Back to Journals » Pragmatic and Observational Research » Volume 13
Real-World Experience with Favipiravir for the Treatment of Mild-to-Moderate COVID-19 in India
Authors Joshi S, Vora A, Venugopal K, Dadhich P, Daxini A, Bhagat S, Patil S, Barkate H
Received 15 March 2022
Accepted for publication 17 May 2022
Published 27 May 2022 Volume 2022:13 Pages 33—41
DOI https://doi.org/10.2147/POR.S364066
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor David Price
Shashank Joshi,1 Agam Vora,2 K Venugopal,3 Pramod Dadhich,4 Anil Daxini,5 Sagar Bhagat,6 Saiprasad Patil,6 Hanmant Barkate6
1Department of Endocrinology, Joshi Clinic and Lilavati Hospital and Research Centre, Mumbai, India; 2Department of Pulmonology, Vora Clinic, Mumbai, India; 3Department of Pulmonology, Sooriya Hospital, Chennai, India; 4Department of Pulmonology, Dadhich Clinic, Ajmer, India; 5Department of Medicine, Fortis Hospital, Mumbai, India; 6Global Medical Affairs, Glenmark Pharmaceuticals Ltd, Mumbai, India
Correspondence: Sagar Bhagat, Global Medical Affairs, Glenmark Pharmaceuticals Ltd, Mumbai, India, Tel +91 9930553638, Email [email protected]
Background: Favipiravir, an RNA-dependent RNA polymerase inhibitor (RdRp), is a broad-spectrum oral antiviral agent approved in India under emergency use authorization, for the treatment of mild-to-moderate coronavirus disease (COVID-19). The present study was planned to evaluate the effectiveness and safety of favipiravir in real-world clinical practice.
Materials and Methods: This was a multicentric, retrospective, single-arm study conducted across four centres in India, after obtaining permission from the independent ethics committee. Medical records were analysed to evaluate effectiveness and safety of patients who were prescribed favipiravir.
Results: The medical records of a total of 360 patients met the inclusion criteria, with 358 of them available for the final analysis. Males made up 58.46% of the study population. The average age of enrolled patients was 51.80 ± 16.45 years. The most common symptoms were fever, cough, and myalgia-fatigue. The median time to clinical cure and fever relief was five and four days, respectively. The average length of stay in the hospital was six days. In total, 8% of the patients experienced adverse events. Hepatic enzyme elevation, diarrhoea, decreased appetite, headache, fatigue, and giddiness were the common symptoms.
Conclusion: In our real-world study, favipiravir was found to have a clinical cure rate of more than 90% in mild-to-moderate COVID-19 patients. This supports the use of favipiravir in the treatment of COVID-19. Favipiravir was well tolerated, with only minimal side effects, which were transient in nature.
Keywords: favipiravir, COVID-19, India, antiviral
Introduction
An unprecedented crisis, which began in Wuhan city of China towards the end of December 2019, was officially declared as a pandemic of novel coronavirus (COVID-19) by the World Health Organization (WHO) on March 11, 2020.1 Globally, as of April 22, 2022, more than 200 countries have been affected by this, causing mortality in 6,213,876 infected individuals.2 India is one of the world’s most impacted countries, with mortality reported in 5,22,223 infected individuals.2 Infected patients often have mild to moderate disease in 80% to 90% of cases, with severe or critical disease in 10% to 20% of cases.3 Not only has the changing face of clinical presentation had a significant impact on the healthcare structure, but so also have emerging variations of concern (VoCs). Early in the course of the disease, replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the primary driver of pathogenesis. Later in the course of the disease, a dysregulated/hyper immunological response causes a multisystem inflammatory syndrome (MIS). The repurposed antiviral medicines are predicted to be useful early in the course of the disease.
