Back to Journals » Pragmatic and Observational Research » Volume 9
Real-life treatment of rhinitis in Australia: a historical cohort study of prescription and over-the-counter therapies for patients with and without additional respiratory disease
Authors Price DB , Smith PK , Harvey RJ, Carney AS , Kritikos V , Bosnic-Anticevich SZ , Christian L, Skinner D , Carter V, Durieux AMS
Received 4 October 2017
Accepted for publication 14 April 2018
Published 15 August 2018 Volume 2018:9 Pages 43—54
DOI https://doi.org/10.2147/POR.S153266
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Christoph R Meier
David B Price,1–3 Pete K Smith,4 Richard John Harvey,5,6 A Simon Carney,7 Vicky Kritikos,8 Sinthia Z Bosnic‑Anticevich,8,9 Louise Christian,10 Derek Skinner,3 Victoria Carter,2,3 Alice MS Durieux2
1Centre of Academic Primary Care, University of Aberdeen, Aberdeen, UK; 2Observational and Pragmatic Research Institute, Singapore, Singapore; 3Optimum Patient Care, Cambridge, UK; 4Clinical Medicine, Griffith University, Southport, QLD, Australia; 5Rhinology and Skull Base, Applied Medical Research Centre, University of New South Wales, Sydney, NSW, Australia; 6Faculty of Medicine and Health Sciences, Macquarie University, Sydney, NSW, Australia; 7Department of Otolaryngology – Head and Neck Surgery, Flinders University, Adelaide, SA, Australia; 8Woolcock Institute of Medical Research, University of Sydney, NSW, Australia; 9Central Sydney Area Health Service, Sydney, NSW, Australia; 10NostraData, Kew, VIC, Australia
Background: The aim of the study was to explore rhinitis therapy purchases in different Australian regions for patients with and without additional respiratory disease, using both doctor’s prescriptions and over-the-counter (OTC) medications.
Patients and methods: It was a historical cohort study of pharmacy-related claims that included prescription or OTC rhinitis therapy, with or without asthma/COPD therapy, from January 2013 to December 2014.
Results: Overall, 4,247,193 prescription and OTC rhinitis treatments were purchased from 909 pharmacies over a calendar year; the majority were single-therapy purchases for rhinitis only patients. More multiple-therapy was purchased for rhinitis and asthma/COPD patients (4.4%) than for rhinitis only patients (4.0%), with a greater proportion purchased in VIC, SA and TAS (4.7% of rhinitis only patients and 4.5% of rhinitis and asthma/COPD patients) than in other areas. Dual therapy of oral antihistamine (OAH) and intranasal corticosteroid (INS) were the most frequently purchased multiple-therapy, with higher purchasing rates for rhinitis and asthma/COPD patients (2.6%) than for rhinitis only patients (1.6%). The most frequently purchased single therapy was OAH (70.1% of rhinitis only patients and 57.3% of rhinitis and asthma/COPD patients). First-line INS therapy was more likely to be purchased for rhinitis and asthma/COPD patients (15.3% by prescription and 11.7% OTC) than for rhinitis only patients (5.0% by prescription and 9.2% OTC); however, geographical differences in the proportion of therapies purchased OTC were noted, with a lower proportion of OTC OAH and INS purchases in Queensland and the Northern Territory for patients with and without comorbid respiratory disease.
Conclusion: Purchases of first-line INS therapy are more likely for patients with comorbid respiratory disease if they have received prescriptions and information/advice from their general practitioner. The study results indicate a need for patient information/education at the point-of-sale of OTC OAHs to enable patients to assess their nasal symptoms and receive treatment support from pharmacists. Greater availability to INSs in pharmacies as well as guidance from current guidelines and instruction in correct intranasal technique may also lead to greater uptake of INSs.
