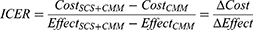Back to Journals » Journal of Pain Research » Volume 14
Real-World Cost-Effectiveness Analysis of Spinal Cord Stimulation vs Conventional Therapy in the Management of Failed Back Surgery Syndrome
Authors Rojo E, Pérez Hernández C , Sánchez Martínez N, Margarit AC, Blanco Arias T, Muñoz Martínez M, Crespo C , Ochoa Mazarro D
Received 23 June 2021
Accepted for publication 5 September 2021
Published 24 September 2021 Volume 2021:14 Pages 3025—3032
DOI https://doi.org/10.2147/JPR.S326092
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Dawood Sayed
Elena Rojo,1 Concepción Pérez Hernández,1 Noelia Sánchez Martínez,1 A César Margarit,2 Tania Blanco Arias,3 Manuel Muñoz Martínez,1 Carlos Crespo,4 Dolores Ochoa Mazarro1
1Pain Unit, La Princesa University Hospital, Madrid, Spain; 2Pain Unit, General University Hospital of Alicante, Alicante, Comunitat Valenciana, Spain; 3Pain Unit, Anderson Clinic, Madrid, Spain; 4Axentiva Solutions, Barcelona, Spain
Correspondence: Concepción Pérez Hernández
Pain Unit, La Princesa University Hospital, Calle Diego de León, 62, Madrid, 28006, Spain
Tel +34 915 20 23 75
Fax +34 914 02 11 69
Email [email protected]
Purpose: Failed back surgery syndrome (FBSS) causes disability and lowers health-related quality of life (HRQoL) for patients. Many patients become refractory to conventional medical management (CMM) and spinal cord stimulation (SCS) is advised. However, comparative cost-effectiveness research of both clinical approaches still lacks further evidence. This probabilistic cost-effectiveness analysis compares CMM versus SCS plus CMM in FBSS patients for a 5-year period in Spain.
Patients and Methods: Patient-level data was obtained from a 2-year real-world study (SEFUDOCE) of adults diagnosed with FBSS who were treated with CMM or SCS. Incremental cost-effectiveness ratios (ICER) were estimated in terms of direct clinical cost and quality-adjusted life years (QALYs). Costs (€ for 2019) were estimated from the Spanish National Health Service (NHS) perspective. We applied a yearly discount rate of 3% to both costs and outcomes and performed a probabilistic sensitivity analysis using bootstrapping.
Results: After 2 years, the health-related quality of life measured by the EQ-5D displayed greater improvements for SCS patients (00.39) than for improved CMM patients (0.01). The proportion of SCS patients using medication fell substantially, particularly for opioids (− 49%). In the statistical model projection, compared with the CMM group at year 5, the SCS group showed an incremental cost of € 15,406 for an incremental gain of 0.56 0.56 QALYs, for an ICER of € 27,330, below the € 30,000 willingness-to-pay threshold for Spain. SCS had a 79% of probability of being cost-effective.
Conclusion: SCS is a cost-effective treatment for FBSS compared to CMM alone based on real-world evidence.
Keywords: cost-effectiveness analysis, failed back surgery syndrome, modelling study, real-world evidence, spinal cord stimulation
Introduction
Failed Back Surgery Syndrome (FBSS), defined as spinal pain persisting or appearing after a surgical procedure that is meant to treat the pain,1 is prevalent in up to 19% of microdiscectomy patients and 40% of lumbar laminectomy patients.2,3 Chronic FBSS patients experience severe pain, disability, insomnia, and anxiety,4 with adverse consequences including worker absenteeism and social isolation.3,5
Spinal cord stimulation (SCS) can be effective at reducing pain and disability while improving Health Related Quality of Life (HRQoL) among FBSS patients.6 However, SCS is still mostly recommended to FBSS patients only after CMM has failed and when the pain has a neuropathic component.5,7
Proper clinical management of FBSS should aim not only to alleviate pain, but also to improve physical function and HRQoL and lower drug dependency. Since narcotic use in FBSS patients has become an issue8 accentuated by the ongoing opioid crisis,9,10 re-evaluation of clinical approaches in the treatment of FBSS is important. SCS is underutilized11,12 and this calls for an additional re-evaluation in a real-world setting. This study offers a cost-effectiveness analysis (CEA) from the perspective of the Spanish National Health System (NHS) of CMM versus SCS+CMM in the treatment of FBSS over a 5-year time frame, using real-world data from a 2-year observational study.
