Back to Journals » Patient Preference and Adherence » Volume 17
Real-World Comparison of First-Line Treatment Adherence Between Single-Agent Ibrutinib and Acalabrutinib in Patients with Chronic Lymphocytic Leukemia
Authors Lu X, Emond B , Morrison L, Kinkead F, Lefebvre P, Lafeuille MH, Khan W, Wu LH, Qureshi ZP, Jacobs R
Received 21 April 2023
Accepted for publication 8 August 2023
Published 23 August 2023 Volume 2023:17 Pages 2073—2084
DOI https://doi.org/10.2147/PPA.S417180
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Xiaoxiao Lu,1 Bruno Emond,2 Laura Morrison,2 Frederic Kinkead,2 Patrick Lefebvre,2 Marie-Hélène Lafeuille,2 Wasiulla Khan,1 Linda H Wu,1 Zaina P Qureshi,1 Ryan Jacobs3
1Real World Value and Evidence, Oncology, Janssen Scientific Affairs, LLC, Horsham, PA, USA; 2Health Economics and Outcomes Research, Analysis Group, Inc, Montréal, Québec, Canada; 3Hematology and Medical Oncology, Atrium Health Levine Cancer Institute, Charlotte, NC, USA
Correspondence: Bruno Emond, Analysis Group, Inc, 1190 avenue des Canadiens-de-Montréal, Tour Deloitte, Suite 1500, Montréal, Quebec, H3B 0G7, Canada, Tel +1-514-394-4455, Fax +1-514-394-4461, Email [email protected]
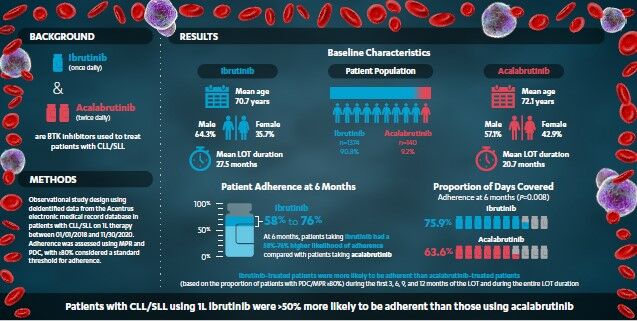
Purpose: Increased dosing frequency adversely affects treatment adherence and outcomes in chronic diseases; however, such data related to treatment adherence is lacking in chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). This study compared adherence between patients treated with ibrutinib (once-daily) versus acalabrutinib (twice-daily) as first-line (1L) therapy for CLL/SLL.
Patients and Methods: Specialty pharmacy electronic medical records were used to identify adults with CLL/SLL initiating 1L ibrutinib or acalabrutinib between 01/01/2018 and 11/30/2020. Adherence was measured by the proportion of days covered (PDC) and medication possession ratio (MPR) and was compared between cohorts using odds ratios (ORs) obtained from logistic regression models adjusted for baseline characteristics.
Results: Between 01/01/2018 and 11/30/2020, 1374 and 140 patients initiated ibrutinib and acalabrutinib, respectively. Based on PDC/MPR ≥ 80%, patients treated with once-daily ibrutinib were more likely to be adherent than those treated with twice-daily acalabrutinib (OR ranges: PDC: 1.04– 1.76; MPR: 1.03– 1.58). At 6 months, patients on ibrutinib had a 58– 76% higher likelihood of staying adherent compared to patients on acalabrutinib (PDC: 75.9% for ibrutinib vs 63.6% for acalabrutinib, OR: 1.76, P= 0.008; MPR: 76.8% vs 66.9%, OR: 1.58, P= 0.036) with a similar trend noted for the entire line of treatment (LOT) (PDC: 53.0% vs 41.4%, OR: 1.53, P= 0.021; MPR: 58.7% vs 47.1%, OR: 1.50, P= 0.027).
Conclusion: In this real-world analysis, CLL/SLL patients initiating 1L once-daily ibrutinib had > 50% higher treatment adherence than those initiating twice-daily acalabrutinib during their LOT. Given the importance of sustained adherence for disease control in CLL/SLL, dosing frequency may be an important consideration for patients and physicians.
