Back to Journals » Journal of Asthma and Allergy » Volume 16
Real-World Safety and Effectiveness of Omalizumab in Moderate to Severe Allergic Asthma Patients in China: A Post-Authorization Study
Authors Su N, Zhi L, Liu F, Wang Y, Zhang Q, Liu X , Wang X, Hao G, Zhang X, Hu Q , Ligueros-Saylan M, Uddin A, Yang J, Liang T, Ding L, Li R, Wang C
Received 24 February 2023
Accepted for publication 2 June 2023
Published 19 June 2023 Volume 2023:16 Pages 625—636
DOI https://doi.org/10.2147/JAA.S406628
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Nan Su,1,2 Lili Zhi,3 Fengxia Liu,4 Yongsheng Wang,5 Qingling Zhang,6 Xiansheng Liu,7 Xueyan Wang,8 Guodong Hao,9 Xiuqin Zhang,10 Qiang Hu,11 Monica Ligueros-Saylan,12 Alkaz Uddin,13 Jing Yang,14 Tiantian Liang,15 Liju Ding,16 Runqin Li,17 Chen Wang1,2,18– 21
1Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, China- Japan Friendship Hospital, Beijing, People’s Republic of China; 2National Center of Respiratory Medicine, Beijing, People’s Republic of China; 3Department of Allergy, Shandong Provincial Qianfoshan Hospital, The First Affiliated Hospital of Shandong First Medical University, Shandong Institute of Respiratory Diseases, Jinan, People’s Republic of China; 4Department of Allergy, Weifang People’s Hospital, Weifang, Shandong, People’s Republic of China; 5Department of Pulmonary and Critical Care Medicine, Chengdu First People’s Hospital, Chengdu, People’s Republic of China; 6Department of Pulmonary and Critical Care Medicine, Guangzhou Institute of Respiratory Health, National Clinical Research Center for Respiratory Disease, National Center for Respiratory Medicine, State Key Laboratory of Respiratory Diseases, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, People’s Republic of China; 7Department of Pulmonary and Critical Care Medicine, Shanxi Bethune Hospital, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, Shanxi Province, People’s Republic of China; 8Allergy Center, Beijing Shijitan Hospital, Capital Medical University, Beijing, People’s Republic of China; 9Department of Allergy, Tangshan Gongren Hospital, Tangshan, Hebei, People’s Republic of China; 10Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Suzhou University, Suzhou, Jiangsu, People’s Republic of China; 11Department of Pulmonary and Critical Care Medicine, Panzhihua Integrated Chinese and Western Medicine Hospital, Panzhihua, Sichuan, People’s Republic of China; 12Respiratory Clinical/Medical 2, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA; 13Analytics - Global Health DU, Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA; 14Neuroscience & Respiratory Development Unit, Beijing Novartis Pharma. Co. Ltd, Beijing, People’s Republic of China; 15Respiratory Clinical Development, China Novartis Institutes for BioMedical Research Co., Ltd, Beijing, People’s Republic of China; 16GCTM-Group-2, China Novartis Institutes for BioMedical Research Co., Ltd, Shanghai, People’s Republic of China; 17New Products, Beijing Novartis Pharma. Co. Ltd, Shanghai, People’s Republic of China; 18National Clinical Research Center for Respiratory Diseases, Beijing, People’s Republic of China; 19Institute of Respiratory Medicine, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 20Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 21Department of Respiratory Medicine, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Chen Wang, China- Japan Friendship Hospital, No. 2, East Yinghua Road, Chaoyang District, Beijing, 100029, People’s Republic of China, Tel +86 10-6510 5565, Fax +86 10-6510 5567, Email [email protected]
Purpose: Omalizumab was first approved in China in 2017 for the treatment of moderate to severe allergic asthma for adult and adolescent patients aged ≥ 12 years. In accordance with the Chinese Health Authority requirement, the post-authorization safety study (PASS) was conducted to evaluate the safety and effectiveness of omalizumab in a real-world setting in patients with moderate to severe allergic asthma in China over a 24-week observation period.
