Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 9
Real-world glycemic outcomes in patients with type 2 diabetes initiating exenatide once weekly and liraglutide once daily: a retrospective cohort study
Authors Saunders W, Nguyen H, Kalsekar I
Received 10 January 2016
Accepted for publication 21 April 2016
Published 15 July 2016 Volume 2016:9 Pages 217—223
DOI https://doi.org/10.2147/DMSO.S103972
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
William B Saunders,1 Hiep Nguyen,2 Iftekhar Kalsekar2
1Department of Public Health Sciences, College of Health and Human Services, The University of North Carolina at Charlotte, Charlotte, NC, 2AstraZeneca, Fort Washington, PA, USA
Aim: The glucagon-like peptide-1 receptor agonists exenatide once weekly (QW) and liraglutide once daily (QD) have demonstrated improvements in glycemic outcomes in patients with type 2 diabetes mellitus in randomized clinical trials. However, little is known about their real-world comparative effectiveness. This retrospective cohort study used the Quintiles Electronic Medical Record database to evaluate the 6-month change in glycated hemoglobin (A1C) for patients initiating exenatide QW or liraglutide QD.
Methods: Patients with type 2 diabetes mellitus prescribed exenatide QW (n=664) or liraglutide QD (n=3,283) between February 1, 2012 and May 31, 2013 were identified. Baseline A1C measures were from 75 days before to 15 days after initiating exenatide QW or liraglutide QD, with follow-up measures documented at 6 months (±45 days). Adjusted linear regression models compared the difference in mean A1C change. A priori defined sensitivity analysis was performed in the subgroup of patients with baseline A1C ≥7.0% and no prescription for insulin during the 12-month pre-index period.
Results: For exenatide QW and liraglutide QD, respectively, mean (SD) age of the main study cohort was 58.01 (10.97) and 58.12 (11.05) years, mean (SD) baseline A1C was 8.4% (1.6) and 8.4% (1.6), and 48.2% and 54.2% of patients were women. In adjusted models, change in A1C did not differ between exenatide QW and liraglutide QD during 6 months of follow-up. Results were consistent in the subgroup analyses.
Conclusion: In a real-world setting, A1C similarly improves in patients initiating exenatide QW or liraglutide QD.
Keywords: diabetes, exenatide, outcomes
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic, progressive, and increasingly prevalent illness.1 In adults, T2DM accounts for ∼90%–95% of all diagnosed cases of diabetes; the remaining are diagnosed cases of type 1 diabetes, a form of diabetes for which the cause is currently unknown.1 As T2DM progresses, attaining and maintaining glycemic control become increasingly challenging; the risk of cardiovascular comorbidities increases and weight gain is common.2 Because of the progressive nature of the disease, 50% of patients eventually require combination therapy to achieve glycated hemoglobin (A1C) goals within 3 years of diagnosis, and this increases to 75% of patients within 9 years.3,4 This has substantial cost implications to health care systems and society.
The treatment for T2DM has become increasingly complex with the introduction of newer therapies in the market. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are a relatively new class of glucose-lowering agents that are increasingly used as second- or third-line therapy in patients with T2DM.5 GLP-1RAs mimic endogenous GLP-1, stimulating insulin release from the pancreas in a glucose-dependent manner and suppressing glucagon secretion.3 GLP-1RAs are associated with high glycemic efficacy, weight loss, and low risk of hypoglycemia, but with some risk of gastrointestinal side effects.6 Depending on the duration of therapy, GLP-1RAs can be classified into short-acting and long-acting agents. Short-acting agents have a half-life of less than 12 hours and up to 24 hours, which requires daily or twice-daily administration.7,8 Long-acting GLP-1RAs have a half-life of greater than 24 hours; so, these agents can be injected as once-weekly doses.9 The short-acting GLP1-RAs tend to have a greater effect on postprandial glycemia, while the long-acting molecules tend to lower fasting glucose to a greater extent.9
Exenatide once weekly (QW) and liraglutide once daily (QD) are two GLP-1RAs that have been demonstrated to be effective in patients with T2DM in numerous clinical studies.10–13; however, the available direct and indirect clinical evidence comparing the efficacy of exenatide QW and liraglutide QD has been mixed. One head-to-head trial compared the efficacy of liraglutide 1.8 mg QD with exenatide QW. Patients receiving liraglutide 1.8 mg QD experienced greater A1C reductions and A1C goal achievement compared with exenatide QW.14 However, Scott et al conducted a network meta-analysis of 22 trials and reported no difference in efficacy between exenatide QW and liraglutide 1.8 mg QD.15
Randomized controlled trials (RCTs) are conducted in a closely controlled, well-defined patient population environment; thus, results from RCTs potentially do not always translate to real-world clinical practice. While RCTs are important for evaluating the clinical efficacy and safety of new interventions, real-world studies yield different and necessary data for clinical and population decision-making. Given that there is limited real-world comparative effectiveness evidence between exenatide QW and liraglutide QD, the primary objective of this paper is to evaluate the comparative effectiveness of exenatide QW and liraglutide QD in GLP-1RA-naïve patients with T2DM in an ambulatory care setting.