Keywords: CLL/SLL, Bruton’s tyrosine kinase inhibitor, treatment compliance, proportion of days covered, medication possession ratio
Graphical Abstract:
Introduction
The need to manage multiple chronic conditions is common in older populations,1 as more than 60% of Americans over the age of 65 have two or more chronic conditions.2 Therefore, polypharmacy (ie, simultaneous treatment with multiple medications) is an important consideration in this population, and consequences of such a phenomenon include increased health-care costs, adverse drug events, increased potential for drug interactions, and treatment nonadherence.3 Simplifying dosing regimens and the frequency of uptake can help avoid subsequent adverse clinical and health-care consequences.
Chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) is a lymphoid malignancy affecting B cells and is the most common leukemia in adults above 65 years old; in the United States (US), the estimated number of new cases of CLL/SLL in the adult population for 2022 was 20,160.4,5 Among available treatment options, once-daily ibrutinib6 and twice-daily acalabrutinib7 are Bruton’s tyrosine kinase inhibitors (BTKis) approved by the US Food and Drug Administration as oral targeted therapies for CLL/SLL, with ibrutinib currently being a standard of care for first-line (1L) treatment in patients with CLL/SLL.8
To date, based on insurance claims data on file from the IQVIA database, more than 250,000 CLL/SLL patients have been treated with ibrutinib, with demonstrable efficacy in patients with high-risk cytogenetics.9,10 Ibrutinib is also the only targeted therapy to demonstrate improved overall survival (OS) compared to chemo and/or immunotherapy in multiple Phase 3 clinical trials in 1L or relapsed/refractory CLL/SLL.11–16 In November 2019, acalabrutinib, a newer BTKi administered twice daily, was approved for 1L or relapsed/refractory CLL/SLL.17–19 While data on progression-free survival (PFS) and response for acalabrutinib has previously been reported in 1L,20 OS data is still accumulating.
The proportion of days covered (PDC) and medication possession ratio (MPR) are both commonly used and validated measures to evaluate adherence using electronic medical records (EMR).21,22 Additionally, PDC is recommended by the Pharmacy Quality Alliance as a measure of treatment adherence, particularly for treatments used over a long period of time (eg, for chronic conditions).21 While clinical outcomes (including safety and efficacy) associated with ibrutinib and acalabrutinib have been evaluated in clinical trials, there is limited data comparing the real-world treatment adherence of these two agents. Considering disease chronicity, assessing adherence becomes important as sustained adherence to medication is essential to achieve and maintain optimal clinical outcomes for patients with CLL/SLL.23,24 Therefore, the current real-world study was conducted to describe and compare treatment adherence between patients with CLL/SLL treated with 1L once-daily ibrutinib or twice-daily acalabrutinib in the US.
Materials and Methods
Data Source
This study used EMR data from the Acentrus database between January 1, 2017, to November 30, 2020, to select patients diagnosed with CLL/SLL who started treatment with 1L ibrutinib or acalabrutinib between January 1, 2018, and November 30, 2020. Acentrus is a health system solution used by specialty pharmacies (including 128,000 prescribers), where BTKis are typically dispensed.25,26 It includes inpatient and outpatient data from 27 sites, including 10 National Cancer Institute designated sites, and 6 National Comprehensive Cancer Network members. Records of patients from 15 academic and 12 non-teaching hospital systems across 15 US states are available (ie, Arizona, California, Florida, Kansas, Massachusetts, Missouri, North Carolina, North Dakota, New York, Ohio, Tennessee, Texas, Virginia, Washington, and West Virginia), thus providing data on the experience of a large number of patients treated in a diverse set of hospital systems across the US. The database provides rich information on medication orders/fills/administrations, which are key to evaluate the main outcome of the study, ie, adherence. It also includes information on patients’ demographic characteristics, insurance plan, medications, visits, date of death, diagnoses, and clinical characteristics including laboratory test results, and vitals. Acentrus is a provider-based data source, in which records are available to the extent that visits are part of the network of academic and non-teaching hospital systems included in the Acentrus data, ie, the database does not capture services that patients receive from a provider outside of the network. Data in the Acentrus database is de-identified and complies with patients’ requirements of the Health Insurance Portability and Accountability Act (HIPAA).