Keywords: asthma, chronic obstructive airways disease, intranasal corticosteroids, medication, oral antihistamines, over-the-counter, pharmacy
Introduction
Rhinitis is a chronic respiratory condition defined by one or more of the following nasal symptoms: nasal congestion, rhinorrhea, sneezing and/or itching.1 Rhinitis is often categorized into allergic rhinitis (AR) and non-allergic rhinitis (NAR), with recent data suggesting that as many as 50–70% of patients suffer from a mixed form known as mixed rhinitis.2 Both AR and NAR are independent risk factors for new-onset asthma.1,3–5 Symptoms of rhinitis can also present in COPD.6 Nasal symptoms can be triggered by exposure to aeroallergens in sensitized individuals with AR, while changes in temperature and humidity can induce symptoms in those with NAR.5 AR is the most common type of rhinitis,7 affecting 19% of the Australian population,8 with different prevalence rates across Australian regions due to significant geographical variability in climatic conditions and allergen exposure.8,9
AR is associated with a variety of comorbidities such as conjunctivitis, rhinosinusitis and otitis media as well as asthma, which is considered to be part of the unified airway.3,5,10 In Australia, at least 30% of patients with known AR also have asthma and as many as 80% of asthma patients have coexisting AR.11 Untreated or poorly managed AR can pose a significant health problem due to its prevalence and negative impact on patients’ quality of life, causing sleep, disturbances resulting in daytime fatigue and affecting work productivity and school performance.12–14 The repercussions of poorly managed AR can also extend into coexisting asthma, where it can worsen asthma control and increase the risk of exacerbations.3,15 Thus, it is fundamental that in asthma patients, coexisting AR is detected and treated with first-line AR therapy, given that over 60% of Australian adults with uncontrolled asthma have moderate-severe AR that is underreported and undertreated.16 It is also important that AR patients allergic to rye grass pollens, with or without a history of asthma, are made aware of the increased risk in certain weather conditions during the pollen season of “thunderstorm asthma” which can trigger severe asthma exacerbations leading to death and the use of preventative measures.17
AR management strategies encompass patient education, allergen minimization, pharmacotherapy and the addition of allergen-specific immunotherapy in severe cases of AR.3–5,18 Intranasal corticosteroids (INSs) are recommended as first-line therapy for moderate-severe and/or persistent AR and NAR in patients with or without lower airways disease. They are considered the most effective monotherapy for AR in both adults and children.3–5,18 INSs are effective in improving all symptoms of AR including ocular symptoms19 and are more effective than oral antihistamines (OAHs) and oral leukotriene receptor antagonists (LTRAs).20,21 Second-generation OAHs are recommended for mild intermittent AR.22 Combination intranasal therapy containing a corticosteroid and an antihistamine in a single device has been shown to deliver added efficacy greater than that attained by INS monotherapy, and for some patients the clinical benefit is significant.23 While there is insufficient clinical evidence available to support the combined use of OAH and INS therapy,3,4 with most published evidence confirming no benefits gained by adding other AR treatments to INS therapy,24 high failure rates of INS monotherapy have been reported, resulting in frequent co-prescription of multiple treatments.25
AR is one of the most underestimated respiratory conditions reported by physicians and patients.26 Its management is often suboptimal and frequently complicated by delayed diagnosis and appropriate treatment because of attempts by patients to self-medicate with a wide range of over-the-counter (OTC) medication available from pharmacies and patients do not consult a general practitioner (GP).26–29 Often the OTC treatments have been self-selected without seeking pharmacist advice.26 In most regions of Australia, many of the treatments for rhinitis are “pharmacy medicines” (Schedule 2), which are stored on open shelves and available OTC, without prescription and without the requirement of pharmacist intervention. Other treatments available without prescription are “pharmacist only medicines” (Schedule 3), which are stored in a secure area away from the public and with the requirement of pharmacist intervention.30 However, in Queensland and the Northern Territory (QLD and NT), both Schedules 2 and 3 medicines are stored in a secure area away from the public and require pharmacist intervention.31
In Australia, apart from reported patterns and costs of single and multiple-therapy purchases from pharmacies for rhinitis,32 little is known about the nature and extent of rhinitis treatments purchases in different regions of Australia for patients with and without additional respiratory disease. Moreover, examining medication use for patients with and without additional respiratory disease may provide evidence for intervention regarding screening and OTC medication counseling protocols for pharmacists, scheduling of medicines and prescribing policies. The study aimed to explore rhinitis therapy purchases in different Australian regions for patients with and without additional respiratory disease using both doctor’s prescriptions and OTC medications.
Patients and methods
This was a historical cohort study of pharmacy-related claims that included both prescription and OTC rhinitis therapy, with or without asthma/COPD therapy, from January 2013 to December 2014. The study was registered with the European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (registration number ENCEPP/SDPP/8507) and approved by the Anonymised Data Ethics Protocols and Transparency (ADEPT) committee (approval reference number ADEPT0215).
Data source
A demographically representative dataset of pharmacy-related claims was provided by NostraData (https://www.nostradata.com.au/Public/Home/About), which included information from both doctor’s prescriptions and OTC supply. Of the estimated 5,240 pharmacies across Australia in 2013, 77% were in QLD, New South Wales and Victoria.33 The NostraData dataset contained data from 909 randomly selected pharmacies across Australia and provided representative geographic coverage of all states/territories and the population as a whole, as shown in Table 1. Information in this dataset describe the details of valid pharmacy transactions, including name(s) of product(s) purchased, prescription or OTC status, postcode of purchase and price paid.
Pharmacy purchases of at least one prescription or OTC rhinitis treatment with or without additional asthma/COPD therapy during 2013 and 2014 were assessed from the pharmacy claims dataset. Prescription or OTC rhinitis treatments were used as a proxy for rhinitis, and asthma/COPD treatments were used as a proxy for comorbid respiratory disease. Therapeutic classes of rhinitis treatments included OAH; INS; intranasal antihistamine and corticosteroid combination; nonsteroidal nasal spray (NNS); LTRA; eye drop (ED) for allergic conjunctivitis; oral corticosteroid and injectable corticosteroid. Table 2 shows a list of drugs included in each therapeutic class, the most representative in terms of prescription and OTC purchases and that are OTC (Schedule 2) and “pharmacist only medicines” (Schedule 3), and that require prescription by a doctor (Schedule 4). LTRAs, oral and injectable corticosteroids were included as rhinitis therapy only if they were purchased without additional asthma/COPD treatment. Therapeutic classes of asthma/COPD treatments included short-acting β2 agonists, inhaled corticosteroids, long-acting β2 agonists, combination inhaled corticosteroids and long-acting β2 agonists therapy, short-acting muscarinic antagonists, long-acting muscarinic antagonists, cromones and theophyllins.