Methods
Data source
This retrospective cohort study utilized the Quintiles Electronic Medical Record (Q-EMR) research database, a large electronic medical record source. The research database contains ambulatory electronic health data for 33 million patients in 49 states, and includes demographic data, vital signs, laboratory orders and results, medication list entries and prescriptions, and diagnoses or problems. This HIPAA (Health Insurance Portability and Accountability Act of 1996)-compliant research dataset includes data contributed by providers who use the Q-EMR system in the ambulatory care setting. Researchers have compared the Q-EMR patient population to the US population (US Census), health care utilization (National Center for Health Statistics [NCHS], National Ambulatory Medical Care Survey [NAMCS]), and disease prevalence (NCHS, National Health and Nutrition Examination Survey [NHANES]), and identified that Q-EMR is similar to the US population. The Q-EMR dataset is well suited to assess clinical outcomes, including glycemic control and weight change in patients with diabetes. The Institutional Review Board of The University of North Carolina at Charlotte deemed approval and patient written informed consent were not required as secondary data containing no protected health information was used.
Study population
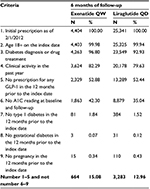
This analysis evaluated patients with diabetes in the Q-EMR database (Table 1). Patients 18 years or older on the index date with T2DM based on at least one of the following criteria were included: 1) diagnosis of T2DM based on an International Classification of Diseases, Ninth Revision (ICD-9) code of 250.X0 or 250.X2; 2) one or more prescription orders for an oral antidiabetes drug; 3) two consecutive fasting blood glucose levels of >126 mg/dL; or 4)A1C at any time ≥7.0%.
Patients were identified based on ≥1 prescription for exenatide QW or liraglutide QD between February 1, 2012 and May 31, 2013. The date of the first prescription was the index date; the specific GLP-1RA identified on the index date was the index medication. Patients were required to have continuous activity in the database for a minimum of 12 months prior to the index date of GLP-1RA initiation and a minimum of 6 months after the index date. Patients were excluded if they had a prescription for exenatide or liraglutide QD during the 12-month pre-index period, or missing baseline and follow-up data for the primary outcome, or a diagnosis of type 1 or gestational diabetes, or who were pregnant.
Outcomes and study measures
Baseline patient characteristics and clinical data (A1C, body mass index [BMI], blood pressure, lipids, comorbidities, and baseline oral antidiabetes drug therapy) were measured. Comorbidities were captured on or prior to the index date using ICD-9 codes. Hyperlipidemia and hypertension were measured using ICD-9 codes and prescriptions.
The primary outcome was change in A1C from index date (75 days prior to 15 days after) to 6 months (±45 days) of follow-up. This range allows for the A1C measurement to be within 2 months of the index date and allows for 2 weeks on either end of the range to detect as many clinically relevant measurements as possible, given the natural variability in patient visits. The primary outcome was evaluated for all study patients and for an a priori subgroup of patients who were insulin-naïve with uncontrolled T2DM (baseline A1C >7.0%) at baseline. Patients for the subgroup were selected based on similar criteria as in pivotal trials.14 In addition, changes in weight, BMI, blood pressure, triglycerides, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol from baseline to 6 months of follow-up were reported.
Statistical methods
Baseline demographic and clinical characteristics for the study cohort were compared with Student’s t-tests for continuous variables and chi-square tests for categorical variables. A generalized linear model was developed to estimate the change in A1C from baseline as the key dependent variable. Covariates in the model included age, sex, race, baseline mean A1C, baseline BMI, number of other antidiabetes medication classes, Deyo–Charlson comorbidity index, health insurance type, region, hyperlipidemia, and hypertension. In addition, we compared the likelihood of patients achieving the American Diabetes Association’s A1C target of less than 7% using logistic regression, adjusting for the variables used in the model. Predicted values were calculated for each index treatment from the multivariate models to report the change in A1C from baseline and the proportion of patients achieving A1C <7% after controlling for confounding factors. Given the study design, power calculations were not performed as all the available data were utilized and sample sizes were sufficiently large. All statistical tests were performed at a priori significance level of 0.05 using SAS STAT software Version 9.3 (SAS Institute, Cary, NC, USA).