Study Design
A retrospective cohort design was used. The index date was defined as the date of initiation of ibrutinib or acalabrutinib in 1L between January 1, 2018, and November 30, 2020. A washout period of ≥12 months of data availability without use of any antineoplastic agents prior to the index date was required to confirm use as 1L therapy. The same period was used to establish the baseline period for the evaluation of patient characteristics. Patients were required to have received no other antineoplastic agents in the 28 days on or after the index date. This time period was selected to ensure that the entire regimen used in the first cycle was observed, since treatment cycles with ibrutinib and acalabrutinib typically last for 28 days.6,7 The follow-up period spanned from the index date to the earliest initiation of second-line (2L) treatment, censoring (based on the rules discussed in the paragraph below), death, or end of study period (November 30, 2020).
Line of Therapy Definition
The 1L therapy period used to evaluate adherence was defined as the period from the initiation of 1L treatment until the earliest of initiation of 2L treatment, death, or end of the study period (November 30, 2022). To allow for a comparison of adherence during a period when patients only received ibrutinib or acalabrutinib, patients with a within-class BTKi switch (ie, 2L treatment is also a BTKi) were censored at the time of switch, as this may have indicated a switch due to tolerability rather than disease progression. Patients who added an anti-CD20 antibody (ie, obinutuzumab or rituximab) or venetoclax to the index BTKi within 180 days post-index were censored at the time of add-on, as these may not have indicated overt disease progression but rather late initiation of a second anti-cancer agent as part of a 1L combination treatment strategy. The 180-day timeframe was selected based on prescribing guidelines, all recommending initiation of the additional agent within the first 6 months (cycles 1–2 for the anti-CD20 agent or cycles 3–4 for venetoclax, allowing for some delay in initiation until 6 months post-index).6,7,27–29 Beyond the first 180 days post-index, anti-CD20 or venetoclax add-ons triggered the start of 2L treatment.
Study Population
Patients were required to meet the following inclusion criteria: ≥2 diagnoses for CLL/SLL ≥30 days apart, including one diagnosis prior to the index date; ≥1 order, fill, or administration for ibrutinib or acalabrutinib; ≥12 months of data availability before the index date (to confirm use of ibrutinib or acalabrutinib in 1L); ≥28 days of data availability after the index date; initiation of ibrutinib or acalabrutinib in 1L between January 1, 2018, and November 30, 2020; and ≥18 years of age as of the index date. Patients were excluded if they had ≥1 diagnosis of end-stage renal disease prior to the index date or ≥2 diagnoses of other blood cancers ≥30 days apart, evaluated from 24 months prior to the index date to 6 months prior to their first CLL/SLL diagnosis.
Study Measures
Patient demographics (ie, age, sex at birth, race, year of the index date, US region and type of insurance) and clinical characteristics (ie, Quan-Charlson Comorbidity Index [Quan-CCI], comorbidities, and use of other medications) were evaluated during the baseline period (up to 12 months prior to the index date). PDC and MPR were used as measures of adherence during the first 3, 6, 9, and 12 months of the ibrutinib or acalabrutinib line of therapy (LOT), as well as over the duration of the entire LOT. PDC was defined as the sum of non-overlapping days of supply over the period of interest (ie, first 3, 6, 9, and 12 months of the LOT, as well as the entire LOT duration), where prescriptions with overlapping days of supply are shifted forward, divided by the duration of the period of interest. MPR was defined as the number of days of medication supplied during the period of interest (ie, first 3, 6, 9, and 12 months of the LOT, as well as the entire LOT duration) divided by the duration of the period of interest and was capped at 100% to account for potential stockpiling of medication. Supplementary Figure 1 provides a summary of the calculation for PDC and MPR.
Statistical Analysis
All analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC). Characteristics reported during the baseline period were described using means, standard deviations (SDs), and medians for continuous variables, and frequencies and proportions for categorical variables. P-values for the comparison of baseline characteristics between cohorts were obtained using t-tests for continuous variables and chi-squared tests for categorical variables.