Patients and Methods: This is a single-arm, non-interventional, multicenter, PASS conducted in adult, adolescent, and pediatric patients (≥ 6 years old) with moderate to severe allergic asthma receiving omalizumab in a real-world clinical setting from 2020 to 2021 in 59 sites of mainland China.
Results: In total, 1546 patients were screened and 1528 were enrolled. They were stratified according to age (6 to < 12 years [n = 191]; ≥ 12 years [n = 1336]; unknown [n = 1]). Among the overall population, 23.6% and 4.5% of patients reported adverse events (AEs) and serious adverse events (SAEs), respectively. Among pediatric patients (6 to < 12 years), 14.1% and 1.6% patients reported AEs and SAEs, respectively. AEs that led to treatment discontinuation in both age groups were < 2%. No new safety signals were reported. Effectiveness results showed improvement in lung function, asthma control, and quality of life (QoL).
Conclusion: The findings of the current study demonstrated that the safety profile of omalizumab was consistent with its known profile in allergic asthma, and no new safety signals were reported. Omalizumab treatment was effective in improving the lung function and QoL in patients with allergic asthma.
Keywords: adverse drug events, allergic asthma, safety, effectiveness, omalizumab
Introduction
Asthma is one of the most common respiratory diseases, characterized by chronic airway inflammation and reversible lower airway obstruction.1 The respiratory symptoms of asthma include cough, chest tightness, wheezing, and shortness of breath.2 Globally, asthma affects 1–18% of the population and is largely associated with socioeconomic implications. Approximately 3–10% of patients with asthma have severe asthma.3 In China, asthma affects 4.2% (45.7 million) of the adult population and 3% of children under 14 years.4,5 Patients with severe asthma had 4.8-fold higher annual treatment cost and 6.7-fold higher hospitalization rate than their mild to moderate counterparts in China.6
Allergic asthma, the most common asthma phenotype, is defined by the presence of sensitization to environmental allergens.7 Nearly two-thirds of asthma patients are considered to have allergic asthma.8 Allergic asthma patients have an earlier age of onset than their non-allergic counterparts, and allergic asthma is more common in male patients.8 In China, the prevalence of allergic asthma ranged between 1.54% and 2.32% during 2000–2010.9
In allergic asthma, immunoglobulin E (IgE) plays a central role in inflammation by binding to FcεRI receptors on mast cells or FcεRII receptors on B lymphocytes and eosinophils to release inflammatory mediators.1,10 Omalizumab is a humanized monoclonal anti-IgE antibody that binds to the Fc region of free IgE. It was first approved in Australia in 2002 for the treatment of adults and adolescents with moderate allergic asthma and in the US for moderate-to-severe allergic asthma in 2003. The biological agent was added to the 2014 Global Initiative for Asthma (GINA) management report for use in patients with uncontrolled allergic asthma. Based on the established efficacy and effectiveness of omalizumab, it has also been approved for the treatment of chronic rhinosinusitis with nasal polyposis and chronic spontaneous urticaria (and allergic rhinitis in Japan and Russia) with an acceptable safety profile and >1.86 million patient-years of exposure.2,11–13
Omalizumab was approved in China in 2017 for the treatment of adult and adolescent (≥12 years of age) patients with moderate to severe persistent IgE-mediated asthma following the completion of a Phase 3 study of omalizumab in Chinese patients.14 Persistent IgE-mediated asthma is defined as asthma that is uncontrolled despite the use of optimal therapy with inhaled corticosteroid (ICS) and long-acting β2 agonists (LABAs).15 In 2018, the Chinese Health Authority (HA) approved to expand the patient population to children with asthma aged 6 to <12 years on the basis of its safety, efficacy, and effectiveness in adult and adolescent patients with moderate to severe allergic asthma in China. The clinical efficacy and effectiveness and safety of omalizumab in allergic asthma have been shown in several clinical trials14 and real-life studies.
In accordance with the requirements of the Chinese HA-National Medical Products Administration (NMPA), we conducted a 24-week post-authorization safety study (PASS) of omalizumab in a real-world setting to evaluate its safety in Chinese patients (≥6 years of age) with moderate to severe allergic asthma.