Results
Sample selection and attrition are detailed in Table 1; 29,745 patients with an initial prescription of exenatide QW or liraglutide QD as of February 1, 2012 were initially identified from the database. After applying the inclusion and exclusion criteria, 3,947 patients remained in the analysis, with 664 patients receiving once-weekly exenatide 2 mg and 3,283 patients receiving once-daily liraglutide 1.8 mg. Among liraglutide QD patients, a subset of patients with available data was prescribed 1.2 mg (n=431) and 1.8 mg (n=507) of the drug. The subgroup of patients who had uncontrolled T2DM with no insulin use at baseline included 304 exenatide QW and 1,472 liraglutide QD patients.
Baseline patient and treatment characteristics
Baseline patient and demographic characteristics are presented for all study patients in Table 2. There were no statistically significant differences in most demographic characteristics between the exenatide QW group and the once-daily liraglutide 1.8 mg group (Table 2). Study cohorts were statistically different with respect to race, sex, systolic blood pressure, and geographic region. Patients who were treated with either exenatide QW or liraglutide QD were severely obese (BMI >40 kg/m2) with mean A1C of 8.4% and on an average aged ∼58 years. The study population was heterogeneous in terms of race and sex. However, more women were treated with liraglutide QD than exenatide QW. Tables 3 and 4 show the baseline clinical characteristics and use of oral antihyperglycemic medications, respectively. Approximately 74% of the study population who were treated with either exenatide QW or liraglutide QD had hypertension, and 70% of those treated with exenatide QW had hyperlipidemia compared with 73% of patients treated with liraglutide QD. Approximately 2.5% of the study population had ischemic heart disease, and fewer than 1% had congestive heart failure, hypertriglyceridemia, and acute coronary syndrome. Except for arthritis, no statistically significant differences in comorbidities were found between the treatment groups. In addition, the previous use of antidiabetic medication was similar between the treatment groups (no statistically significant difference) with the exception of the use of thiazolidinediones.
Study outcome
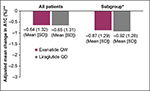
Results from the adjusted linear regression models suggest no difference in mean A1C change from baseline to 6 months between exenatide QW and liraglutide QD for all study patients and in the subgroup (Figure 1). Unadjusted results were consistent with results adjusted for age, sex, race, baseline A1C, BMI, number of other antidiabetes medication classes, Deyo–Charlson comorbidity index, insurance type, region, hyperlipidemia, and hypertension (not reported). Similarly, in adjusted logistic regression models comparing the likelihood of achieving target A1C goal of less than 7%, adjusted results were similar between exenatide QW and liraglutide QD in all study patients (odds ratio =1.01, 95% confidence interval [CI] =0.83–1.23) and the subgroup (odds ratio =0.95, 95% CI: 0.76–1.18). Table 5 also shows the changes in weight, BMI, blood pressure, triglycerides, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol from baseline to 6-month follow-up. No statistically significant differences were found when comparing exenatide QW to liraglutide QD with the exception of systolic blood pressure where liraglutide QD patients had slightly elevated baseline levels and subsequently a higher average decrease (129.0 mmHg versus 126.8 mmHg and –1.68 mmHg versus –0.28 mmHg, respectively).
Discussion
The results of this real-world, observational study indicate similar reductions in A1C in patients with T2DM initiating either exenatide QW or liraglutide QD. Among patients who were not at goal at baseline with no prior insulin use, the results demonstrated that A1C reductions in registration trials are similar to those achieved in a clinical setting.
In this study, GLP-1RAs were generally prescribed as second- or third-line therapy, which is consistent with the current consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes about the medical management of hyperglycemia in T2DM.16,17
This study is the first A1C comparative effectiveness analysis between exenatide QW and liraglutide QD in the ambulatory care setting. Patients in our study had mean baseline A1C of 8.4%, which was similar to clinical trial patients in core registration programs for exenatide QW and liraglutide QD,10,11 including the only head-to-head, prospective clinical trial of liraglutide and once-weekly exenatide (DURATION-6).14 However, patients were heavier than those studied in registration clinical trials, with an average BMI of 38.8 versus 34 kg/m2 in registration trials. In addition, patients had different effectiveness-based results comparing twice-daily and once-weekly formulations.15 Approximately two-thirds had a BMI ≥35 kg/m2. Also, results in the overall patient population in our study suggest moderate HbA1C reduction, but not of the magnitude observed in RCTs.10,11 Unlike the overall patient population from our study, patients included in RCTs generally have uncontrolled T2DM (HbA1C ≥7%) and are naïve to insulin therapy at baseline. When we applied similar patient criteria, our results in the subgroup of insulin-naïve patients who had uncontrolled T2DM at baseline were consistent with previous RCTs for exenatide QW and liraglutide.14
While our study demonstrated that exenatide and liraglutide improved glycemic control with associated weight loss, our findings differ from those of DURATION-6.14 In the DURATION-6 study by Buse et al, there were greater reductions noted with liraglutide (0.21%).14 DURATION-6 was a 26-week, open-label, randomized, parallel-group study at 105 sites in 19 countries outside of the US, and patients in the liraglutide arm were uptitrated to the 1.8 mg dose within a week.14
The difference in the findings between our study and DURATION-6 may be partially explained by differences in the study setting and treatment dose. Moreover, there are differences in dosing frequency between the formulations, which may have a greater impact on real-world A1C reduction compared with A1C reduction in a controlled trial of motivated patients receiving higher than normal intensity clinical attention. Our study represented the routine care setting where adult patients with T2DM received either exenatide QW or liraglutide QD in different dosages.