Mean, SD, and median PDC and MPR were reported, in addition to the proportion of patients with PDC or MPR above specific thresholds (ie, ≥50%, ≥60%, ≥70%, ≥80%, and ≥90%; with ≥80% considered a standard threshold to consider patients as adherent).30,31 Mean differences (MDs) with 95% confidence intervals (CIs) and P-values obtained from ordinary least-squares regression models were used to compare mean PDC and MPR between cohorts. Odds ratios (ORs) with 95% CIs and P-values obtained from logistic regression models were used to compare the proportion of patients with PDC or MPR above specific thresholds. Models were adjusted for baseline characteristics, which included standard controls of age, sex at birth, region, race, year of index date, and baseline Quan-CCI, with the addition of other variables that may have also affected differences in adherence between the two cohorts such as cardiopulmonary disease (CPD), peripheral vascular disease, hypertension, atrial fibrillation (AF), metastatic cancer, use of corticosteroids, and use of antiplatelets.
Results
The Acentrus EMR database contained 3712 patients with ≥2 CLL/SLL diagnoses. In total, 2824 patients met the study inclusion/exclusion criteria (Figure 1). Among them, 1374 and 140 patients initiated 1L ibrutinib and 1L acalabrutinib, respectively, between January 1, 2018, and November 30, 2020.
Patient Baseline Characteristics
Among the ibrutinib and acalabrutinib cohorts, respectively, the mean age was 70.7 and 72.1 years (P=0.109), 35.7% and 42.9% were female (P=0.095), the mean (median) length of follow-up time was 32.0 (33.2) and 23.8 (22.2) months, and the mean (median) LOT duration was 27.5 (28.9) and 20.7 (21.1) months (Table 1 and Table 2). US region varied between ibrutinib and acalabrutinib cohorts (South: 27.8% vs 41.4% [P<0.001]; Midwest: 26.1% vs 16.4% [P=0.012]). The mean Quan-CCI was 3.0 for ibrutinib and acalabrutinib (P=0.707) and a higher proportion of patients in the ibrutinib cohort had hypertension (37.8% vs 29.3%; P=0.046) in the baseline period. Baseline corticosteroid use was lower in the ibrutinib cohort (13.7% vs 25.0%; P<0.001).
 |
Table 1 Baseline Demographic and Clinical Characteristics Evaluated in the 12-Month Baseline Period |
 |
Table 2 Comparison of Mean Adherence Between Patients Treated with 1L Ibrutinib and Acalabrutinib |
Treatment Adherence
Overall, at 6 months, patients on ibrutinib had a 58–76% higher likelihood of staying adherent as compared to patients on acalabrutinib, with a similar trend observed for the entire LOT (ibrutinib 50–53% more likely to be adherent).
Based on the proportion of patients with a PDC ≥ 80%, during the first 3, 6, 9, and 12 months of the LOT, and over the entire LOT duration, ibrutinib-treated patients were consistently more adherent than acalabrutinib-treated patients. ORs for the time points considered ranged from 1.04 to 1.76 (Figure 2). Results were statistically significant at 6 months into the LOT with 75.9% and 63.6% of patients adherent in the ibrutinib and acalabrutinib cohorts, respectively (OR: 1.76, P=0.008), and over the entire LOT duration, with 53.0% and 41.4% of patients adherent (OR: 1.53, P=0.021). Mean PDC for ibrutinib-treated patients was also higher than for acalabrutinib-treated patients at all time points (MD range of 0.03 to 0.07), with results reaching statistical significance at 6 months (0.85 for ibrutinib vs 0.77 for acalabrutinib; MD: 0.07, P=0.010), 9 months (0.80 for ibrutinib vs 0.73 for acalabrutinib; MD: 0.07, P=0.017), and over the entire LOT duration (0.69 for ibrutinib vs 0.62 for acalabrutinib; MD: 0.07, P=0.034) (Table 2).
