The mean change in post-voiding residual urine volume from baseline was significant at each time point and was − 9.8 mL at 18 months (P < 0.001); there were no significant differences from baseline in maximum urinary flow rate.
Conclusion: This surveillance demonstrated that tadalafil has favorable safety and effectiveness profiles for long-term use in Japanese men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. In addition, safety profiles in patients aged ≥ 75 years were similar to patients aged < 75 years.
Keywords: elderly patient, long-term, observational study, phosphodiesterase type 5 inhibitor
Introduction
Benign prostatic hyperplasia (BPH) is a common urinary condition in aging men, which is characterized by an enlarged prostate gland. This often leads to lower urinary tract symptoms (LUTS), such as increased daytime frequency, nocturia, urinary urgency, and voiding difficulties.1–3 Although LUTS secondary to BPH (BPH/LUTS) is not often life-threatening, it can be bothersome and negatively affect patient quality of life (QOL) by interfering with daily activities and sleep.4 The prevalence of BPH increases after the age of 40, with a prevalence rate of 73% in men aged ≥70.5 In 2017, over 473,000 men were estimated to suffer from BPH in Japan,5 a number that is expected to increase as the elderly population grows.
Until recently, alpha-adrenoreceptor antagonists (alpha-blockers) and 5-alpha reductase inhibitors (5-ARIs) were considered a first-line medical treatment in men with moderate to severe BPH/LUTS. However, these treatments are not effective in all patients and are associated with adverse sexual outcomes (alpha-blockers and 5-ARIs), orthostatic hypotension (alpha-blockers), and fatigue (alpha-blockers), which all frequently lead to discontinuation of treatment. Tadalafil, a selective phosphodiesterase type 5 inhibitor (PDE-5i), was first approved as an alternative treatment for BPH/LUTS in October 2011 in the United States and was subsequently approved in numerous countries including Japan.1,6,7 Recently, tadalafil has been accepted by the Japanese Urological Association as a first-line treatment for BPH/LUTS.1 Previous randomized, placebo-controlled clinical studies investigating 12-week tadalafil treatment in Asians8–12 and Caucasians13,14 have shown that the mean change from baseline in total International Prostate Symptom Score (IPSS) is significantly greater in patients treated with tadalafil compared with placebo. Furthermore, tadalafil was rarely linked to sexual dysfunction or orthostatic blood pressure adverse events that have been previously reported with other BPH treatments.6,7 While these previous studies show potential for tadalafil as a treatment for BPH/LUTS, few studies have been conducted with large sample sizes, and there is still a lack of information on the long-term use and safety of tadalafil among elderly patients in real-world clinical settings. Although >50% of patients with BPH in Japan are aged ≥75 years,5 this age group is under-represented in clinical trials (~10% of subjects) and no real-world data are available for this age group.15
Here, we report the results of a post-marketing surveillance study aimed to evaluate the safety and effectiveness of tadalafil among Japanese men with BPH/LUTS in real-world clinical practice. Because age-related physiologic changes may affect drug safety, we also targeted patients aged ≥75 years and observed differences and similarities to those aged <75 years to further clarify the safety profile of tadalafil. Hence, our results provide insights not only into the long-term safety and effectiveness of this treatment but also into the safety profile of tadalafil in patients ≥75 years.
Patients and Methods
Study Design and Patients
This was a prospective, non-interventional, multicenter, post-marketing surveillance study of Japanese men treated with tadalafil for BPH/LUTS conducted from September 2014 to March 2017 (enrollment from September 2014 to September 2015). The observation period was a maximum of 18 months’ follow-up, or up to the last day of treatment (if patients discontinued treatment before 18 months).
This study was conducted in accordance with the standards of Good Post-marketing Study Practice for Drugs (Ordinance No. 171, issued 20 December 2004, the Japanese Ministry of Health, Labour and Welfare). The study protocol adhered to applicable local and country-specific laws and regulations pertaining to the protection of patient privacy and safety. In accordance with these laws and regulations, this study did not obtain written informed consent from enrolled patients and was exempt from World Health Organization registration criteria.
All sites that purchased tadalafil and were equipped with a web-based system to collect clinical data were invited to participate and a written contract was provided only after the sites agreed to cooperate in this surveillance.