Methods
We performed a CEA based on the SEFUDOCE prospective observational study13 whose population received CMM or SCS+CMM in accordance with physician criteria. Both the CMM and SCS groups received pharmacological treatments.
The study was carried out on adult patients treated in the Pain Unit at La Princesa Hospital (Madrid, Spain) and at General University Hospital of Alicante (Comunidad Valenciana, Spain) between 2012 and 2016. All participants provided written informed consent; the study protocol was approved by the Ethic Committees of both institutions and was carried out in full observance of the Ethical Principles for Medical Research Involving Human Subjects (WMA Helsinki).
Study Design
SEFUDOCE patients attended 5 monitoring visits at months 3, 6, 12, 18, and 24. Direct clinical resource consumption data and effectiveness data were collected at baseline and at each monitoring visit. Beyond the 24-month observation time, costs and effects were based on the mean for the second year of observation (Figure 1). There were no statistically significant differences between groups apart from age and opioid consumption (Supplementary Figure 1, CMM-34.15; SCS-79.49, p<0.001).
 |
Figure 1 Schematic representation of the study design. Abbreviations: CMM, conventional medical management; SCS, spinal cord stimulation. |
The EQ-5D-3L assesses 5 dimensions of health, for which there are 3 levels of severity.14 We estimated utility values from the SEFUDOCE study.15 To extrapolate beyond the 24-month observed time horizon, we used the average six-monthly cost and Quality-Adjusted Life Year (QALY) measures observed in the second year (Figure 1).
Costs
SCS patients were implanted with one of two distinct rechargeable (12–25 years of battery life), percutaneous SCS systems, 83% of patients were programmed with conventional SCS systems (Tonic stimulation: 40–70 Hz; 280–420 microsec; 3,8–6 ma) and 17% with high-frequency stimulation (1000 Hz; 200 microsec; 2 ma). Additionally, we collected information on the use of other direct medical resources attributable to FBSS: medication, specialists’ visits, primary care visits, emergency room visits, ambulatory care, diagnostics tests, interventions, and hospitalisations.
We also considered the costs of patients who crossed from SCS to CMM and vice versa. Costs (€2019) came from official taxes of Madrid (Spain)16 and the drug cost database.17 We estimated the total cost per patient based on the Lin method for censored medical cost.18
The results were expressed as the incremental cost and effect ratio (ICER) between SCS+CMM vs CMM19 (costs per QALY gained).
SCS+CMM was considered cost-effective if: a) the cost was less and more effective than CMM or; b) its ICER fell below €30,000/QALY.20
We applied a yearly discount rate of 3% after the first year to both costs and effects.21
Sensitivity Analysis
We performed a probabilistic sensitivity analyses (PSA)22 by bootstrapping individual patient data.23 The bootstrap approach employs the original data in a resampling with replacement exercise to give an empirical estimate of the distribution.
For each of the 10,000 bootstrap subsamples, we calculated the mean cost and utility over time. The resulting distribution of outputs (point cloud) are shown in the cost-effectiveness plane, and cost-effectiveness acceptability curve. Additionally, we estimated the temporal distribution of the probability to be cost-effective (€20,000 and €30,000).
Results
2-Year Results
The 2-year follow-up showed that the SCS+CMM patients had an improved overall HRQoL, as measured by EQ-5D-3L utility values (from 0.22 to 0.61), while the CMM patients remained close to baseline (from 0.32 to 0.33), which translates to 0.184 QALYs more for patients in the SCS group over CMM. Moreover, the SCS+CMM patients maintained a steady increase in measured HRQoL while CMM patients followed an irregular trend (Table 1 and Figure 1). The improved HRQoL in the SCS group is corroborated by EQ-VAS (21.36 at baseline to 46.3 at 2 years for SCS+CMM, and 17.2 at baseline to 27.13 at 2 years for CMM).