Similar outcomes were observed using MPR to measure adherence. Based on the proportion of patients with an MPR of ≥80%, ibrutinib-treated patients were more adherent than acalabrutinib-treated patients during the first 3, 6, 9, and 12 months of the LOT, and over the entire LOT duration. ORs for the time points considered ranged from 1.03 to 1.58 (Figure 3). Results were statistically significant at 6 months with 76.8% and 66.9% of patients adherent in the ibrutinib and acalabrutinib cohorts, respectively (OR: 1.58, P=0.036), and over the entire LOT duration, with 58.7% and 47.1% of patients adherent (OR: 1.50, P=0.027). Mean MPR for ibrutinib-treated patients was also higher than for acalabrutinib-treated patients at all time points (MD range of 0.03 to 0.06), with results reaching statistical significance at 6 months (0.85 for ibrutinib vs 0.78 for acalabrutinib; MD: 0.06, P=0.016), and 9 months (0.82 for ibrutinib vs 0.75 for acalabrutinib; MD: 0.06, P=0.041) (Table 2).
Discussion
For chronic diseases like CLL/SLL requiring continuous BTKi therapy, several factors can impact adherence, and sustained medication adherence is needed to achieve optimal outcomes. Thus, it becomes imperative to understand real-world adherence patterns with continuous BTKi therapies. This real-world study compared treatment adherence of patients in the US diagnosed with CLL/SLL who were treated with once-daily ibrutinib or twice-daily acalabrutinib in 1L. Using PDC and MPR as measures of adherence, results show that at 6 months and during the entire LOT, patients on ibrutinib had >50% higher likelihood of staying adherent compared to patients on acalabrutinib, with consistently higher adherence at all other time points evaluated as well.
To our knowledge, this is the first real-world study comparing treatment adherence among BTKis in 1L patients with CLL. Real-world adherence to ibrutinib has been previously characterized, but in different settings. One retrospective chart review study found that among patients treated at North Carolina Cancer Hospital for at least 6 months, the mean adherence rate measured by MPR was 91.7% (range: 84.4% to 100%).32 A second follow-up study reported a mean PDC of 95% (range: 65–100%) among patients treated with ibrutinib for at least 6 months.33 The mean PDC and MPR reported in the current study for patients treated with ibrutinib with at least 6 months of LOT duration were similarly high at 85% and may be more generalizable, as the data came from a larger cohort of 1374 patients treated with ibrutinib in 15 academic and 12 non-teaching hospital systems across 15 US states (compared to only 32–149 patients from one or two centers).
Our finding that treatment adherence was higher among patients treated with once-daily ibrutinib than those treated with twice-daily acalabrutinib is consistent with what has been observed for the treatment of other chronic diseases. A meta-analysis investigating dosing frequency and medication adherence across 51 studies of various chronic disease states reported a statistically significant relationship between medication adherence and dosing frequency, where patients treated for chronic diseases are more adherent to treatments that require less frequent dosing.34 Specifically, regimen adherence was 13.5% lower among patients on twice-daily treatment regimens than those on once-daily regimens.34 These findings are likely associated with the convenience of having to take a treatment less frequently.35
Adherence to a prescribed treatment regimen is a key component of the management of chronic diseases. High adherence to treatment has been associated with longer time to disease progression,24 fewer acute or adverse events,36,37 and overall increased quality of life.38 In a retrospective analysis of a phase 3 clinical trial for ibrutinib, patients missing ≥8 consecutive days of medication were associated with a shorter median PFS.24 More generally, one meta-analysis across 63 chronic disease studies showed that adherence to treatment reduces the risk for a null or poor treatment outcome by 26% relative to nonadherence, and the odds of a good outcome were almost three times higher for adherent patients.39 Additionally, the economic costs of treatment nonadherence are high, with an estimated US $100 to $300 billion avoidable health-care costs attributed to medication nonadherence annually.40,41 Thus, treatments requiring less frequent dosing such as ibrutinib that are associated with higher patient adherence, and that offer the option of dose flexibility6 may result in more favorable patient outcomes and reduced health-care costs relative to their twice-daily counterparts, which require more frequent administration.