Our study results are consistent with findings from a previous report in which a systematic review and network meta-analysis of RCTs of ≥24 weeks that compared exenatide QW, liraglutide (1.2, 1.8 mg), insulin glargine, exenatide BID, or placebo were conducted.15 After pooling 11,049 patients with T2DM from 22 clinical studies, the network meta-analysis did not identify meaningful differences in A1C lowering between exenatide QW and both liraglutide doses, suggesting that these GLP-1RAs have similar glycemic effects.15 Estimated mean differences in A1C versus placebo were –1.15% (95% CI: –1.31 to –1.00) for exenatide QW, –1.01% (95% CI: –1.18 to –0.85) for liraglutide 1.2 mg, and –1.18% (95% CI: –1.32 to –1.04) for liraglutide 1.8 mg. A1C differences for exenatide QW versus liraglutide 1.2 and 1.8 mg were –0.14% (95% CI: –0.34 to 0.06) and 0.03% (95% CI: –0.14 to 0.18), respectively.
This study has the following limitations to the analysis, which need to be considered. First, not all clinical data may be captured, such as laboratory test results. Second, data were limited to office-based treatments, and other services (eg, hospitalizations and emergency department visits) were not captured. Third, medication data are based on prescription orders and not prescription dispensed. Fourth, we did not include all possible GLP-1RAs. Fifth, we did not evaluate differences in adverse events. Sixth, it is not possible to confirm that the drug was actually used by the patient, and finally, as an observational study, the accuracy and completeness of data cannot be confirmed due to the retrospective nature of the data collection using EMRs.
Conclusion
In summary, this study provides important information for decision makers, clinicians, and adult patients with T2DM on the real-world use of two GLP-1RAs that demonstrate similar effectiveness and glycemic control in an ambulatory care setting. Future research is warranted to understand the long-term comparative effectiveness of GLP-1RAs and factors associated with achieving glycemic control in the real-world setting.
Acknowledgments
AstraZeneca provided funding for this manuscript. The authors would like to thank Cynthiya Ruban for editorial assistance provided during the preparation of the manuscript.
Disclosure
HN is an employee of AstraZeneca. The authors report no other conflicts of interest in this work.
References
Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services; 2014. | ||
Wysham CH. New perspectives in type 2 diabetes, cardiovascular risk, and treatment goals. Postgrad Med. 2010;122:52–60. | ||
Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999:281(12):2005–2012. | ||
Centers for Disease Control and Prevention (CDC). National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. | ||
Inzucchi SE, Bergenstal, RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55:1577–1596. | ||
Divino V, DeKoven M, Hallinan S, et al. Glucagon-like peptide-1 receptor agonist treatment patterns among type 2 diabetes patients in six European countries. Diabetes Ther. 2014;5(2):499–520. | ||
Fineman MS, Bicsak TA, Shen LZ, et al. Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care. 2003;26:2370–2377. | ||
Degn KB, Juhl CB, Sturis J, et al. One week’s treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and α- and β-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes. 2004;53:1187–1194. | ||
Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007;30:1487–1493. | ||
Bydureon [package insert]. San Diego, CA: Amylin Pharmaceuticals Inc; 2012. | ||
Victoza [package insert]. Plainsboro, NJ: Novo Nordisk; 2013. | ||
Bergenstal RM, Wysham C, MacConnel L, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2013;376:431–439. | ||
Hall GC, McMahon AD, Dain MP, Wang E, Home P. Primary-care observational database study of the efficacy of GLP-1 receptor agonists and insulin in the UK. Diabet Med. 2013;30(6):681–686. | ||
Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomized, open-label study. Lancet. 2013;381(9861):117–124. | ||
Scott DA, Boye KS, Timlin L, Clark JF, Best JH. A network meta-analysis to compare glycaemic control in patients with type 2 diabetes treated with exenatide once weekly or liraglutide once daily in comparison with insulin glargine, exenatide twice daily or placebo. Diabetes Obes Metab. 2013;15(3):213–23. | ||
Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364–1379. | ||
Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15:540–559. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.