According to the SEFUDOCE study, the SCS+CMM patients showed greater impairments in physical functioning, lower pain sensation, and higher HRQoL at baseline compared to the CMM patients. Final outcomes indicate that the SCS+CMM group achieved greater overall improvement compared to the CMM group, starting from a baseline of greater issues, and improving to levels similar to or, in the case of EQ-5D-3L, significantly better than the CMM group.
The SCS+CMM patients reported likely presence (>90%) of neuropathic pain at baseline measured with the Pain Detect Questionnaire; while for the CMM group, results were unclear. After 2 years, the SCS+CMM arm had achieved a reduction of 10.19 points (19.49 vs 9.3) in the Pain Detect Questionnaire average score in comparison to a decrease in 0.48 for the CMM arm (14.46 vs 14.08). These improvements were achieved in term of pain at the present moment, strongest pain in the past month, average intensity of pain in the past month, burning sensation, and light touching and numbness sensation. At the last monitoring visit, the SCS+CMM patients had moved to unlikely evidence (<15%) of neuropathic pain components, and from severe to moderate disability, while the CMM patients remained in a severe state according to the Oswestry Disability Index.
The SCS+CMM patients used more healthcare resources than the CMM group at baseline (+€875) and 3-month visits (+€18,148). The consumption for the SCS+CMM group was noticeably greater in drugs (€57 at baseline, €18 at 3-months, €45 at 6-months, €65 at 12-months and €132 at 18-months), in non-pharmacological treatments (€161 at baseline), primary care visits (€153 at baseline), specialists visits (for referral visits it was €173 higher at baseline and for non-referral visits it was €138 higher at month 3), and in diagnostic tests (€135 greater at baseline) and hospitalizations (€18,108 at month 3). At this point, higher costs for the neurostimulation group were mainly due to the costs of the SCS+CMM devices and to the implant/reoperation costs included in the hospitalization category, as well as the corresponding medical tests and visits needed beforehand. Nonetheless, from the 12-month visit onwards, the CMM group showed greater direct costs due to a higher use of specialist’s visits, ambulatory care, diagnostic tests, minimally invasive techniques, and hospitalizations. At month 18, hospitalization costs were higher for the CMM group, and one patient was switched to SCS+CMM (Table 2).
 |
Table 2 Discounted Costs of FBSS Treatments (Average Cost in Year 2019€) |
A higher number of the SCS+CMM patients needed drugs to deal with pain and anxiety/depression at baseline, 79% of these patients were taking opioids and 54% took anticonvulsants. After 2 years, the number of patients in the SCS+CMM group taking opioids, sedatives, anticonvulsants, and antidepressants had dropped overall and achieved similar percentages to the CMM group.
Drug use dropped throughout the observational period in both groups and after 2 years, the proportion of SCS+CMM patients using drugs approached the levels of the CMM group. The percentage of the SCM+CMM (vs CMM group) on medication fell by 49% (5%) opioids, 18% (12%) sedatives, 31% (7%) anticonvulsants, and 13% (7%) antidepressants. (Supplementary Figure 1).
5-Year Results
CMM patients accrued 1.90 QALYs whilst the SCS+CMM patients accrued 2.46 QALYs on average, for a difference of 0.56 QALYs over for the 5-year period. The discounted costs were €9,383 for the CMM group and €24,789.90 for the SCS+CMM group, an incremental difference of €14,406, yielding an ICER of €27,330/QALY gained. This ICER falls below the commonly accepted willingness to pay threshold of €30,000 for the Spanish NHS24 (see Table 3).