Study outcomes
The following medication-related datasets were analyzed for patients with and without additional asthma/COPD therapy in each geographic region of Australia:
- Count of therapies (ie, frequency of single and multiple rhinitis therapies of distinct drug class purchased in the same transaction).
- Drug class of rhinitis therapy purchased as single therapy in the same transaction.
- Drug class of rhinitis therapy purchased as multiple-therapy in the same transaction.
- Prescription and OTC “classes of therapies” purchased as single therapy in the same transaction.
Data analysis
Data were analyzed using MySQL and Microsoft Excel 2011 software (Microsoft Corporation, Redmond, WA, USA). The sample characteristics of pharmacy transactions in Australia were summarized using descriptive statistics. Pharmacy transactions during the study period were analyzed together and reported as average numbers and percentages per calendar year. To account for differences in climate and drug provision policies across Australia, data were analyzed separately for the following geographic areas: New South Wales and Australian Capital Territory; QLD and NT; Victoria, South Australia and Tasmania (VIC, SA and TAS); Western Australia. Count of therapies are shown as single, multiple and total number of therapies and recorded as absolute numbers and percentages. Combinations of different classes of therapies are recorded as absolute numbers and percentages. Numbers of OTC therapies were tallied for each combination of therapies.
Results
Table 1 shows the sample characteristics of pharmacy transactions across different Australian regions over 12 months. Of the 8,334,472 pharmacy transactions assessed, 4,247,193 (51%) included rhinitis therapy, of which 4,074,496 (96%) were without asthma/COPD therapy and 172,697 (4%) were with additional asthma/COPD therapy.
Count of rhinitis therapies of distinct drug class in the same transaction with and without additional asthma/COPD therapy
Of the 4,074,496 transactions for which rhinitis only therapy was purchased, 3,909,923 (96.0%) were transactions that included a single drug class and 164,573 (4.0%) included multiple drug classes in the same transaction from pharmacies across all Australian regions.
Of the 172,697 transactions for which rhinitis and asthma/COPD therapy was purchased, 165,064 (95.6%) were transactions that included a single drug class and 7,633 (4.4%) included multiple drug classes in the same transaction from pharmacies across all Australian regions.
Differences between Australian regions
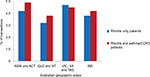
Figure 1 shows the proportion of transactions where multiple “classes” of rhinitis therapy were purchased for rhinitis only patients and those with asthma/COPD from pharmacies in each Australian region. More multiple-therapy (4.7% of rhinitis only patients and 4.5% of rhinitis and asthma/COPD patients) was purchased in VIC, SA and TAS than in other areas.
Drug class of rhinitis therapy purchased as single therapy with and without additional asthma/COPD therapy in the same transaction
Of the 4,074,496 transactions for which rhinitis only therapy was purchased, 2,856,342 (70.1%) were single-therapy purchases of OAH, 555,672 (13.6%) were INS purchases and 415,670 (10.2%) were NNS purchases from pharmacies across all Australian regions. LTRA, intranasal combination antihistamine/corticosteroid and ED drug classes accounted for the remaining 2.1% transactions for rhinitis only single-therapy purchases.
Of the 172,697 transactions for which rhinitis and asthma/COPD therapy was purchased, 99,027 (57.3%) were single-therapy purchases of OAH, 44,501 (25.8%) were INS purchases and 12,913 (7.5%) were NNS purchases from pharmacies across all Australian regions. ED drug classes and an intranasal combination of antihistamine with corticosteroid accounted for the remaining 5.4% transactions for rhinitis and asthma/COPD therapy single-therapy purchases.
Differences between Australian regions
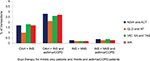
Figure 2 shows the proportion of transactions where OAH, INS and NNS were purchased as single therapy for rhinitis only patients and those with asthma/COPD from pharmacies in each Australian region. More OAHs (73.5% of rhinitis only patients and 60.1% of rhinitis and asthma/COPD patients) and fewer INSs (11.0% of rhinitis only patients and 23.6% of rhinitis and asthma/COPD patients) were purchased in QLD and NT than in other areas.
Drug class of rhinitis therapy purchased as multiple-therapy with and without additional asthma/COPD therapy in the same transaction
Of the 4,074,496 transactions for which rhinitis only therapy was purchased, 64,391 (1.6%) were multiple-therapy purchases of both OAH and INS and 9,171 (0.2%) were multiple-therapy purchases of both INS and NNS from pharmacies across all Australian regions. Other combinations accounted for the remaining 2.2% of multiple-therapy transactions.