Patients eligible to enroll in the study were those diagnosed with BPH/LUTS by each principal investigator and who had no history of tadalafil use. Patients were excluded if they had contraindications to tadalafil. Dosage of tadalafil, concomitant medication, and treatment for adverse reactions were based on the investigators’ discretion. Data were reported in a case report form (CRF) by each investigator and missing laboratory effectiveness data were imputed using the Last Observation Carried Forward (LOCF) approach.
Safety and Effectiveness
Persistence of treatment was estimated by the Kaplan–Meier method. Assessments were conducted at baseline and at each time point during the observation period. Safety data were collected continuously and reported as the frequency of adverse drug reactions (ADRs), which were classified using the Medical Dictionary for Regulatory Activities (MedDRA, version 20.0) preferred terms, and were defined as events for which a causal relation to tadalafil could not be ruled out by investigators. Effectiveness outcomes were assessed at 1 (only for IPSS), 3, 6, 12, and 18 months following treatment initiation. Effectiveness outcomes included the change from baseline at each time point in IPSS,16 IPSS-quality of life (IPSS-QOL), post-voiding residual urine volume (PVR), and maximum urinary flow rate (Qmax).
Statistical Analysis
Since this was a noncomparative observational study, statistical analysis was not used for hypothesis testing. Data are reported mainly using estimated means, incidence proportion, mean change from baseline, and 95% confidence intervals (95% CI). Differences between groups were conducted using a one-sample t-test, fisher exact test, or chi-square test to support the evaluations. All analyses were performed using SAS version 9.2 or above (SAS Institute, Cary, NC, USA).
Results
Patient Disposition
A total of 1449 CRFs were collected from 1499 patients who registered for enrollment (Figure 1). The safety analysis set included 1393 patients, of whom 1387 were included in the effectiveness analysis set (Figure 1). For the safety analysis set, 56 patients were excluded mainly due to “no-show post-enrollment”, while an additional 6 patients were excluded from the effectiveness analysis set due to “unsigned CRF for effectiveness”.
 |
Figure 1 Patient population for the safety and effectiveness analysis. Abbreviations: CRF, case report form; n, number of patients. |
Patient Demographics and Clinical Characteristics
The mean age ± standard deviation (SD) of BPH/LUTS patients was 69.3 ± 8.8 years with 70.1% (n = 976/1393) aged <75 and 29.9% (n = 417/1393) aged ≥75 years (Table 1). More than half the patients (58.7%, n = 817/1393) had BPH/LUTS for ≥1 year and 9.4% (n = 131/1393), 48.4% (n = 674/1393), and 25.2% (n = 351/1393) experienced mild (total IPSS ≤7), moderate (total IPSS 8−19), and severe (total IPSS 20−35) symptoms, respectively (Table 1). Among the safety analysis set, 45.3% (n = 631/1393) of patients reported some kind of comorbidity including hypertension (55.8%, n = 352/631), diabetes (18.1%, n = 114/631), hypertonic bladder (11.9%, n = 75/631), hyperuricemia (9.4%, n = 59/631), and hyperlipidemia (9.2%, n = 58/631) (Table 2). In addition, 1.1% (n = 16/1393) and 0.6% (n = 9/1393) of patients had complications of liver or kidney function, respectively (data not shown).
 |
Table 1 Baseline Demographics and Patient Characteristics |
 |
Table 2 Patient’s Medical Background |
Of the 42.6% (n = 593/1393) of patients who received medication for BPH/LUTS treatment before the study (ie, previous medication), alpha-blockers (86.2%, n = 511/593) and/or 5-ARIs (18.0%, n = 107/593) were most often prescribed (Table 2). Notably, these two drugs were also the most frequently used concomitant medications throughout the observation period; 61.2% (n = 482/788) of patients used alpha-blockers and 18.0% (n = 142/788) used 5-ARIs (Table 2). In contrast, only 2.7% (n = 38/1393) of patients had a history of surgical treatment (ie, previous surgical treatment) and 1.1% (n = 16/1393) were on concurrent therapy during the observation period.