Favipiravir, an RNA-dependent RNA polymerase (RdRp) inhibitor, is a broad-spectrum oral antiviral drug approved in India for the treatment of mild to moderate COVID-19 disease under emergency use authorisation.4 It inhibits RdRp, which is an important step in viral replication, and hence, plays an important role in preventing viral replication and transmission. The clinical use and benefit of Favipiravir in COVID-19 infection was first demonstrated by a study conducted by Cai et al5 and Chen et al.6 In an Indian phase 3 randomised control trial (RCT), Favipiravir demonstrated faster clinical cure in patients with mild-to-moderate COVID-19, when compared to standard supportive therapy, with good tolerability.7 In addition, a recent meta-analysis from the international database also reported that favipiravir significantly increases viral clearance and decreases hospitalization in mild-to-moderate COVID-19 patients.8 Efficacy and safety trials on Favipiravir in COVID-19 have been reported from China, Japan, Russia, India, Egypt, and Oman to date.
Current COVID-19 trials, such as the PRINCIPLE trial9 by Oxford University UK, are looking at its usage for at-home recovery, whilst the PRESECO phase 3 study, which is taking place in the United States, Mexico, and Brazil, is looking into its involvement in the prevention of severe COVID-19.10
Currently, Favipiravir is authorised for emergency or compassionate use in a number of countries.11 Favipiravir has been included in several state COVID-19 protocols in India, including Maharashtra, Karnataka, Kerala, Gujarat, and Orissa. The present study was designed to assess the effectiveness and safety of Favipiravir in real-world clinical practice in India, as the earlier data from India was part of a controlled clinical trial.
Materials and Methods
This was a multicenter, retrospective, single-arm study conducted across four centres in India after obtaining permission from Independent ethics committee. (Suraksha Ethics Committee: ECR/644/Inst/MH/2014/RR-17). The study was conducted in accordance with the Declaration of Helsinki and adhered to ICH-Good Clinical Practice guidelines. Confidentiality of data was maintained throughout the study period. Because this was a retrospective study in which participants were de-identified or could not be contacted, informed consent was not required, and waiver of consent was considered for the study in accordance with the Indian Council of Medical Research (ICMR) National Ethical Guidelines for Biomedical and Health Research involving Human Participants, 2017.
The study was conducted among patients with mild to moderate COVID-19 infection. Key inclusion criteria were adult patients (≥ 18 years) with clinical symptoms of COVID-19 or SARS-CoV-2 virus infected, confirmed by RT-PCR, and who were prescribed Favipiravir (Fabiflu® 200mg/400mg/800mg). The recommended dose of Favipiravir is 1800mg twice daily on day 1 and 800 mg twice daily day 2 onwards for a maximum period of 14 days.
Mild cases were defined as real time reverse transcription–polymerase chain reaction (RT-PCR) positive with oxygen saturation (SpO2) ≥ 94% on room air with or without upper respiratory symptoms (and/or fever), without breathlessness or hypoxia, whereas moderate cases were defined as RT-PCR positive with SpO2 < 94% on room air and/or presence of breathlessness or hypoxia.
Medical records from October 2020 to March 2021 were evaluated for information such as medical history, symptoms, treatment details, laboratory data, clinical result, and adverse events. Effectiveness was assessed in terms of time to clinical cure, rate of clinical cure at days 7 and 14, time to resolution of pyrexia, percentage of patients who required Auxiliary Oxygen therapy (AOT)/ Non Invasive Ventilation (NIV)/Mechanical ventilation (MV)/ Extracorporeal membrane oxygenation (ECMO) support during the study period, time from enrolment to hospital discharge, all-cause mortality rate and physician global assessment of effectiveness of Favipiravir at the end of the treatment.
Safety was assessed by lab tests (if available) and patient-reported symptoms, and recorded by the treating physician who evaluated if there were any adverse events experienced by the patients (Adverse Event (AE)/ Serious Adverse Event (SAE) /treatment discontinuation), and also, if the physician deemed the event to be related to Favipiravir. The physician’s and patients’ global assessment was also collected in order to better understand their experiences with Favipiravir in general.
Data was collected in Microsoft excel version 2019. Descriptive statistics for quantitative variables were represented as mean ± SD, median. The normality of the distribution was determined, and the mean ± SD or median was calculated based on the distribution. Qualitative variables were represented as frequency and percentages. Graphical representations will be done wherever applicable. The software used for analysis was GraphPad Instat version 3.06.