Of the 172,697 transactions for which rhinitis and asthma/COPD therapy was purchased, 4,449 (2.6%) were multiple-therapy purchases of both OAH and INS and 560 (0.3%) were multiple-therapy purchases of both INS and NNS from pharmacies across all Australian regions. Other combinations accounted for the remaining 1.1% of multiple-therapy transactions.
Differences between Australian regions
Figure 3 shows the proportion of transactions where treatment combinations of OAH and INS and INS and NNS were purchased for rhinitis only patients and those with asthma/COPD from pharmacies in each Australian region. A lower proportion of dual OAH and INS treatment (1.0% of rhinitis only patients and 2.1% of rhinitis and asthma/COPD patients) was purchased in QLD and NT than in other areas.
Prescription and OTC “classes of therapies” purchased as single therapy without additional asthma/COPD therapy in the same transaction
Of the 3,909,923 transactions for which rhinitis only therapy was purchased that included a single drug class, 2,626,934 (67.2%) and 236,680 (6.0%) were OTC and prescription OAH purchases, respectively; 359,899 (9.2%) and 196,226 (5.0%) were OTC and prescription INS purchases, respectively; and 412,788 (10.6%) and 2,898 (0.07%) were OTC and prescription NNS purchases, respectively. For rhinitis only patients, more single therapy was purchased OTC than by prescription, and the most frequently purchased single therapy was OAH (67.2%), followed by NNS (10.6%) and INS (9.2%), all OTC.
Differences between Australian regions
Figure 4 shows the proportion of transactions for which both OTC and prescription OAH, INS and NNS were purchased as single therapy for rhinitis only patients from pharmacies in each Australian region. Fewer OTC OAHs (55.8%), more prescription OAHs (20.3%) and fewer OTC INSs (6.6%) were purchased for rhinitis only patients in QLD and NT than in other areas.
Prescription and OTC “classes of therapies” purchased as single therapy with additional asthma/COPD therapy in the same transaction
Of the 165,064 transactions for which rhinitis only therapy was purchased that included a single drug class, 85,420 (51.7%) and 14,011 (8.5%) were OTC and prescription OAH purchases, respectively; 19,265 (11.7%) and 25,326 (15.3%) were OTC and prescription INS purchases, respectively; and 12,538 (7.6%) and 376 (0.2%) were OTC and prescription NNS purchases, respectively. For rhinitis and asthma/COPD patients, the most frequently purchased single therapy was OTC OAH (51.7%), followed by prescription INS (15.3%) and OTC INS (11.7%).
Differences between Australian regions
Figure 5 shows the proportion of transactions for which both OTC and prescription OAH, INS and NNS were purchased as single therapy for rhinitis and asthma/COPD patients from pharmacies in each Australian region. In QLD and NT, fewer OTC OAHs and INSs (39.8% and 8.5%, respectively) and more prescription OAHs (23.0%) were purchased for rhinitis and asthma/COPD patients than in other areas.
Discussion
This study provides valuable insight into the nature and extent of rhinitis therapy purchases in different Australian regions for patients with and without comorbid respiratory disease during a calendar year. It provides valid transaction data on single- and multiple-therapy purchases in different Australian regions, the way in which rhinitis therapy is purchased, the prescribing behaviors of GPs and self-medication behaviors of patients in a real-life setting. Our study revealed that the majority of transactions were single-therapy purchases for rhinitis only patients. More multiple-therapy was purchased for rhinitis and asthma/COPD patients (4.4%) than for rhinitis only patients (4.0%), with a greater proportion purchased in VIC, SA and TAS (4.7% of rhinitis only patients and 4.5% of rhinitis and asthma/COPD patients) than in other areas. Dual therapies of OAH and INS were the most frequently purchased multiple-therapy, with higher purchasing rates for rhinitis and asthma/COPD patients (2.6%) than for rhinitis only patients (1.6%). OAHs were the most frequently purchased single therapy (70.1% of rhinitis only patients and 57.3% of rhinitis and asthma/COPD patients). INS therapy was more likely to be purchased for rhinitis and asthma/COPD patients (15.3% by prescription and 11.7% OTC) than for rhinitis only patients (5.0% by prescription and 9.2% OTC); however, there were geographical differences in the proportion of therapies purchased OTC, with a lower proportion of OTC OAH and INS purchases in QLD and NT for both patients with and without comorbid respiratory disease.