Tadalafil Treatment
Most patients received tadalafil 5 mg per day throughout the observation period: 94.5% of patients (n = 1316/1393) at the beginning of treatment and 94.6% (n = 1318/1393) on the final day of treatment, while 4.5% (n = 63/1393) received 2.5 mg, and 0.9% (n = 12/1393) received <2.5 mg at the final day of treatment, respectively (data not shown).
Among the safety analysis set, 48.2% (n = 672/1393) of patients discontinued tadalafil treatment during the 18-month observation period; 14.6% (n = 98/672) due to ADRs (Figure 2). The major ADRs (≥5 incidence) that resulted in discontinuation were headache (n = 17/98) and diarrhea (n = 5/98). Other major reasons for discontinuation were patient decision (not related to ADRs) (31.7%, n = 213/672), lack of efficacy (19.3%, n = 130/672), and lost to follow-up due to hospital transfer (18.8%, n = 126/672).
Safety
Adverse Drug Reactions
Of 1393 patients included in the safety analysis set, 115 reported at least 1 ADR (8.3%). The main ADRs reported (≥5 incidence) were headache (1.5%, n = 21/1393), dyspepsia, and spontaneous penile erection (each 0.5%, n = 7/1393), palpitations, diarrhea, pollakiuria, and blood pressure decreased (each 0.4%, n = 5/1393), all of which were not serious (Table 3).
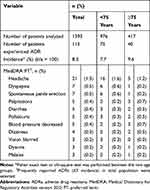 |
Table 3 Number and Frequency of Adverse Drug Reactions During Tadalafil Treatment |
The incidence of ADRs in patients aged <75 and ≥75 years was 7.7% (n = 75/976) and 9.6% (n = 40/417), respectively (Table 3). Reported ADRs (≥3 incidence) were headache (1.6%, n = 16/976), dyspepsia, and spontaneous penile erection (each 0.6%, n = 6/976), vision blurred, diarrhea, and pollakiuria (each 0.3%, n = 3/976) in patients <75 years; headache (1.2%, n = 5/417), palpitations, and blood pressure decreased (each 0.7%, n = 3/417) in patients ≥75 years (Table 3). There was no statistical difference in the frequency of ADRs between patients aged <75 and ≥75 years.
Two serious ADRs were reported: atrial fibrillation with complete right bundle branch block in a patient aged 81 years, and myocardial infarction in a patient aged 73 years, both of whom had preexisting cardiovascular risk factors.
The incidence of ADRs was 8.3% (n = 115/1393) during the whole observation period, 6.5% (n = 90/1393) during the first 3 months, and 0.4% (n = 3/817) after 12 months’ observation (Table 4). Notably, most ADRs appeared in the earlier period and few new ADRs were observed after 7 months (Table 4). Three ADRs were reported after the 12-month observation period: abdominal discomfort (n = 1), constipation (n = 1), and myocardial infarction (n = 1) (Table 4). Among these, only myocardial infarction was newly reported after 12 months (Table 4).
 |
Table 4 Long-Term Safety During Tadalafil Treatment |
Vital Signs
Because elderly patients are susceptible to adverse drug effects, particularly increased risk of hypotension and related symptoms,17 the influence of tadalafil on blood pressure reduction was carefully monitored during the observation period as part of the safety measurements. Systolic blood pressure before tadalafil treatment was 133.9 ± 16.3 (mean ± SD) mmHg (n = 681) and diastolic blood pressure was 78.1 ± 10.8 mmHg (n = 681). The mean change from baseline to 18 months (LOCF) was −2.1 mmHg (n = 587, P < 0.01) and −2.8 mmHg (n = 586, P < 0.001), respectively (Table 5). However, none of these changes were considered clinically meaningful or significant, and there was no major difference between patients aged <75 and ≥75 years (See Table S1 in the Supplementary material).
 |
Table 5 Mean Change from Baseline in Vital Signs During Tadalafil Treatment |
Concomitant Use of Alpha-Blockers and 5-ARIs
The percentage of patients taking ≥1 concomitant medication, including alpha-blockers and/or 5-ARIs, during the observation period was 56.6% (n = 788/1393) (Table 2). The incidence of ADRs in alpha-blocker users and nonusers was 8.7% (n = 42/482) and 8.0% (n = 73/909), respectively. Reported ADRs (≥3 incidence) in alpha-blocker users were headache (1.0%, n = 5/482), palpitations, spontaneous penile erection (each 0.8%, n = 4/482), and blood pressure decreased (0.6%, n = 3/482). Orthostatic hypotension was not reported and no serious ADRs were noted among alpha-blocker users (data not shown).