This study was subject to certain limitations. For instance, the analyses were based on EMR data, which may contain inaccuracies or omissions; however, these are expected to be random and affect all patients equally and should not bias the conclusions made here. In addition, Acentrus is a provider-based data source in which records are only captured for visits to a network of academic and non-teaching hospital centers included in the Acentrus data. Therefore, any services that patients receive from providers outside of the network are not captured in the database. However, even if patients may change providers to obtain different medications, they tend to obtain all prescriptions for the same medication through a single provider, which allows the analysis of treatment patterns for a specific medication. Prescription fills were assumed to indicate that patients were utilizing their medication; however, patients may not necessarily adhere to the treatment regimen as prescribed. There may also be residual confounding remaining in adjusted analyses due to unmeasured confounders. However, the Acentrus database was rich in demographic and clinical information which, for example, enabled the calculation of the Quan-CCI and reporting of individual comorbidities and use of other medications. This allowed models to be adjusted for many key confounders, which would not be possible in other databases. Of note, in the current study, the number of patients initiated on ibrutinib was much higher than the number of patients initiated on acalabrutinib, given that the period available for this analysis was from January 1, 2018, to November 30, 2020. However, this was adjusted for in regression analyses by accounting for the year during which treatment was initiated. Additionally, the study used a washout period of 12 months to identify the use of ibrutinib or acalabrutinib in 1L. While this is a common definition used extensively in real-world studies,42–44 it is possible that patients with longer remission who received a prior line of therapy were included in the sample. However, the expectation would be that these patients, if present, would be balanced between the two groups. Finally, while results may not be generalizable to all patients treated with 1L ibrutinib or acalabrutinib, this real-world study is one of the largest reflecting the experience of patients treated in academic and non-teaching centers, as it covered many sites across the US.
Conclusion
In this real-world study of patients with CLL/SLL in the US treated with once-daily ibrutinib or twice-daily acalabrutinib in 1L, patients who were treated with ibrutinib demonstrated higher adherence to treatment than those treated with acalabrutinib. Specifically, patients on ibrutinib had a >50% higher likelihood of staying adherent compared to patients on acalabrutinib during their LOT. These findings support what is known about the role of lower dosing frequency in achieving higher treatment adherence and better outcomes in patients with chronic diseases. Given the importance of medication adherence in achieving disease control in CLL/SLL (in addition to other factors such as efficacy, tolerability, safety, and past experience treating patients with the medication), dosing frequency may be an important consideration for patients and physicians when considering treatment options in the 1L setting.
Abbreviations
1L, first-line; 2L, second-line; 2L+, second or later-line; AF, atrial fibrillation; BTKi, Bruton’s tyrosine kinase inhibitor; CI, confidence interval; CIT, chemoimmunotherapy; CLL, chronic lymphocytic leukemia; CPD, cardiopulmonary disease; EMR, electronic medical records; HIPAA, Health Insurance Portability and Accountability Act; KM, Kaplan–Meier; LOT, line of therapy; MD, mean difference; MPR, medication possession ratio; OR, odds ratio; OS, overall survival; PDC, proportion of days covered; PFS, progression-free survival; Quan-CCI, Quan-Charlson Comorbidity Index; SD, standard deviation; SLL, small lymphocytic lymphoma; US, United States.
Data Sharing Statement
The data that support the findings of this study are available from Acentrus, but restrictions apply to the availability of these data, which were used pursuant to a data use agreement. The data are available through requests made directly to Acentrus, subject to Acentrus’ requirements for data access.
Ethics Approval and Informed Consent
The data used in this study were anonymized and comply with the Health Insurance Portability and Accountability Act; therefore, no reviews by an institutional review board were required per Title 45 of CFR, Part 46.101(b)(4).
Acknowledgments
Medical writing assistance was provided by Lilian Diaz, MScPH, and Christine Tam, MSc, employees of Analysis Group, Inc, which has provided paid consulting services to Janssen Scientific Affairs, LLC. Part of the material in this manuscript was presented at the Lymphoma, Leukemia & Myeloma Congress, held in New York, NY, October 18–22, 2022.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Janssen Scientific Affairs, LLC. The sponsor was involved in the study design; data collection, analysis, and interpretation; manuscript writing; and the decision to publish the article.