Materials and Methods
Study Design
This is a single-arm, non-interventional, multicenter, PASS conducted between 27-Dec-2019 (first patient first visit) and 07-Jul-2021 (last patient last visit) in a real-world clinical setting in 59 hospitals in mainland China. The study protocol was approved by Independent Ethics Committees in the participating sites (Table S1). All procedures in this study comply with the Declaration of Helsinki, International Council for Harmonisation Guidelines for Good Clinical Practice, and applicable local regulations. The actual dosage of omalizumab was recorded as per the local prescribing information according to the approved dosing tables.
The analysis was based on a fixed duration of treatment for the cohort, and patient-years of exposure were also planned accounting for the dropout rate. The index date referred to the date of start point of the analysis, which was the last patient last visit on 07-Jul-2021.
Patients
Patients enrolled in this study were male or female adults aged ≥18 years, adolescents, and pediatric (≥6 years) patients with a confirmed diagnosis of moderate to severe allergic asthma whose symptoms were inadequately controlled with ICS plus a LABA. Adult patients signed the informed consent form, and for those aged 6 to <18 years, parent or a legal representative had provided their consent prior to initiation of omalizumab on physician decision.
Patients were treated according to the local prescribing information, and routine medical practice in terms of visit frequency and types of assessments performed. Patients who had contraindications according to locally approved label including hypersensitivity to the active substance or to any of the excipients or participation in any investigational clinical trial within 30 days prior to enrollment or previous treatment with omalizumab within 1 year prior to enrollment were excluded.
Study Endpoints
The primary endpoint of the study was the incidence of adverse events (AEs) and serious adverse events (SAEs) over 24 weeks during omalizumab therapy. Key secondary endpoints included response to treatment as measured by investigator’s and patient’s Global Evaluation of Treatment Effectiveness (GETE)16 at Week 16 and 24, health-related QoL as measured by Mini Asthma Quality of Life Questionnaire (mini-AQLQ)17 and the Pediatric Asthma Quality of Life Questionnaire (PAQLQ)18 at Week 16 and 24, and the rate of asthma exacerbations over Week 16 and 24. An asthma exacerbation is defined as a worsening of asthma symptoms requiring either additional systemic steroid therapy, hospitalization due to asthma, emergency visit due to asthma, unscheduled visit due to asthma, or absence from school/work including housework due to asthma.
The GETE questionnaire is an assessment of asthma symptom control and overall response to asthma treatment and was performed by both investigator and patient, each using the same 5-point scale. The GETE scale ranges were as follows: Excellent, Good, Moderate, Poor, and Worsening. Excellent and Good were defined as responder, while Moderate, Poor, and Worsening were defined as non-responder.16
AQLQ is a disease-specific questionnaire that evaluates the impact of treatment on QoL. The Mini-AQLQ is a shorter validated version of the AQLQ that has been developed and has 15 questions in the same domains as the original AQLQ (symptoms, activities, emotions, and environment). Clinically important differences in scores between any 2 assessments had been determined by the authors of the AQLQ questionnaire. Changes in scores of 0.5–1.0 were considered clinically meaningful, 1.0–1.5 as moderate, and >1.5 as marked clinically important differences for any individual domain or for the overall summary score.17
The pediatric QoL was measured using the self-administered Juniper’s PAQLQ. The PAQLQ contained 23 items that children with asthma have identified as troublesome in their daily lives. PAQLQ domains include activity limitation, emotional function, symptoms, and overall. It has been previously documented that mean changes of 0.5, 1.0, and 1.5 represent small, medium, and large effects, respectively.18
Data
The baseline parameters collected from medical records included patient demographics, disease characteristics, medical history and previous treatment, concomitant medications, comorbidities, laboratory values, treatment dose, interval and duration, withdrawal/discontinuation, and reason for withdrawal/discontinuation. The baseline event rate in the patient population was based on a China Market Access Health Economics and Outcomes (HEOR) study.19
Per protocol, data from clinic visit/hospitalization/emergency room visit and telephone contact were all accepted and recorded into the eCRF. Some study assessments were done via telephone. Safety data collected at each monthly visit included the type, severity, and duration of AEs, SAEs, and AEs of special interest (AESI). Effectiveness data comprised physicians’ and patients’ GETE questionnaire, mini-AQLQ, PAQLQ, and the number, severity, and treatment of asthma exacerbation events.