Our study revealed that patients overall purchased OTC OAHs (70.1% of rhinitis only patients and 57.3% of rhinitis and asthma/COPD patients) and that first-line INS therapy was more likely to be purchased for rhinitis and asthma/COPD patients (15.3% by prescription and 11.7% OTC) than for rhinitis only patients (5.0% by prescription and 9.2% OTC). Many studies have shown that most single therapy is purchased OTC than by prescription, and most often OAHs are purchased,14,25,28,34–37 which are not clinically effective or cost-effective.3,5,18 Our results are consistent with those from a recent community pharmacy-based study in Australia, where the majority of AR patients who had visited the pharmacy self-selected their medications, most of which were OTC OAHs, and 71% of OTC purchases of OAHs were self-selected by patients without seeking pharmacist advice.26 Therefore, we can hypothesize that if patients had interacted with the pharmacist, the proportion of patients with comorbid respiratory disease who select OTC first-line INS would increase. Our study showed that first-line INS therapy was more likely to be purchased for patients with comorbid respiratory disease, which could be due to that fact that these patients are more likely to visit their GP to receive prescriptions and information/education, are better informed and thus more likely to purchase appropriate OTC treatment and less likely to switch prescription medications.14,25,35 By contrast, rhinitis only patients are more likely to self-medicate with OTC medication, often self-selecting and switching medication, formulations and brands without being informed or seeking medical advice, in pursuit of finding a treatment that they perceive effectively controls their symptoms.14,26,36,38 Given that INSs are first-line rhinitis therapy for patients with and without comorbid respiratory disease,3,5,18 INS therapy continues to be underused (and OAHs overused) by patients overall. Underuse of INSs has been previously reported in the Australian population, and major reasons identified for not using INSs included a dislike for nasal sprays, lack of knowledge about INS use, lack of perceived effectiveness and possible side effects.37 Discontinuation of INS therapy is also a common phenomenon among AR sufferers, with major reasons for stopping INS therapy being lack of acceptable efficacy, decline in effect over time, unpleasant side effects and concerns about dependence.37,39 As adequate adherence to continuous maintenance INS therapy and correct intranasal technique are factors that can contribute to rhinitis symptom control, appropriate patient information/education at the point-of-sale of OTC treatments and pharmacist support may lead to greater uptake of INSs, higher patient satisfaction rates with INS therapy and, by extension, improvements in the long-term management of rhinitis in primary care.
In this study, there were geographical differences in the proportion of therapies purchased OTC, with a lower proportion of OTC OAH and INS purchases in QLD and NT for rhinitis patients with and without comorbid respiratory disease. In QLD and NT, OTC Schedule 2 medicines (which are accessible to the public in all other regions) must be stored in a secure area away from the public with the requirement of pharmacist intervention31 and may explain the lower proportion of OTC OAH and OTC INS purchases (as single and dual therapy) in QLD and NT for patients with and without comorbid respiratory disease. Thus, information and advice on appropriate medication use for rhinitis is most likely to be provided by primary care providers in QLD and NT, as fewer OTC treatments are available on open shelves for self-selection by patients, with most of the treatments available requiring pharmacist intervention or by prescription.
This large-scale study showed that more multiple-therapy was purchased for rhinitis and asthma/COPD patients (4.4%) than for rhinitis only patients (4.0%), with a greater proportion purchased in VIC, SA and TAS (4.7% of rhinitis only patients and 4.5% of rhinitis and asthma/COPD patients) than in other areas. The use of multiple-therapy to manage AR symptoms has been well documented by a number of studies.14,25,40,41 Almost 15% of patients add OTC medications to supplement prescribed or OTC medication for symptoms that are no longer managed with current treatment.14,25 Also, many patients receiving prescribed monotherapy at the start of the pollen season frequently return to their GP as the pollen season progresses, to co-prescribe multiple treatments or change treatment.25 Patient adherence to treatment appropriately prescribed by their GP is also known to be poor.14,34,40 Uncontrolled rhinitis symptoms can worsen asthma control as well as increasing the number of GP visits35,42 and may offer a possible explanation for higher rates of multiple-therapy purchases for rhinitis and asthma/COPD patients, as this patient cohort is more likely to have moderate-severe disease with persistent symptoms and be prescribed multiple-therapy by their GP.12,25,43 Compared to other Australian regions, VIC, SA and TAS had the highest multiple-therapy purchase rate, which is not surprising given that the prevalence rates of AR in VIC and SA are significantly higher than the rate for all of Australia,9 and possibly a reflection of high exposure to common clinically relevant seasonal allergens such as grass pollens including perennial ryegrass, timothy grass and small wind-distributed pollens from trees and weeds.44 Moreover, VIC has higher peak pollen counts than many other regions of Australia, due to the impact of springtime northerly winds that carry pollen from grasslands north of its border.44
Dual therapy of OAH and INS was the most common multiple-therapy purchased in this study, with higher purchasing rates for rhinitis and asthma/COPD patients (2.6%) than for rhinitis only patients (1.6%), despite the lack of clinical evidence supporting this practice,3,4 which can incur additional costs (including costs associated with side effects from inappropriate medication use) to the patient.14,29,32,38 This dual-therapy regimen frequently used by patients when monotherapy fails (whether OAH or INS) has been well documented by a number of studies.25,40,41 Failure of INS monotherapy in controlling rhinitis symptoms could be due to poor intranasal device technique,37 poor adherence to long-term therapy, polysensitization,45 comorbidities,3–5,18 mixed rhinitis,1,2 misdiagnosis of rhinitis46 and severe chronic upper airways disease.12 Since dual-therapy purchase rates were the highest for patients with comorbid respiratory disease, health care provider (HCP) educational activities targeting appropriate investigation and management of rhinitis are required to ensure that patients use guideline-recommended medications appropriately.