Disclosure
XL, WK, LHW, and ZPQ are employees of Janssen Scientific Affairs, LLC, and stockholders of Johnson & Johnson. BE, LM, FK, PL, and MHL are employees of Analysis Group, Inc., which has provided paid consulting services to Janssen Scientific Affairs, LLC. RJ reports the following: consultancy (Pharmacyclics, AbbVie, AstraZeneca, Verastem, Genentech), research funding (Pharmacyclics, TG Therapeutics, Tenobio, and MEI Pharma), and speakers bureau (Pharmacyclics, AbbVie, AstraZeneca, Janssen, and BeiGene). He also reports personal fees from Securabio and Lilly, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Jaul E, Barron J. Age-related diseases and clinical and public health implications for the 85 years old and over population. Front Public Health. 2017;5:335. doi:10.3389/fpubh.2017.00335
2. Ward B, Schiller J. Prevalence of multiple chronic conditions among us adults: estimates from the national health interview survey. Prev Chronic Dis. 2013;10:E65. doi:10.5888/pcd10.120203
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57–65. doi:10.1517/14740338.2013.827660
4. Hallek M, Al-Sawaf O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol. 2021;96(12):1679–1705. doi:10.1002/ajh.26367
5. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708
6. Janssen Biotech, Inc.. IMBRUVICA (Ibrutinib) Prescribing Information. Horsham, PA: Janssen Biotech, Inc.; 2022.
7. AstraZeneca Pharmaceuticals LP. CALQUENCE (Acalabrutinib) Prescribing Information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2022.
8. Patel K, Pagel JM. Current and future treatment strategies in chronic lymphocytic leukemia. J Hematol Oncol. 2021;14(1):1–20. doi:10.1186/s13045-021-01054-w
9. Ahn IE, Tian X, Wiestner A. Ibrutinib for chronic lymphocytic leukemia with TP53 alterations. N Engl J Med. 2020;383(5):498–500. doi:10.1056/NEJMc2005943
10. O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a Phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–1418. doi:10.1016/s1470-2045(16)30212-1
11. Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. doi:10.1056/NEJMoa1400376
12. Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437. doi:10.1056/NEJMoa1509388
13. Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(1):43–56. doi:10.1016/S1470-2045(18)30788-5
14. Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432–443. doi:10.1056/NEJMoa1817073
15. Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–2528. doi:10.1056/NEJMoa1812836
16. Fraser G, Cramer P, Demirkan F, et al. Updated results from the phase 3 HELIOS study of ibrutinib, bendamustine, and rituximab in relapsed chronic lymphocytic leukemia/small lymphocytic lymphoma. Leukemia. 2019;33(4):969–980. doi:10.1038/s41375-018-0276-9
17. AstraZeneca. Calquence approved in the US for adult patients with chronic lymphocytic leukaemia. Available from: https://www.astrazeneca.com/media-centre/press-releases/2019/calquence-approved-in-the-us-for-adult-patients-with-chronic-lymphocytic-leukaemia-21112019.html#!.
18. Ghia P, Pluta A, Wach M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2849–2861. doi:10.1200/JCO.19.03355
19. Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278–1291. doi:10.1016/S0140-6736(20)30262-2
20. Sharman JP, Egyed M, Wojciech J, et al. Acalabrutinib ± obinutuzumab versus obinutuzumab + chlorambucil in treatment-naïve chronic lymphocytic leukemia: five-year follow-up of ELEVATE-TN. In:
21. Forbes CA, Deshpande S, Sorio-Vilela F, et al. A systematic literature review comparing methods for the measurement of patient persistence and adherence. Curr Med Res Opin. 2018;34(9):1613–1625. doi:10.1080/03007995.2018.1477747
22. Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7–8):1280–1288. doi:10.1345/aph.1H018
23. Barr PM, Robak T, Owen C, et al. Sustained efficacy and detailed clinical follow-up of first-line ibrutinib treatment in older patients with chronic lymphocytic leukemia: extended phase 3 results from RESONATE-2. Haematologica. 2018;103(9):1502–1510. doi:10.3324/haematol.2018.192328
24. Barr PM, Brown JR, Hillmen P, et al. Impact of ibrutinib dose adherence on therapeutic efficacy in patients with previously treated CLL/SLL. Blood. 2017;129(19):2612–2615. doi:10.1182/blood-2016-12-737346