Result
The study evaluated medical records of 360 consecutive mild to moderate COVID-19 patients treated with Favipiravir, who met the inclusion criteria. For the final analysis, 358 of the 360 patients were considered. Two patients were lost to follow-up, and their data was not included in the final analysis. There were 214 males (58.46%) and 144 females (39.28%) among the 358 participants. The average age of the patients in the study was 51.80±16.45 years. Patients with mild severity made up 81.56% (n=292) of the cohort, while those with moderate severity made up 18.43% (n=66). 208 (58.10%) patients presented at least one comorbid condition. Among them, 123 (59.13%) patients had ≥2 comorbid conditions. Overall Hypertension (130, 40%) and diabetes (116,35.69%) were the two most common comorbidities reported. The mean oxygen saturation (SpO2) and respiratory rate among enrolled patients was 95.69 ± 3.16% and 21.97 ± 10.81 per min respectively (Table 1). The mean SpO2 among patients with mild and moderate COVID-19 was 96.93 ± 1.45% (median-97%) and 90.37 ± 3.08% (median-91%) respectively.
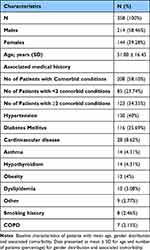 |
Table 1 Baseline Demographic Characteristics |
The most common symptom was fever, which was recorded in 325 (90.78%) patients. Cough (60.05%), myalgia (35.20%), fatigue (33.24%), sore throat (19.55%), and loss of taste (19.27%) were the other common symptoms. Dyspnoea was reported in 65 (18.15%) of the patients (Table 2). At the baseline serum ferritin, LDH, D-Dimer and CRP were 385.17 ± 475.51 ng/mL, 249.69 ± 107.77 ng/mL, 481.92 ± 737.55 and 37.01 ± 47.01 mg/L respectively (Table 3).
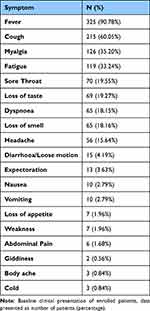 |
Table 2 Symptoms at Baseline |
 |
Table 3 Biomarkers at Baseline |
The median time to clinical cure was five days (Table 4). Clinical cure was attained by 77.93% of patients on day 7, and 93.30% by day 14 (Figure 1). The median time for fever resolution was four days. By day 7, 96% had fever resolution, and by day 10, all of them had fever resolution. (Figure 2).
 |
Table 4 Comparison of Mild and Moderate and Overall Patient Population |
At baseline, 68 (18.99%) of the patients required some form of respiratory support, which was reduced by 89.70% by day 10, and 94.11% by day 14 (Figure 3). Hospitalisation was required for 205 (57.26%) of all recruited patients. Overall, 199 (97.03%) of the 205 hospitalised patients were discharged. The median length of stay in the hospital was six days.
Mortality was reported in three patients due to disease progression. Overall, Favipiravir was well tolerated. One or more adverse events were reported by 8% of patients. The most common adverse event recorded was an increase in hepatic enzymes (4.61%), followed by diarrhoea (1.67%), decreased appetite (0.5%), headache (0.2%), fatigue (0.2%) and giddiness (0.2%). All the adverse effects were mild to moderate in severity, not necessitating the cessation of Favipiravir treatment.
A physician’s global assessment was available in 273 (76.25%) patients. According to the physician’s global assessment, the effectiveness of Favipiravir was good to very good in 88.27% (n = 241) of patients. The patients’ global assessment of effectiveness was available in 272 (75.97%) patients. According to the patients’ global assessments, 89.2% (n = 223) of Favipiravir effectiveness was good or very good.
Discussion
The present study was carried out in a real-world setting to assess the therapeutic benefit of Favipiravir in the treatment of COVID-19 patients with mild to moderate disease. The baseline characteristics of individuals involved in our study indicate that covid-19 infection appears to be more common in the elderly, men, and those with comorbidities. Favipiravir was prescribed to all patients at the appropriate dose. This was similar to a Japanese observational study12 that revealed equivalent results, with more than half (52.3%) of the patients being 60 years or older and 67.1% being male. At least one of the four comorbidities (diabetes, cardiovascular disease, chronic lung disease, and immunosuppression) was present in 49.2% of the participants. A recent meta-analysis of 9 RCTs has shown that hypertension and diabetes significantly enhanced the risk of mortality in COVID-19 patients.13
In the current study, the time for clinical cure was five days. A meta-analysis of nine clinical trials involving Favipiravir found that the drug was associated with a significant clinical improvement (vs control) after seven days of hospitalisation (RR=1.24).13 Udwadia et al7 reported a median period of three days for clinical improvement, in patients who were symptomatic at the start of the study.