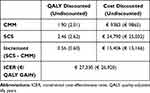 |
Table 3 Cost-Effectiveness Results at 5 Years Follow-Up |
In the PSA, the mean of the 10,000 bootstrap-based samples was cost-effective, and the 95% confidence ellipse for the mean fell fully within the cost-effective range of the CEA plane (see Figure 2). Furthermore, 99% of simulations were found in the first quadrant of the CEA plane, meaning that SCS+CMM is more effective than CMM. At 5 years, the cost-effectiveness acceptability curve indicated that SCS+CMM had a 79% probability of being cost-effective given a WTP threshold of €30,000, and a 51.7% probability given a threshold of €20,000 (see Figure 3).
 |
Figure 3 Cost-effectiveness acceptability curve for SCS treatment in failed back surgery syndrome. Abbreviations: SCS, spinal cord stimulation; QALY, quality-adjusted life years. |
As the SCS+CMM systems used in the SEFUDOCE study have an estimated battery life of at least 12 years, we have estimated the probability for the neurostimulation approach to be cost-effective for a range of time horizons of up to 12 years based on the results of our PSA. For a willingness-to-pay threshold of €20,000, the probability of the SCS+CMM to be cost-effective in the treatment of FBSS is over 70% (point estimate of 78.5%) after 6.5 years and over 90% (point estimate 92.9%) after 9.5 years. For a willingness-to-pay threshold of €30,000, the number of years for SCS+CMM to achieve greater than 70% probability to be cost-effective is below 4.5 years (point estimate 75.5%), and greater than 90% after 6.5 years (point estimate of 92%, see Figure 4).
 |
Figure 4 Cost-effectiveness probability of SCS treatment in failed back surgery syndrome per year. Abbreviation: SCS, spinal cord stimulation; QALY, quality-adjusted life years. |
Discussion
SCS treatment is still usually offered to chronic FBSS patients only after intensive and unsuccessful use of CMM alone, even though the clinical superiority of neurostimulation has been demonstrated.12 The present work adds to existing evidence on the superior results of SCS for the treatment of FBSS patients in the long-term in a Spanish setting.
The findings reported here are well-aligned with previous cost-effectiveness studies of SCS within the UK,25 Canadian,26 and Italian settings.27 However, our study is based on a distinct profile of patients: the lumbar zone is the main focus of pain in a much greater number of patients than in previous studies, all SCS patient received rechargeable batteries, and participants were not just included after a failure of conventional treatment. These studies were all based on the PROCESS trial,11,28 estimated costs and effects of the CMM and the SCS approaches over a 24-month period. Their findings suggested that SCS was an effective treatment for chronic FBSS patients, which would be cost-effective compared to CMM for thresholds of £30,000 and of $50,000 per QALY gained. Krames et al29 suggested that SCS could result in longer term cost savings due to a reduction in healthcare resources in the future. Two recent published reviews30,31 also support SCS’s cost-effectiveness against CMM in FBSS in a long-term time horizon, with Odonkor et al31 suggesting, as well, shorter hospital stays and lower complication rates and healthcare costs at 90-days.
SCS+CMM treatment showed its superiority in terms of outcomes over the conventional approach: SCS+CMM patients reported reductions in pain, functional disability, and the presence of neuropathic pain components. Manca et al32 offered evidence of statistically significant associations between generic HRQoL as measured by the EQ-5D; and measures of pain and reduced functional ability from the Oswestry Questionnaire. We found that HRQoL was considerably higher for the SCS+CMM patients in comparison to the CMM group.
As drug use in FBSS patients has increasingly become a concern,8 it is significant that the proportion of the SCS+CMM group using opioids to manage pain dropped to 30.77%. The corresponding decrease in anticonvulsant use to 23.08% in the SCS+CMM group is consistent with the Pain Detect Questionnaire results at the end of the observational period, even though SCS+CMM patients reported more pain at baseline than the CMM group.
Also, due to the number of professionals working together, pain unit management might result in an increase of effectiveness of therapy for FBSS patients as, regardless of the treatment option, a multidisciplinary approach is recommended.
Strengths and Limitations
Our analysis is in a real-world setting, to better reflect FBSS patients in a real clinical practice within a 5-year time frame.