25. Janssen Biotech. IMBRUVICA (Ibrutinib) Specialty Pharmacies and Distributors. Janssen Biotech; 2021.
26. AstraZeneca. CALQUENCE (Acalabrutinib) Distribution Card. AstraZeneca; 2022.
27. Davids M, Lampson B, Tyekucheva S, et al. Acalabrutinib, venetoclax, and obinutuzumab as frontline treatment for chronic lymphocytic leukaemia: a single-arm, open-label, phase 2 study. Lancet Oncol. 2021;22(10):1391–1402. doi:10.1016/S1470-2045(21)00455-1
28. Davids M, Mato A, Hum J, et al. Majic: a phase 3 prospective, multicenter, randomized, open-label trial of acalabrutinib plus venetoclax versus venetoclax plus obinutuzumab in previously untreated chronic lymphocytic leukemia or small lymphocytic lymphoma. Blood. 2021;138(Supplement 1):1553. doi:10.1182/blood-2021-148155
29. Jain N, Keating M, Thompson P, et al. Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med. 2019;380(22):2095–2103. doi:10.1056/NEJMoa1900574
30. Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25(9):2303–2310. doi:10.1185/03007990903126833
31. Pednekar P, Malmenas M, Agh T, Bennett B, Peterson A. Measuring multiple medication adherence–which measure when? Value Outcomes Spotlight. 2017;2017:17–20.
32. Garner LM, Kline T, Miller J, et al. Impact of adherence to ibrutinib on clinical outcomes in real-world patients with chronic lymphocytic leukemia. J Adv Pract Oncol. 2021;12(1):20–28. doi:10.6004/jadpro.2021.12.1.2
33. Collins J, Stump SE, Heiling H, et al. Impact of adherence to ibrutinib on clinical outcomes in real-world patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2022;63(8):1823–1830. doi:10.1080/10428194.2022.2045597
34. Coleman CI, Limone B, Sobieraj DM, et al. Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm. 2012;18(7):527–539. doi:10.18553/jmcp.2012.18.7.527
35. Reginster JY, Rabenda V, Neuprez A. Adherence, patient preference and dosing frequency: understanding the relationship. Bone. 2006;38(4 Suppl 1):S2–6. doi:10.1016/j.bone.2006.01.150
36. Santoleri F, Sorice P, Lasala R, Rizzo RC, Costantini A. Patient adherence and persistence with Imatinib, Nilotinib, Dasatinib in clinical practice. PLoS One. 2013;8(2):e56813. doi:10.1371/journal.pone.0056813
37. Gillespie CW, Morin PE, Tucker JM, Purvis L. Medication adherence, health care utilization, and spending among privately insured adults with chronic conditions in the United States, 2010–2016. Am J Med. 2020;133(6):690–704 e19. doi:10.1016/j.amjmed.2019.12.021
38. Fernandez-Lazaro CI, Garcia-Gonzalez JM, Adams DP, et al. Adherence to treatment and related factors among patients with chronic conditions in primary care: a cross-sectional study. BMC Fam Pract. 2019;20(1):132. doi:10.1186/s12875-019-1019-3
39. DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811. doi:10.1097/00005650-200209000-00009
40. Cutler RL, Fernandez-Llimos F, Frommer M, Benrimoj C, Garcia-Cardenas V. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open. 2018;8(1):e016982. doi:10.1136/bmjopen-2017-016982
41. Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35–44. doi:10.2147/RMHP.S19801
42. Huang Q, Emond B, Lafeuille MH, et al. Healthcare resource utilization and costs associated with first-line ibrutinib compared to chemoimmunotherapy treatment among Medicare beneficiaries with chronic lymphocytic leukemia. In:
43. Narezkina A, Akhter N, Lu X, et al. Real-world persistence and time to next treatment with ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma including patients at high risk for atrial fibrillation or stroke. Clin Lymphoma Myeloma Leuk. 2022;22(11):e959–e971. doi:10.1016/j.clml.2022.07.004
44. Kabadi SM, Near A, Wada K, Burudpakdee C. Treatment patterns, adverse events, healthcare resource use and costs among commercially insured patients with mantle cell lymphoma in the United States. Cancer Med. 2019;8(17):7174–7185. doi:10.1002/cam4.2559
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