This was also reported in a multicentric double-blind randomised controlled trial, where the median time to clinical recovery was seven days.14 Similarly, in a phase III placebo-controlled RCT of Moderate COVID-19 patients who did not require oxygen, patients who received Favipiravir had a three-day shorter clinical cure, with a significant difference (p = 0.0136) and a hazard ratio of 1.59 (1.02–2.48).15 In another metaanalysis16 of 11 eligible studies, 5 of which included a comparison group, the Favipiravir group had significantly greater clinical improvement on days 7 and 14. Another meta-analysis that examined standard of care (SOC) and Favipiravir as the treatment group and SOC with other antivirals and supportive care as the control group found that Favipiravir groups improved significantly on both day 7 and day 14 of the therapy (Day 7: RR 1.25, 95% CI 1.01 to 1.53; Day 14: RR 1.29, 95% CI 1.08 to 1.54).17 A randomized controlled trial evaluating the efficacy of Hydroxychloroquine (HCQ) reported median time to clinical cure as 10.5 days with HCQ and 11.5 in the placebo arm.18
In the current study, 77.93% of patients had a clinical cure by day 7, and 93.30% had it by day 14. When compared to a large observational study from Japan,12 the rates of clinical improvement at day 7 in mild, moderate, and COVID-19 patients treated with Favipiravir were 73.8%, 66.6%, and 73.8%, respectively, whereas at day 14, the rates were 87.8%, 84.5%, and 73.8%, respectively. A double-blind RCT19 comparing Ivermectin with or without doxycycline, discovered clinical cure in 60.7% and 44.4% of patients by day 7.
The efficacy of Favipiravir in COVID patients hospitalised with severe pneumonia was also studied; there was no change in the need for mechanical ventilation, dialysis, or sepsis with Favipiravir, compared to alternative active treatment. A tendency towards mortality benefit with Favipiravir was seen; however, it was not statistically significant (26.3% versus 37.2%, p>0.05).20
In this study, the most common symptom was fever. Data from the literature6 also showed that fever resolution is associated with PCR negativity in upper respiratory samples and radiological improvement. In the current study, the time for fever remission was reported to be around four days. In one study, Chen et al6 reported a six-day fever resolution with Favipiravir and an eight-day fever resolution with Umifenovir. In contrast, Favipiravir treated mild to moderate patients in a Russian RCT, which achieved fever remission by the second day.21
The need for supplemental oxygen in patients undergoing treatment may suggest disease progression. In our study, supplemental oxygen was required by 68 (18.99%) of the patients at baseline, and the need steadily dropped to 1.12% after Favipiravir treatment. This was similar to the Chen et al6 study, in which the Favipiravir arm was associated with an 18.1% necessity for supplementary oxygen therapy or non-invasive mechanical ventilation. In the Indian phase 3 RCT, 9.7% of patients receiving Favipiravir, required oxygen support.7
A meta-analysis of nine clinical trials13 on Favipiravir use found that the need for supplementary oxygen therapy was 7% lower in the Favipiravir group than in the control group (RR=0.93, 95% CI: 0.67–128; P=0.664). Early clinical cure and decreased need for oxygen has led to early hospital discharge among patients enrolled in the present study. The median time for hospital discharge in the current study was six days. This was relatively faster than the Indian phase III study,7 where the median time for hospital discharge in the Favipiravir arm was nine days, and 10 days in the standard of care arm. It could be influenced by a change in the RT-PCR testing recommendation during discharge. This was also faster than the mean hospitalisation time following Ivermectin + doxycycline, which were 10.1 days (8.5–11.8 days) and 9.6 days (7.7–11.7 days) after Ivermectin alone.22