Statistical Analysis
All safety and effectiveness analyses were descriptive in nature and performed for overall population (≥6 years), as well as for pediatric (6 to <12 years) population separately. All analyses were performed by a designated contract research organization (Taimei Technology) using SAS 9.4 or the latest version.
Continuous variables were summarized using descriptive statistics (number of non-missing data, mean, standard deviation, median, minimum, and maximum). Categorical variables were summarized in terms of the number and percentage of patients in each category including a category for missing data if any.
The full analysis set (FAS) consisted of all patients who received at least 1 dose of omalizumab in this study. Safety set (SS) consisted of all patients who had at least 1 post-baseline safety assessment and were exposed to at least 1 dose of omalizumab.
Results
Overall Population
A total of 1546 patients were screened, and 1528 patients were enrolled in the study. All enrolled patients were included in both the FAS and SS. Of the 1528 patients, 690 (45.2%) patients completed the treatment on or before Week 24, and 71 (4.6%) patients discontinued the study with the main reason being lost to follow-up (2.3%, Figure 1). The proportion of male and female patients was similar among the enrolled patients (female: 50.6%; male 49.3%) with mean (standard deviation [SD]) age of 40.0 (20.04) years. In total, 191 (12.5%) were pediatric patients aged <12 years.
 |
Figure 1 Patient disposition. |
Majority of patients had moderate allergic asthma (74.9%), while the others had severe allergic asthma (25.1%). The mean (SD) duration of allergic asthma was 7.64 (9.96) years. Among 1514 patients analyzed for IgE, the mean (SD) of total serum IgE was 457.01 (703.17) IU/mL. The most commonly received asthma-related concomitant medications were drugs for obstructive airway diseases (95.7%), antihistamines for systemic use (23.6%), and corticosteroids for systemic use (11.3%).
Patient demographics and baseline characteristics are presented in detail in Table 1. Asthma-related prior and concomitant medications are presented in Table S2.
 |
Table 1 Patient Demographics and Clinical Characteristics at Baseline |
Safety
Duration of Drug Exposure
The mean (SD) duration of omalizumab exposure was 133.1 (63.50) days and 129.3 (63.36) days in the overall and pediatric population, respectively.
Incidence of AEs
A total of 361 (23.6%) patients in the overall population and 27 (14.1%) patients in the pediatric population (6 to <12 years) experienced AEs. Among these, 58 patients (3.8%) and 5 patients (2.6%) reported AEs that were related to omalizumab in the overall population and pediatric population, respectively. The most commonly reported AEs by preferred term (PT) in the overall population were upper respiratory tract infection, asthma, and nasopharyngitis and their respective incidence was 3.5%, 3.0%, and 2.5%. The most commonly reported AEs by PT in the pediatric population (6 to <12 years) were upper respiratory tract infection and nasopharyngitis and their respective incidence was 4.7% and 1.6% (Table 2).
 |
Table 2 Overview of Adverse Events (Safety Analysis Set) |
In the overall population, among those who experienced AEs (23.6%), the severity of AEs was mostly mild (206 patients, 13.5%) and moderate (121 patients, 7.9%). Only 2.2% of the patients had severe AEs. Two patients (0.1%) in the overall population reported AESI, of which one was lung adenocarcinoma and the other malignant neoplasm of the lung. Both AESIs were not related to the study drug as per the discretion of the investigator. No anaphylaxis events were reported in this large cohort followed for 24 months.
Incidence of SAEs
A total of 68 (4.5%) patients in the overall population and 3 (1.6%) patients in the pediatric population experienced SAEs. Only 1 SAE (0.1%) was related to omalizumab. The most commonly reported SAEs by PT in the overall population were asthma (1.9%) and chronic obstructive pulmonary disease (COPD) (0.5%). The most commonly reported SAEs by PT in the pediatric population were asthma, hydrocele, and bacterial gastroenteritis (0.5% each). No SAEs in the pediatric population were related to the study treatment.