The statistical power may be weaker due to the smaller sample size. There was a loss of follow-up information during the observational period, which was significantly greater within the CMM group at months 12 (p= 0.0291), 18 (p= 0.0024) and 24 (p= 0.0096). Moreover, the SCS+CMM patients were 7.78 years younger than CMM patients on average which could have had a potential impact on comparative results.
FBSS patients were treated with CMM or SCS+CMM based on medical criteria and not randomization. Therefore, the study shows two real populations that are not equal or idealy comparable, but it allowed for more reliable data to be obtained according to real clinical practice.
Conclusion
SCS+CMM offers improved pain relief, physical functioning, and HRQoL in FBSS patients in comparison with CMM. SCS+CMM could be cost-effective during a longer time span.
Disclosure
Dr Carlos Crespo is a member of Axentiva Solutions SL, which has received consulting fees from Boston Scientific Iberica S.A. The authors report no other potential conflicts of interest for this work.
References
1. Thomson S. Failed back surgery syndrome – definition, epidemiology and demographics. Br J Pain. 2013;7(1):56–59. doi:10.1177/2049463713479096
2. Sebaaly A, Lahoud MJ, Rizkallah M, Kreichati G, Kharrat K. Etiology, evaluation, and treatment of failed back surgery syndrome. Asian Spine J. 2018;12(23):574–585. doi:10.4184/asj.2018.12.3.574
3. Thomson S, Jacques L. Demographic characteristics of patients with severe neuropathic pain secondary to failed back surgery syndrome. Pain Pract. 2009;9(3):206–215. doi:10.1111/j.1533-2500.2009.00276.x
4. Yun SY, Kim DH, Do HY, Kim SH. Clinical insomnia and associated factors in failed back surgery syndrome: a retrospective cross-sectional study. Int J Med Sci. 2017;14(6):536–542. doi:10.7150/ijms.18926
5. Jeon YH. Spinal cord stimulation in pain management: a review. Korean J Pain. 2012;25(3):143. doi:10.3344/kjp.2012.25.3.143
6. Mekhail N, Visnjevac O, Azer G, Mehanny DS, Agrawal P, Foorsov V. Spinal cord stimulation 50 years later. Reg Anesth Pain Med. 2018;43(4):391–406. doi:10.1097/AAP.0000000000000744
7. Hussain A, Erdek M. Interventional pain management for failed back surgery syndrome. Pain Pract. 2014;14(1):64–78. doi:10.1111/papr.12035
8. Chen Y-C, Lee C-Y, Chen S-J. Narcotic addiction in failed back surgery syndrome. Cell Transplant. 2019;28(3):239–247. doi:10.1177/0963689718796072
9. Council of Economic Adviser of the White House of the United States of America. The full cost of the opioid crisis: $2.5 trillion over four years; 2019. Available from: https://trumpwhitehouse.archives.gov/articles/full-cost-opioid-crisis-2-5-trillion-four-years/. Accessed September 20, 2021.
10. National Institute on Drug Abuse. Opioid Overdose Crisis. National Institute of Health (NIH), U.S. Department of Health and Human Services; 2019. Available from: https://www.drugabuse.gov/drug-topics/opioids/opioid-overdose-crisis. Accessed September 20, 2021.
11. Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132(1):179–188. doi:10.1016/j.pain.2007.07.028
12. Dones I, Levi V. Spinal cord stimulation for neuropathic pain: current trends and future applications. Brain Sci. 2018;8(8):138. doi:10.3390/brainsci8080138
13. Perez C, Rojo E, Margarit C, et al. 24-month Real-World study of Spinal Cord Stimulation in Failed Back Surgery patients with refractory pain. Pain Physician. 2021;24(6):479–488.
14. Devlin NJ, Brooks R. EQ-5D and the EuroQol group: past, present and future. Appl Health Econ Health Policy. 2017;15(2):127–137. doi:10.1007/s40258-017-0310-5
15. Szende A, Oppe M, Devlin N. EQ-5D Value Sets: Inventory, Comparative Review and User Guide. ©2007 EuroQol Group; 2007:13–38.
16. Official Bulletin of Madrid. ORDER 727/2017, of August 7, of the minister of health, by which the public prices for the provision of the services and activities of sanitary nature of the network of centers of the community of Madrid are fixed. Madrid; 2017.