Despite the fact that as many as 80% of patients with allergic asthma have coexisting AR in Australia,11 our study found that the majority or rhinitis therapy transactions were without additional asthma/COPD therapy. A possible explanation for this finding could be that a proportion of rhinitis only transactions were for patients with undiagnosed respiratory conditions such as asthma and COPD. Given that asthma and rhinitis are considered as part of a unified airway,10 it is recommended that in patients with persistent AR, HCPs should screen for asthma, and in those with asthma, they should screen for rhinitis.3,5,18 Other possible explanations for this finding were that a proportion of rhinitis only transactions were destined to individuals with lower respiratory conditions or that most people with rhinitis and comorbid respiratory disease may not manage their symptoms with pharmacotherapy from pharmacies but rather immunotherapy.
Strengths and limitations of this study
The strength of this study lies in the fact that it is the first large-scale Australian study that used a demographically representative dataset of valid pharmacy-related claims, providing information on 4,247,193 rhinitis treatments for “real-life” patients over a calendar year. A major strength of this study was its observational nature, which enabled a depiction of real-life rhinitis treatment purchases via doctor’s prescriptions and OTC supply across Australia. This would have been difficult to capture through surveys that rely on prescriber and patient recall for reported outcomes, which can underestimate or overestimate patient and prescriber behavior. Moreover, the study captured valuable information regarding prescribing and self-medication trends, as well as the extent to which rhinitis management occurs within the Australian community pharmacy setting, as the majority of treatments options for rhinitis are available without a prescription.
There were several limitations of the study, which included the cross-sectional design, lack of demographic characteristics and lack of follow-up data, which may have resulted in multiple-therapy rates being underestimated, as individual patients may have purchased additional rhinitis treatments at different time points. This has been reported in a community-based longitudinal study in the UK, whereby 16% of patients purchased additional therapies 5 days after the original purchase, with 16% and 18% purchasing additional therapies 4 and 8 weeks later, respectively.36 Another limitation of the study was the use of prescription and OTC rhinitis therapy purchases as a proxy for a diagnosis of rhinitis as well as the use of asthma/COPD therapy purchases as a proxy for a diagnosis of asthma or COPD. However, it is plausible that treatments classified as rhinitis therapy may have been purchased OTC or by prescription for another indication such as eczema or food allergy, even though a study based in the UK had found that over 60% of patients who were prescribed OAHs had a rhinitis diagnosis.47 Moreover, it may be plausible that EDs classified as rhinitis therapy may have been purchased for allergic conjunctivitis in the absence of rhinitis, even though a population-based study had found that up to 70% of patients with AR also had allergic conjunctivitis.48 Finally, it was not possible to confirm whether multiple-therapy purchases in the same transaction were all intended for the same patient, or to record purchases of rhinitis therapy from pharmacies outside NostraData coverage.
Conclusion
Patients overall are purchasing OTC OAHs, which are not clinically effective or cost-effective. Purchases of first-line INS therapy are more likely for patients with comorbid respiratory disease if they have received prescriptions and information/advice from their GP. The study results indicate a need for patient information/education at the point-of-sale of OTC OAHs to enable patients to assess their nasal symptoms and receive treatment support from pharmacists. Greater availability to INSs in pharmacies as well as guidance from current guidelines and instruction in correct intranasal technique may also lead to greater uptake of INSs.
Acknowledgments
The abstract of this paper was presented at the Respiratory Effectiveness Group 2016 Summit, April 15–16, 2016, as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in The Journal of Thoracic Disease (Vol. 8, Supplement 5, July 5, 2016) http://jtd.amegroups.com/article/view/8504/html. This study was part funded by Meda, Australia. The study was conducted by the Observational and Pragmatic Research Institute Pte Ltd (OPRI) as an independent research organization; Meda had no role in the conduct or reporting of the study.
Disclosure
David B Price has board membership with Aerocrine, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Mundipharma, Napp, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, and Teva Pharmaceuticals; consultancy agreements with Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Napp, Novartis, Pfizer, Teva Pharmaceuticals and Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from Aerocrine, AKL Research and Development Ltd, AstraZeneca, Boehringer Ingelheim, British Lung Foundation, Chiesi, Mylan, Mundipharma, Napp, Novartis, Pfizer, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Teva Pharmaceuticals, Theravance, UK National Health Service and Zentiva (Sanofi Generics); payment for lectures/speaking engagements from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Merck, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, Skyepharma and Teva Pharmaceuticals; payment for manuscript preparation from Mundipharma and Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma and Novartis; payment for travel/accommodation/meeting expenses from Aerocrine, AstraZeneca, Boehringer Ingelheim, Mundipharma, Napp, Novartis and Teva Pharmaceuticals; funding for patient enrolment or completion of research from Chiesi, Novartis, Teva Pharmaceuticals and Zentiva (Sanofi Generics); stock/stock options from AKL Research and Development Ltd, which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); and is peer reviewer for grant committees of the Efficacy and Mechanism Evaluation programme.