Death
Three deaths caused by lung infection, heart failure, and COPD were reported in the overall population, and these events were considered not to be related to the study drug. No deaths were reported in the pediatric population.
Discontinuation of Study and Treatment Due to AEs/SAEs
Study discontinuation: In the overall population, two patients (0.1%) reported AEs and SAEs, leading to study discontinuation. There were no AEs/SAEs leading to study discontinuation in the pediatric population.
Treatment discontinuation: The AEs leading to discontinuation from the treatment were reported in 29 patients (1.9%), including 3 pediatric patients (6 to <12 years, 1.6%). Among those who reported SAEs, 9 patients (0.6%), including 1 pediatric patient (0.5%), discontinued from the treatment.
Effectiveness
Investigator’s and Patient’s GETE
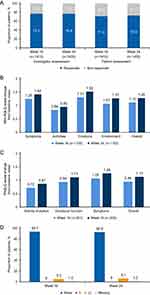
A total of 1419 and 1426 patients were evaluated for treatment effectiveness by investigators and patients, respectively. Majority of the patients were responders at Week 16 (77.2% based on the investigators’ GETE; 71.4% based on the patients’ GETE) and Week 24 (76.8% based on the investigators’ GETE; 72.9% based on the patients’ GETE, Figure 2A). There was a slightly lower but overall good proportion of responders in the pediatric population (more than 50% in both investigator and patient assessment) at Week 16 and Week 24.
Mini-AQLQ
Among 1528 patients, a total of 1207 patients, 1105 patients, and 1101 patients completed the mini-AQLQ at Day 1, Week 16, and Week 24, respectively. The mean (SD) change in mini-AQLQ scores from baseline in the overall domain was 1.12 (1.12) at Week 16 and 1.28 (1.18) at Week 24 (Figure 2B). At Week 16, 70.8% of patients showed an improvement of ≥0.5 from baseline in the total score, with 34.1% of patients showing clinically important differences (>1.5 from baseline). At Week 24, 76.8% of patients showed an improvement of ≥0.5 from baseline in the total score, with 41.0% of patients showing clinically important differences (>1.5 from baseline).
PAQLQ
A total of 316 patients, 301 patients, and 306 patients completed the PAQLQ at Day 1, Week 16, and Week 24, respectively. For patients <18 years old, the mean (SD) change of PAQLQ scores from baseline of the overall domain was 0.95 (0.97) at Week 16 and 1.13 (1.03) at Week 24 (Figure 2C). For patients aged 12 to <18 years old, the mean (SD) change of PAQLQ scores from baseline of the overall domain was 1.05 (1.02) at Week 16 and 1.24 (1.07) at Week 24. A similar outcome was observed in the pediatric (6 to <12 years) population: mean (SD) change of PAQLQ scores from baseline of the overall domain was 0.89 (0.93) at Week 16 and 1.05 (1.01) at Week 24.
Asthma Exacerbation
In the overall population (1528 patients), during pre-treatment, the mean (SD) of annualized number of asthma exacerbation events was 0.50 (1.05) times. At Week 16 and 24, the mean (SD) of number of annualized asthma exacerbation events was 0.24 (1.12) times and 0.19 (0.87) times, respectively. At Week 16 and 24, the majority of patients (93.70% and 92.90%) did not experience asthma exacerbation events. Only 5.3% and 6.1% of the patients experienced more than 2 times of asthma exacerbation events at Week 16 and 24, respectively (Figure 2D).
Pulmonary Function Test
Forced expiratory volume in 1 second (FEV1), FEV1% predicted, and peak expiratory flow (PEF) showed slight improvement at Week 16 and 24 (Table 3).
 |
Table 3 Summary of Pulmonary Function Test |
Treatment Pattern
Approximately half of the patients (overall: 56.9% and pediatric: 48.2% patients) adhered to the recommended dose of omalizumab. Reason for non-adherence in the overall population included financial reasons (17.3%) and other diseases (13.2%). Similarly, in the pediatric population, financial reasons (23.0%) and other diseases (16.2%) were the most frequently cited reason for non-adherence.