The incidence of ADRs among 5-ARI users and nonusers was 9.9% (n = 14/142) and 8.1% (n = 101/1249), respectively. There were fewer ADR reports of headache among 5-ARI users (0.7%, n = 1/142) than nonusers (1.6%, n = 20/1249). Pollakiuria was the only nonserious ADR with more than 1 report (1.4%, n = 2/142) in 5-ARI users. As reported above, for serious ADRs, only one incidence each for atrial fibrillation and myocardial infarction was reported.
Effectiveness
Maximum Urinary Flow Rate and Post-Voiding Residual Urine Volume
No difference from baseline in Qmax was observed during the observation period (Table 6). However, PVR showed a marked decrease from baseline at each visit, including at LOCF (Table 6). The mean change in PVR from baseline was −12.7 mL at 3 months (n = 484, P < 0.001), −8.0 mL at 6 months (n = 486, P < 0.01), −9.0 mL at 12 months (n = 409, P < 0.01), −9.8 mL at 18 months (n = 371, P < 0.001), and −7.6 mL at LOCF (n = 699, P < 0.01) (Table 6).
 |
Table 6 Effect of Tadalafil on Maximum Urinary Flow Rate and Post-Voiding Residual Urine Volume |
Total IPSS and IPSS-QOL
During the 18-month observation period, there was a significant decrease in total IPSS (Table 7). The mean change in total IPSS from baseline was −3.4 at 1 month (n = 818, P < 0.001), −4.5 at 3 months (n = 685, P < 0.001), −4.7 at 6 months (n = 663, P < 0.001), −4.7 at 12 months (n = 553, P < 0.001), −5.0 at 18 months (n = 467, P < 0.001), and −4.3 at LOCF (n = 988, P < 0.001) (Table 7). Similarly, the mean change in IPSS-QOL score from baseline improved to −0.9 at 1 month (n = 815, P < 0.001), −1.2 at 3 months (n = 686, P < 0.001), −1.4 at 6 months (n = 664, P < 0.001), −1.5 at 12 months (n = 554, P < 0.001), −1.5 at 18 months (n = 465, P < 0.001), and −1.3 at LOCF (n = 989, P < 0.001) (Table 7).
 |
Table 7 Effect of Tadalafil on Total IPSS and IPSS-QOL |
Discussion
In this post-marketing surveillance study, we examined the real-world safety and effectiveness of tadalafil in Japanese men with BPH/LUTS. In general, ADRs were consistent with the known safety profile of tadalafil from previous clinical trials and no new safety risks were identified.11 Similarly, there were no additional safety concerns observed with long-term use (18 months) of tadalafil, which provides further reassurance that the safety profile of tadalafil is favorable (Table 4). Since most previous clinical trials have focused on 12 weeks of treatment8–14 or an open-label extension period of up to 42 weeks8 our observational findings provide extended insights into the long-term safety of tadalafil. Among the frequently reported ADRs (≥3 incidence), headache, dyspepsia, spontaneous penile erection, palpitations, diarrhea, pollakiuria, and blood pressure decreased occurred relatively early after tadalafil was administered (within 3 months) and decreased over time (Table 4). This could imply that ADRs associated with tadalafil in BPH/LUTS treatment are often seen at the beginning of treatment. Moreover, more than 50% of patients were able to continue tadalafil treatment for 18 months (Figure 2), which might have been influenced by the low incidence of ADRs and the fact that there were only few reports of serious ADRs (Table 3). However, we cannot conclude that tadalafil is safer or has more effect than other urinary function-improving drugs because the present study lacks concurrent control and comparator groups.