The safety assessment of our study population was consistent with the available literature. Gastrointestinal adverse effects such as a rise in hepatic transaminase (4.61%), diarrhoea (1.67%), decreased appetite (0.5%), fatigue (0.2%), and giddiness (0.2%) were noted in 8% of the patients. These side effects, however, were transient, and did not warrant drug withdrawal. A systematic review23 of the safety analysis of Favipiravir in >4000 patients found that the drug has an established and well-characterised safety profile, with common adverse events (AEs) being gastrointestinal AEs, uric acid elevations, neutrophil count decrease, increase of aspartate aminotransferase (SGOT), increase of alanine transaminase (SGPT), psychiatric symptom reactions, and an increase in blood triglycerides. In an Indian phase III7 clinical study, AEs were recorded in 2.7% patients, with gastrointestinal and elevated hepatic enzymes being the most common; no SAEs were reported. Cai et al5 observed comparable findings in his trial, with AEs significantly lower with Favipiravir compared to the control arm (11.23% vs 55.56%, p 0.0001), with diarrhoea, anorexia, and hepatic injury recorded. A meta-analysis13 that compared adverse events in the Favipiravir and control groups reported that the Favipiravir arm had a decreased risk of adverse events, although this was not statistically significant (RR=0.77, 95% CI: 0.34–1.70; P=0.524). A recent meta-analysis12 reported that Favipiravir had tolerable safety in terms of overall and serious adverse reactions, with the most common reactions being gastrointestinal, such as nausea, diarrhoea, elevated transaminases, elevated blood uric acid, and so on, which was consistent with the conclusion of a review article.
Mortality was reported in three patients, where the investigator has linked the association with the disease progression. A Meta-analysis13 conducted revealed similar findings, namely, that the mortality rate in the Favipiravir group was approximately 30% lower than in the control group.
Conclusion
In our real-world study, Favipiravir was found to have a clinical resolution rate of more than 90% in mild to moderate COVID-19 patients. This supports the use of Favipiravir in the treatment of COVID-19. Reduced oxygen requirement has been linked to a reduced risk of illness progression. Favipiravir was well tolerated, with side effects, which were quite mild. Our findings need to be evaluated in a larger population.
Ethical Approval
The study was initiated after taking approval from the Independent ethics committee. (ECR/644/Inst/MH/2014/RR-17).
Acknowledgment
We would like to thank all the authors, and all the patients, who participated in this study, and Dr. Sagar Panchal for his assistance throughout the study process.
The abstract of this paper was presented at the National critical care conference in India, named Criticare 2021 conference, as a poster presentation with interim findings. The poster’s abstract was published in “Abstracts” in the Indian Journal of Critical Care Medicine. 2021; DOI: 10.5005/jp-journals-10071-23711.20.
Funding
No funds, grants, or other support was received.
Disclosure
Dr. Sagar Bhagat, Dr. Saiprasad Patil and Dr. Hanmant Barkate are employees of Glenmark Pharmaceutical and had helped in the data analysis and manuscript writing. Dr Shashank Joshi reports speaker fees from Glenmark, during the conduct of the study; advisor, speaker fees consultancy from Glenmark, Novo, Twin Health, Marico, PHFI, Astra Zeneca, Roche Diabetes Care, Abbott, Biocon, Novo Nordisk, Sanofi, Torrent, Zydus Cadila, Bayer Zydus, MSD, Boehringer Ingelheim, Franco India, Alkem, Cipla, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. World Health Organization. IHR emergency committee on novel coronavirus (2019-nCoV) [internet]. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-ihr-emergency-committee-on-novel-coronavirus-(2019-ncov);.
2. World Health Organization. Coronavirus (COVID-19) Dashboard [Internet]. Available from: https://covid19.who.int/.
3. Joshi S, Parkar J, Ansari A, et al. Role of Favipiravir in the treatment of COVID-19. Int J Infect Dis. 2021;102:501–508. doi:10.1016/j.ijid.2020.10.069
4. Approval of favipiravir tablets to Glenmark Pharmaceuticals and Remdesivir Injection to Cipla Ltd, Hetero Drugs and Mylan Labs. Available from: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=NjIxMw==;.