Majority of the patients (overall: 76.2% and pediatric: 72.8%) did not require any dose adjustment. The most commonly reported (>2%) reason for dose adjustment (for the first-time occurrence) was improvement of illnesses (2.1%) in the overall population and financial reason (4.2%) and improvement of illnesses (2.6%) in the pediatric population.
Within the overall population with at least one of the following asthma-related events (occurring over 24 weeks) due to an asthma exacerbation, the mean (SD) number of hospitalizations was 1.2 (0.50) times, the mean (SD) number of emergency visits was 1.3 (0.48) times, and the mean (SD) of number of unscheduled visits was 1.0 (0.00). In the pediatric population, the mean (SD) of number of hospitalizations was 1.0 (-) time and none of the pediatric patients had emergency visits or unscheduled visits during the 24 weeks.
Discussion
The current PASS evaluated the real-world safety and effectiveness of omalizumab in adult, adolescent, and pediatric patients with moderate to severe allergic asthma in China. To our knowledge, this is the largest real-world omalizumab study conducted in China with more than 1500 patients enrolled.
Overall, the demographics and baseline characteristics of the patients in this study were comparable to other real-life studies outside of China (mean age: current study 40.0 years vs other studies 45–47.8 years).20,21 A similar proportion of male and female patients were included in this study; however, other similar studies reported a lower proportion of pediatric patients compared to this study.
Another real-world study conducted in China by Zhang et al evaluated the effectiveness of omalizumab in 139 patients based on Asthma Control Test and lung function tests. Regarding the mini-AQLQ score, the improvement from baseline (1.12) is comparable with the study conducted by Zhang et al (0.09).19 Other findings from this PASS that were comparable to the real-world study include PEF by Week 16 (mean change from baseline: current study 0.42 vs real-world study 0.91) and GETE responders by Week 16 (% responders: current study Physicians’ 77.2% and Patients’ 71.4% vs real-world study Zhang et al overall 91.5%). However, the current study reported a higher percentage of patients that did not experience asthma exacerbations compared to the real-world study19 (92.4% vs 80.5%). A lower exacerbations rate observed in this study could be due to avoidance by asthmatics.22 In the COVID-19 epidemic environment, patients with asthma might have strengthened their self-protection, thereby having reduced risk of respiratory tract infection, which could also be one of the possible reasons for less asthma exacerbations. It should be noted that our study enrolled a total of 1528 patients compared to 139 in the real-world study.19 Furthermore, we included adolescent and pediatric patients (<18 years), which was not included in the real-world study by Zhang et al.19 The current study also covered a longer treatment period (24 weeks) versus Zhang et al (16 weeks) study.19
From the eXpeRience registry, Braunstahl et al provided the global real-world effectiveness of omalizumab in patients with allergic asthma.20 This current study was in line with the effectiveness findings from the eXpeRience registry20 such as the responder rate with Physician’s GETE at Week 16 (eXpeRience 69.9% vs current study 77.2%). Lung function improvements were also similar in both studies, in terms of FEV1% predicted change from baseline (eXpeRience 9.8% by Month 12 vs current study 7.48% by Week 24) and the mean PEF change from baseline (eXpeRience 40.4 L/min [equivalent to 0.67 L/s] by Month 12 vs current study 0.51 L/s by Week 24).20
The results from the current study were also in line with another real-world study reported in Japan.23 Patient characteristics were different in Japan versus China (mean age: Japan 59.3 years vs China 40.0 years; gender: Japan, female 64.45% vs China, female 50.1%). The study23 only included 0.19% pediatric patients by Japanese definition (<15 years) versus 12.5% in the current study (<12 years). AEs were reported in 32.24% in the Japan study and 23.6% in the current study. SAEs were reported in 15.3% patients and 4.5% patients in the Japan study and the current study, respectively. Physician-assessed GETE was also consistent with previously reported findings: Japan 59.29% vs China 77.2% by Week 16. In the Japanese study, about 47.96% patients were free from asthma exacerbations after therapy compared to the current study (93.7%).