In patients aged ≥75 years, the frequency of ADRs was slightly higher than in those aged <75 years; however, there were no clinically relevant differences between the two age groups (Table 3). These results thus suggest that tadalafil is well tolerated not only for patients aged <75 years but also for those aged ≥75 years. In a meta-analysis report based on Phase 2 and Phase 3 clinical studies, tadalafil was well tolerated regardless of age, which supports our observational results in a real-world clinical setting.15
In terms of effectiveness, PVR decreased significantly after 3 months of treatment and the effects were sustained for a maximum of 18 months (Table 6). In contrast, Qmax showed no changes from baseline (Table 6). Similar outcomes were also observed in other 12-week clinical trials where Qmax remained largely unchanged during the study with no statistically significant or clinically meaningful difference between tadalafil and placebo.8–10 Interestingly, though, in a one-armed, open-label, prospective study, both PVR and Qmax significantly improved during 12 months of treatment.18 This could be because patients in this 12-month study were carefully selected to have a relatively high Bladder Outlet Obstruction index.18
In addition to PVR, other measures of effectiveness such as total IPSS and IPSS-QOL improved during the current study. Both total IPSS and IPSS-QOL scores decreased significantly after only 1 month of treatment and continued to improve with time (Table 7). These results are consistent with the 12-week findings from previous randomized, placebo-controlled clinical studies investigating tadalafil treatment in Asians8–12 and Caucasians13,14 with BPH/LUTS. A series of analyses integrating the data pooled from different subsets of these clinical trials consistently found that tadalafil progressively and significantly improved total IPSS scores over 12 weeks compared with placebo.15 Similar results were also observed in a Korean post-marketing surveillance study, which had a shorter investigation period from the present study, and was mainly investigating 12-week tadalafil treatment.19
Due to the observational nature of post-marketing surveillance studies, they can be prone to bias and findings cannot be attributed to causation. Notably, the present study lacked concurrent control and comparator groups, and the outcomes of tadalafil could only be determined based on the mean change from baseline. Additionally, the need to address missing data is frequently encountered in longitudinal studies where outcome data are collected repeatedly at several time points, and in observational studies, including those conducted in registries.
Conclusion
In conclusion, this post-marketing surveillance study has demonstrated that the safety and effectiveness profiles of tadalafil are favorable during long-term use in Japanese men with BPH/LUTS in real-world clinical practice, and that the characteristics of ADRs during long-term use in patients aged ≥75 years are similar to those aged <75 years.
Abbreviations
ADR, adverse drug reactions; alpha-blockers, alpha-adrenoreceptor antagonists; 5-ARIs, 5-alpha reductase inhibitors; BPH, benign prostatic hyperplasia; CI, confidence interval; CRF, case report form; IPSS, International Prostate Symptom Score; LUTS, lower urinary tract symptoms; PDE-5i, phosphodiesterase type 5 inhibitor; LOCF, last observation carried forward; QOL, quality of life; PVR, post-voiding residual urine volume; Qmax, maximum urinary flow rate; SD, standard deviation.
Acknowledgments
We would like to thank all patients, physicians, and institutions who participated in this post-marketing surveillance study.
Disclosure
HY, NT, MKoy, MCK, and MKom are current employees of Eli Lilly Japan K.K.; and KT is an employee of Nippon Shinyaku Co., Ltd. Both companies were involved in the preparation of the manuscript and sponsored the study. The authors report no other conflicts of interest in this work.
References
1. Homma Y, Gotoh M, Kawauchi A, et al. Clinical guidelines for male lower urinary tract symptoms and benign prostatic hyperplasia. Int J Urol. 2017;24(10):716–729. doi:10.1111/iju.13401
2. Gratzke C, Bachmann A, Descazeaud A, et al. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2015;67(6):1099–1109. doi:10.1016/j.eururo.2014.12.038
3. AUA Practice Guidelines Committee. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: diagnosis and treatment recommendations. J Urol. 2003;170(2):530–547. doi:10.1097/01.ju.0000078083.38675.79
4. Welch G, Weinger K, Barry MJ. Quality-of-life impact of lower urinary tract symptom severity: results from the health professionals follow-up study. Urology. 2002;59(2):245–250. doi:10.1016/S0090-4295(01)01506-0
5. Japanese Ministry of Health, Labour and Welfare. The results of the 2017 patient survey. Japanese government official statistics of Japan website. [Updated March 29, 2019]. Available from: https://www.e-stat.go.jp/dbview?sid=0003318621.