17. General Council of Official Colleges of Pharmacists of Spain. Database of Medicines. Bot PLUS 2019.
18. Lin DY. Linear regression analysis of censored medical costs. Biostatistics. 2000;1(1):35–47. doi:10.1093/biostatistics/1.1.35
19. Incremental Cost-Effectiveness Ratio (ICER). York; York Health Economics Consortium; 2016. Available from: https://yhec.co.uk/glossary/incremental-cost-effectiveness-ratio-icer/. Accessed September 20, 2021.
20. Sacristán JA, Oliva J, Del Llano J, Prieto L, Pinto JL. ¿Qué es una tecnología sanitaria eficiente en España? [What is an efficient health technology in Spain?]. Gac Sanit. 2002;16(4):334–343. Spanish. doi:10.1016/S0213-9111(02)71933-X
21. López-Bastida J, Oliva J, Antoñanzas F, et al. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ. 2010;11(5):513–520. doi:10.1007/s10198-010-0244-4
22. Gray AM, Clarke PM, Wolstenholme JL, Wordsworth S. Applied Methods of Cost-Effectiveness Analysis in Health Care. Oxford University Press; 2011.
23. Campbell MK, Torgerson DJ. Bootstrapping: estimating confidence intervals for cost-effectiveness ratios. QJM. 1999;92(3):177–182. doi:10.1093/qjmed/92.3.177
24. Sacristán JA, Oliva J, Campillo-Artero C, et al. ¿Qué es una intervención sanitaria eficiente en España en 2020? [What is an efficient health technology in Spain in 2020?]. Gac Sanit. 2020;34(2):189–193. Spanish. doi:10.1016/j.gaceta.2019.06.007
25. Taylor RS, Ryan J, O’Donnell R, Eldabe S, Kumar K, North RB. The cost-effectiveness of spinal cord stimulation in the treatment of failed back surgery syndrome. Clin J Pain. 2010;26(6):463–469. doi:10.1097/AJP.0b013e3181daccec
26. Kumar K, Rizvi S. Cost-effectiveness of spinal cord stimulation therapy in management of chronic pain. Pain Med. 2013;14(11):1631–1649. doi:10.1111/pme.12146
27. Zucco F, Ciampichini R, Lavano A, et al. Cost-effectiveness and cost-utility analysis of spinal cord stimulation in patients with failed back surgery syndrome: results from the PRECISE study. Neuromodulation. 2015;18(4):266–276. doi:10.1111/ner.12292
28. Kumar K, North R, Taylor R, et al. Spinal cord stimulation vs. conventional medical management: a Prospective, Randomized, Controlled, Multicenter Study of Patients with Failed Back Surgery Syndrome (PROCESS study). Neuromodulation. 2005;8(4):213–218. doi:10.1111/j.1525-1403.2005.00027.x
29. Krames ES, Monis S, Poree L, Deer T, Levy R. Using the SAFE principles when evaluating electrical stimulation therapies for the pain of failed back surgery syndrome. Neuromodulation. 2011;14(4):299–311. doi:10.1111/j.1525-1403.2011.00373.x
30. Niyomsri S, Duarte RV, Eldabe S, et al. A systematic review of economic evaluations reporting the cost-effectiveness of spinal cord stimulation. Value Health. 2020;23(5):656–665. doi:10.1016/j.jval.2020.02.005
31. Odonkor CA, Orman S, Orhurhu V, Stone ME, Ahmed S. Spinal cord stimulation vs conventional therapies for the treatment of chronic low back and leg pain: a systematic review of health care resource utilization and outcomes in the last decade. Pain Med. 2019;20(12):2479–2494. doi:10.1093/pm/pnz185
32. Manca A, Eldabe S, Buchser E, Kumar K, Taylor RS. Relationship between health-related quality of life, pain, and functional disability in neuropathic pain patients with failed back surgery syndrome. Value Health. 2010;13(1):95–102. doi:10.1111/j.1524-4733.2009.00588.x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.