The ASTERIX study21 reported the real-world effectiveness of omalizumab in Canada, in which 56.3% of patients remained exacerbation free in the 12-month observation period versus the current study in China (93.7% in Week 16 or 92.9% in Week 24). The Canada study included only 99 patients aged ≥12 years compared to the current China study with over 1500 patients aged ≥6 years. The AQLQ improvement was also in line with the published Canada real-world study21 (AQLQ change from baseline at Week 16: Canada 0.9 vs China 1.12).
The open-label study design of the current PASS with no control group for comparison could be a limitation for the evaluation of effectiveness and safety. However, it was considered that the results of this study reflected the actual safety and effectiveness of product in real-world medical practice. Considering that the current study did not sponsor the study treatment, and that patients paid for the products, it was important to ensure the enrolled patients complete a 24-week observation to avoid a high dropout rate and impact on data quality and assessment. It should be noted that only 57% of patients adhered to the study and financial reason was the most common cause for non-adherence (17.3%). Some of the strengths of the study include the enrollment of a majority of patients from centers of excellence in asthma management and the inclusion of patients from different age groups, representative of the asthma patient population treated with omalizumab.
Conclusion
The findings of the current study conducted in Chinese population demonstrated that the safety profile of omalizumab was consistent with its known profile in allergic asthma, and no new safety signals were reported.24 Omalizumab treatment was effective in improving lung function and QoL in patients with allergic asthma.
Acknowledgments
Investigators Yi Liu, Jie Song, Kewu Huang, Suting Xi, Hua Liu, Weining Xiong, Yimin Lu, Changshan Liu, Leping Ye, Wen Li, Yongchang Sun, Guangfa Wang, Yong Huang, Yanming Li, Lianglu Wang, Jianqing Zhang, Junfei Zhu, Shaoxi Cai, Xiangdong Mu, Limin Wang, Xianmei Huang, Xin Zhou, Jianchu Zhang, Jie Cao, Siqin Wang, Qiang Li, Zheng Liu, Zeying Liu, Qun Lv, Huapeng Yu, Minhua Shi, Tao Zhang, Pusheng Xu, Xiaoluan Li, Shumei Sun, Xiwen Gao, Kang Ning, Ning Li, Jie Lin, Yongjian Huang, Hui Li, Songming Zhuo, Ke Hu, Jian Feng, Yi Xiao, Xiuhua Fu, Yanping Lai, Zhongmin Qiu, Xiaoju Zhang, Lihong Wang are thanked for their contributions to study enrollment in this study. The authors would like to thank Ian Chun Tan, Preethi B, and Poh Sien Ooi (Novartis Corporation Sdn Bhd) for providing medical writing assistance in accordance with the Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Lili Zhi has moved to a different institution before this article is completed, author would like to thank the Center Hospital of Zibo, where the work was carried out.
Funding
This work was supported by Beijing Novartis Pharma Co., Ltd., Beijing, China.
Disclosure
Monica Ligueros-Saylan, Alkaz Uddin, Jing Yang, Tiantian Liang, Liju Ding, and Runqin Li are/were employees of Novartis during the time of the study and the manuscript writing. The authors report no other conflicts of interest in this work.
References
1. Holgate ST, Djukanović R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35(4):408–416. doi:10.1111/j.1365-2222.2005.02191.x
2. Global Initiative for Asthma. Global strategy for asthma management and prevention revised 2014; 2014. Available from: https://ginasthma.org/wp-content/uploads/2019/01/2014-GINA.pdf.