5. Cai Q, Yang M, Liu D, et al. Experimental treatment with Favipiravir for COVID-19: an open-label control study. Engineering. 2020;10:1192–1198. doi:10.1016/j.eng.2020.03.007
6. Chen C, Zhang Y, Huang Y, et al. Favipiravir versus Arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020;03. doi:10.1101/2020.03.17.20037432
7. Udwadia ZF, Singh P, Barkate H, et al. Efficacy and safety of Favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis. 2021;103:62–71. doi:10.1016/j.ijid.2020.11.142
8. Deng W, Yang C, Yang S, Chen H, Qiu Z, Chen J. Evaluation of favipiravir in the treatment of COVID-19 based on the real-world. Expert Rev Anti Infect Ther. 2022;20(4):555–565. doi:10.1080/14787210.2022.2012155
9. Favipiravir to be investigated as a possible COVID-19 treatment for at-home recovery in the PRINCIPLE trial. Available from: https://www.principletrial.org/news/Favipiravir-to-be-investigated-as-a-possible-covid-19-treatment-for-at-home-recovery-in-the-principle-trial;.
10. The prevent severe Covid-19 (PRESECO) study (PRESECO). Available from: https://clinicaltrials.gov/ct2/show/NCT04600895;.
11. Agrawal U, Raju R, Udwadia ZF. Favipiravir: a new and emerging antiviral option in COVID-19. Med J Armed Forces India. 2020;76(4):370–376. doi:10.1016/j.mjafi.2020.08.004
12. Doi Y, Hibino M, Hase R, et al. A prospective, randomized, open-label trial of early versus late Favipiravir in hospitalized patients with COVID-19. Antimicrob Agents Chemother. 2020;64. doi:10.1128/AAC.01897-20
13. Hassanipour S, Arab-Zozani M, Amani B, Heidarzad F, Fathalipour M, Martinez-de-Hoyo R. The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. Sci Rep. 2021;11(1):11022. doi:10.1038/s41598-021-90551-6
14. Bosaeed M, Alharbi A, Mahmoud E, et al. Efficacy of Favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial. Clin Microbiol Infect. 2022;28(4):602–608. doi:10.1016/j.cmi.2021.12.026
15. Shinkai M, Tsushima K, Tanaka S, et al. Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, phase III clinical trial. Infect Dis Ther. 2021;10(4):2489–2509. doi:10.1007/s40121-021-00517-4
16. Manabe T, Kambayashi D, Akatsu H, Kudo K. Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2021;21(1):489. doi:10.1186/s12879-021-06164-x
17. Shrestha DB, Budhathoki P, Khadka S, Shah PB, Pokharel N, Rashmi P. Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis. Virol J. 2020;17:141. doi:10.1186/s12985-020-01412-z
18. Johnston C, Brown ER, Stewart J, et al. COVID-19 early treatment study team. Hydroxychloroquine with or without azithromycin for treatment of early SARS-CoV-2 infection among high-risk outpatient adults: a randomized clinical trial. E Clinl Med. 2021;33:100773.
19. Mahumad R. Clinical trial of ivermectin plus doxycycline for the treatment of confirmed Covid-19 infection. Available from: https://clinicaltrials.gov/ct2/show/NCT04523831;.
20. Dheir H, Yaylaci S, Sipahi S, et al. The effectiveness of favipiravir treatment in severe COVID-19 pneumonia: a single centre experience. Konuralp Med J. 2021;13(1):4–10.
21. Ivashchenko AA, Dmitriev KA, Vostokova NV, et al. AVIFAVIR for treatment of patients with moderate COVID-19: interim results of a phase II/III multicenter randomized clinical trial. Clin Infect Dis. 2020;73:ciaa1176.
22. Ahmed S, Karim MM, Ross AG, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021;103:214–216. doi:10.1016/j.ijid.2020.11.191
23. Pilkington V, Pepperrell T, Hill A. A review of the safety of Favipiravir - a potential treatment in the COVID-19 pandemic? J Virus Erad. 2020;6(2):45. doi:10.1016/S2055-6640(20)30016-9
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