6. Gacci M, Andersson KE, Chapple C, et al. Latest evidence on the use of phosphodiesterase type 5 inhibitors for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol. 2016;70(1):124–133. doi:10.1016/j.eururo.2015.12.048
7. Yokoyama O, Igawa Y, Takeda M, Yamaguchi T, Murakami M, Viktrup L. Tadalafil for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a review of clinical data in Asian men and an update on the mechanism of action. Ther Adv Urol. 2015;7(5):249–264. doi:10.1177/1756287215589238
8. Takeda M, Nishizawa O, Imaoka T, Morisaki Y, Viktrup L. Tadalafil for the treatment of lower urinary tract symptoms in Japanese men with benign prostatic hyperplasia: results from a 12-week placebo-controlled dose-finding study with a 42-week open-label extension. Low Urin Tract Symptoms. 2012;4(3):110–119. doi:10.1111/j.1757-5672.2012.00144.x
9. Yokoyama O, Yoshida M, Kim SC, et al. Tadalafil once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a randomized placebo- and tamsulosin-controlled 12-week study in Asian men. Int J Urol. 2013;20(2):193–201. doi:10.1111/j.1442-2042.2012.03130.x
10. Takeda M, Yokoyama O, Lee SW, Murakami M, Morisaki Y, Viktrup L. Tadalafil 5 mg once-daily therapy for men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: results from a randomized, double-blind, placebo-controlled trial carried out in Japan and Korea. Int J Urol. 2014;21(7):670–675. doi:10.1111/iju.12410
11. Nishizawa O, Yoshida M, Takeda M, et al. Tadalafil 5 mg once daily for the treatment of Asian men with lower urinary tract symptoms secondary to benign prostatic hyperplasia: analyses of data pooled from three randomized, double-blind, placebo-controlled studies. Int J Urol. 2015;22(4):378–384. doi:10.1111/iju.12699
12. Zhang Z, Li H, Zhang X, et al. Efficacy and safety of tadalafil 5 mg once-daily in Asian men with both lower urinary tract symptoms associated with benign prostatic hyperplasia and erectile dysfunction: a phase 3, randomized, double-blind, parallel, placebo- and tamsulosin-controlled study. Int J Urol. 2019;26(2):192–200. doi:10.1111/iju.13828
13. Porst H, Kim ED, Casabé AR, et al. Efficacy and safety of tadalafil once daily in the treatment of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: results of an international randomized, double-blind, placebo-controlled trial. Eur Urol. 2011;60(5):1105–1113. doi:10.1016/j.eururo.2011.08.005
14. Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol. 2012;61(5):917–925. doi:10.1016/j.eururo.2012.01.013
15. Oelke M, Wagg A, Takita Y, Büttner H, Viktrup L. Efficacy and safety of tadalafil 5 mg once daily in the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia in men aged ≥75 years: integrated analyses of pooled data from multinational, randomized, placebo-controlled clinical studies. BJU Int. 2017;119(5):793–803. doi:10.1111/bju.13744
16. Homma Y, Tsukamoto T, Yasuda K, Ozono S, Yoshida M, Shinji M. Linguistic validation of Japanese version of international prostate symptom score and BPH impact index. Jpn J Urol. 2002;93(6):669–680. doi:10.5980/jpnjurol1989.93.669
17. Jansen PA, Brouwers JR. Clinical pharmacology in old persons. Scientifica. 2012;2012:723678. doi:10.6064/2012/723678
18. Matsukawa Y, Takai S, Majima T, et al. Objective impacts of tadalafil on storage and voiding function in male patients with benign prostatic hyperplasia: 1-year outcomes from a prospective urodynamic study. World J Urol. 2019;37(5):867–872. doi:10.1007/s00345-018-2453-x
19. Won JE, Chu JY, Choi HC, Chen Y, Park HJ, Dueñas HJ. Safety and effectiveness of once-daily tadalafil (5 mg) therapy in Korean men with benign prostatic hyperplasia/lower urinary tract symptoms in a real-world clinical setting: results from a post-marketing surveillance study. World J Mens Health. 2018;36(2):161–170. doi:10.5534/wjmh.17017
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