3. Lee JK, Amin S, Erdmann M, et al. Real-world observational study on the characteristics and treatment patterns of allergic asthma patients receiving omalizumab in Canada. Patient Prefer Adherence. 2020;14:725–735. doi:10.2147/PPA.S248324
4. Huang K, Yang T, Xu J, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet. 2019;394(10196):407–418. doi:10.1016/S0140-6736(19)31147-X
5. Global Asthma Network. The global asthma report 2018; 2018. Available from: www.globalasthmanetwork.org.
6. Wang P, Wang L, Ni Q, Shi G. Factors of high asthma expenditure in China: an 1-year retrospective study. Value Health. 2018;21:S104. doi:10.1016/j.jval.2018.07.788
7. Schatz M, Rosenwasser L. The allergic asthma phenotype. J Allergy Clin Immunol. 2014;2(6):645–648.
8. Novak N, Bieber T. Allergic and nonallergic forms of atopic diseases. J Allergy Clin Immunol. 2003;112(2):252–262. doi:10.1067/mai.2003.1595
9. Mao D, Tang R, Wu R, et al. Prevalence trends in the characteristics of patients with allergic asthma in Beijing, 1994 to 2014. Medicine. 2017;96:22.
10. Platt-mills TAE. The role of immunoglobulin E in allergy and asthma. Am J Respir Crit Care Med. 2001;164:51–55.
11. Omalizumab [package insert]. South San Francisco, CA: Genentech, Inc; 2021. Available from: www.fda.gov/medwatch.
12. Omalizumab 75 mg Solution. Summary of product characteristics (SPC) - (eMC); 2021. Availabe from: https://www.medicines.org.uk/emc/product/5327/ampc#gref.
13. Xolair (omalizumab). Data on file. Safety update. Period covered; 2018.
14. Li J, Kang J, Wang C, et al. Omalizumab improves quality of life and asthma control in Chinese patients with moderate to severe asthma: a randomized Phase III study. Allergy Asthma Immunol Res. 2016;8(4):319–328. doi:10.4168/aair.2016.8.4.319
15. Chan J. Novartis’ asthma therapy Xolair gets approval in China; 2017. Available from: https://www.spglobal.com/marketintelligence/en/news-insights/trending/ygigsltbpjsgzlzabvyzfq2.
16. Lloyd A, Turk F, Leighton T, Canonica W. Psychometric evaluation of global evaluation of treatment effectiveness: a tool to assess patients with moderate-to-severe allergic asthma. J Med Econ. 2007;10(3):285–296. doi:10.3111/13696990701478856
17. Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma quality of life questionnaire. Eur Respir J. 1999;14:32–38. doi:10.1034/j.1399-3003.1999.14a08.x
18. Juniper EF, Guyatt GH, Feeny DH, et al. Measuring quality of life in children with asthma. Qual Life Res. 1996;5(1):35–46. doi:10.1007/BF00435967
19. Zhang M, Jin M, Zhou X, et al. Effectiveness of omalizumab in patients with severe allergic asthma: a retrospective study in China. Respir Med. 2021;186:106522. doi:10.1016/j.rmed.2021.106522
20. Braunstahl GJ, Chen CW, Maykut R, Georgiou P, Peachey G, Bruce J. The eXpeRience registry: the “real-world” effectiveness of omalizumab in allergic asthma. Respir Med. 2013;107(8):1141–1151. doi:10.1016/j.rmed.2013.04.017
21. Bhutani M, Yang WH, Hébert J, De Takacsy F, Stril JL. The real world effect of omalizumab add on therapy for patients with moderate to severe allergic asthma: the ASTERIX observational study. PLoS One. 2017;12(8):e0183869. doi:10.1371/journal.pone.0183869
22. Luskin AT, Chipps BE, Rasouliyan L, Miller DP, Haselkorn T, Dorenbaum A. Impact of asthma exacerbations and asthma triggers on asthma-related quality of life in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol Pract. 2014;2(5):544–552.e2. doi:10.1016/j.jaip.2014.02.011
23. Adachi M, Kozawa M, Yoshisue H, et al. Real-world safety and efficacy of omalizumab in patients with severe allergic asthma: a long-term post-marketing study in Japan. Respir Med. 2018;141:56–63. doi:10.1016/j.rmed.2018.06.021
24. Di Bona D, Fiorino I, Taurino M, et al. Long-term “real-life” safety of omalizumab in patients with severe uncontrolled asthma: a nine-year study. Respir Med. 2017;130:55–60. doi:10.1016/j.rmed.2017.07.013
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.