Pete K Smith has received honoraria from AstraZeneca, GlaxoSmithKline, MEDA Pharmaceuticals and Mundipharma.
Richard John Harvey is a consultant for Medtronic, NeilMed and Olympus, and has received honoraria from Seqirus and grant support from MEDA Pharmaceuticals, NeilMed and Stallergenes.
A Simon Carney is a consultant for Olympus and Smith & Nephew and has received honoraria from MEDA Pharmaceuticals.
Vicky Kritikos has received honoraria from AstraZeneca, GlaxoSmithKline and Pfizer.
Sinthia Z Bosnic-Anticevich has received honoraria from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Mundipharma and TEVA Pharmaceuticals for her contribution to advisory boards/key international expert forum.
Victoria Carter is an employee of Observational and Pragmatic Research Institute Pte Ltd, which has conducted paid research in respiratory disease on behalf of the following organizations in the past 5 years: Aerocrine, AKL Research and Development Ltd, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Orion, Takeda, Teva and Zentiva (a Sanofi company).
Alice MS Durieux was an employee of Observational and Pragmatic Research Institute Pte Ltd.
The authors report no other conflicts of interest in this work.
References
Bernstein JA. Characterizing rhinitis subtypes. Am J Rhinol Allergy. 2013;27(6):457–460. | ||
Bernstein JA. Allergic and mixed rhinitis: epidemiology and natural history. Allergy Asthma Proc. 2010;31(5):365–369. | ||
Bousquet J, Khaltaev N, Cruz AA, et al; World Health Organization; GA(2)LEN; AllerGen. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(Suppl 86):8–160. | ||
Brozek JL, Bousquet J, Baena-Cagnani CE, et al; Global Allergy and Asthma European Network; Grading of Recommendations Assessment, Development and Evaluation Working Group. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126(3):466–476. | ||
Scadding GK, Kariyawasam HH, Scadding G, et al. BSACI guideline for the diagnosis and management of allergic and non-allergic rhinitis (Revised Edition 2017; First edition 2007). Clin Exp Allergy. 2017;47(7):856–889. | ||
Hens G, Vanaudenaerde BM, Bullens DM, et al. Sinonasal pathology in nonallergic asthma and COPD: ‘united airway disease’ beyond the scope of allergy. Allergy. 2008;63(3):261–267. | ||
Mygind N. Allergic rhinitis. Chem Immunol Allergy. 2014;100:62–68. | ||
Australian Institute of Health and Welfare. Australia’s Health 2016. Australia’s health series no. 15. Cat. no. AUS 199. Canberra: AIHW; 2016. Available from: https://www.aihw.gov.au/reports/australias-health/australias-health-2016/contents/summary. Accessed June 20, 2018. | ||
Australian Institute of Health and Welfare. Allergic Rhinitis (‘Hay Fever’) in Australia. Cat. no. ACM 23. Canberra, ACT: AIHW; 2011. Available from: https://www.aihw.gov.au/reports/asthma-other-chronic-respiratory-conditions/allergic-rhinitis-hay-fever/contents/allergic-rhinitis-by-the-numbers. Accessed June 20, 2018. | ||
Giavina-Bianchi P, Aun MV, Takejima P, Kalil J, Agondi RC. United airway disease: current perspectives. J Asthma Allergy. 2016;9:93–100. | ||
National Asthma Council Australia [webpage on the Internet]. Australian Asthma Handbook. Version 1.1. Melbourne, VIC: National Asthma Council Australia; 2015. Available from: http://www.asthmahandbook.org.au. Accessed March 16, 2018. | ||
Bousquet PJ, Bachert C, Canonica GW, et al. Uncontrolled allergic rhinitis during treatment and its impact on quality of life: a cluster randomized trial. J Allergy Clin Immunol. 2010;126(3):666–668.e1–e5. | ||
Valovirta E, Myrseth SE, Palkonen S. The voice of the patients: allergic rhinitis is not a trivial disease. Curr Opin Allergy Clin Immunol. 2008;8(1):1–9. | ||
Meltzer EO, Farrar JR, Sennett C. Findings from an online survey assessing the burden and management of seasonal allergic rhinoconjunctivitis in US patients. J Allergy Clin Immunol Pract. 2017;5(3):779–789.e6. | ||
Lakhani N, North M, Ellis AK. Clinical manifestations of allergic rhinitis. J Aller Ther. 2012;S5:007. | ||
Bosnic-Anticevich S, Kritikos V, Carter V, et al. Lack of asthma and rhinitis control in general practitioner-managed patients prescribed fixed-dose combination therapy in Australia. J Asthma. 2017:1–11. | ||
Thien F. Thunderstorm asthma: potential danger but a unique opportunity. Asia Pac Allergy. 2017;7(2):55–56. | ||
Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015;152(1 Suppl):S1–S43. | ||
Bielory L, Chun Y, Bielory BP, Canonica GW. Impact of mometasone furoate nasal spray on individual ocular symptoms of allergic rhinitis: a meta-analysis. Allergy. 2011;66(5):686–693. | ||
Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ. 1998;317(7173):1624–1629. | ||
Ratner PH, Howland WC 3rd, Arastu R, et al. Fluticasone propionate aqueous nasal spray provided significantly greater improvement in daytime and nightime nasal symptoms of seasonal allergic rhinitis compared with montelukast. Ann Allergy Asthma Immunol. 2003;90(5):536–542. | ||
Simons FE, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. 2011;128(6):1139–1150.e4. | ||
Meltzer EO, Wallace D, Dykewicz M, Shneyer L. Minimal clinically important difference (MCID) in allergic rhinitis: agency for healthcare research and quality or anchor-based thresholds? J Allergy Clin Immunol Pract. 2016;4(4):682–688.e6. | ||
Benninger M, Farrar JR, Blaiss M, et al. Evaluating approved medications to treat allergic rhinitis in the United States: an evidence-based review of efficacy for nasal symptoms by class. Ann Allergy Asthma Immunol. 2010;104(1):13–29. | ||
Price DB, Scadding G, Bachert C, et al. UK prescribing practices as proxy markers of unmet need in allergic rhinitis: a restrospective observational study. NPJ Prim Care Respir Med. 2016;26:16033. | ||
Tan R, Cvetkovski B, Kritikos V, et al. Identifying the hidden burden of allergic rhinitis (AR) in community pharmacy: a global phenomenon. Asthma Res Pract. 2017;3:8. | ||
Nolte H, Nepper-Christensen S, Backer V. Unawareness and undertreatment of asthma and allergic rhinitis in a general population. Respir Med. 2006;100(2):354–362. | ||
Maurer M, Zuberbier T. Undertreatment of rhinitis symptoms in Europe: findings from a cross-sectional questionnaire survey. Allergy. 2007;62(9):1057–1063. | ||
Bousquet J, Neukirch F, Bousquet PJ, et al. Severity and impairment of allergic rhinitis in patients consulting in primary care. J Allergy Clin Immunol. 2006;117(1):158–162. | ||
Australian regulation of over-the-counter medicines 2016 [webpage on the Internet]. Available from: https://www.tga.gov.au/node/4206. Accessed March 16, 2018. | ||
Medicines and poisons: retailers, wholesalers and manufacturers [webpage on the Internet]. Available from: https://nt.gov.au/industry/licences/medicines-and-poisons-retailers-wholesalers-and-manufacturers. Accessed March 16, 2018. | ||
Smith P, Price D, Harvey H, et al. Medication-related costs of rhinitis in Australia: a NostraData cross-sectional study of pharmacy purchases. J Asthma Allergy. 2017;10:153–161. | ||
Retail and Personal Services (RAPS) Training Council. Community Pharmacy Environmental Scan 2013. Available from: http://rapstc.com.au/wp-content/uploads/2011/12/Community-Pharmacy-Environmental-Scan-2013.pdf. Accessed March 16, 2018. | ||
Storms W, Meltzer EO, Nathan RA, Selner JC. Allergic rhinitis: the patient’s perspective. J Allergy Clin Immunol. 1997;99(6):S825–S828. | ||
Price D, Scadding G, Ryan D, et al. The hidden burden of adult allergic rhinitis: UK healthcare resource utilisation survey. Clin Transl Allergy. 2015;5:39. | ||
Sinclair H, Bond C, Largue G, Price D, Hannaford P. Community pharmacy provision of allergic rhinitis treatments: a longitudinal study of patient reported outcome. Int J Pharm Pract. 2005;13(4):249–256. | ||
Katelaris CH, Sacks R, Theron PN. Allergic rhinoconjunctivitis in the Australian population: burden of disease and attitudes to intranasal corticosteroid treatment. Am J Rhinol Allergy. 2013;27(6):506–509. | ||
Mehuys E, Gevaert P, Brusselle G, et al. Self-medication in persistent rhinitis: overuse of decongestants in half of the patients. J Allergy Clin Immunol Pract. 2014;2(3):313–319. | ||
Fromer LM, Ortiz G, Ryan SF, Stoloff SW. Insights on allergic rhinitis from the patient perspective. J Fam Pract. 2012;61(2 Suppl):S16–S22. | ||
Navarro A, Valero A, Rosales MJ, Mullol J. Clinical use of oral antihistamines and intranasal corticosteroids in patients with allergic rhinitis. J Investig Allergol Clin Immunol. 2011;21(5):363–369. | ||
Canonica GW, Bousquet J, Mullol J, Scadding G, Virchow JC. A survey of the burden of allergic rhinitis in Europe. Allergy. 2007;62(Suppl 85):17–25. | ||
Price D, Zhang Q, Kocevar VS, Yin DD, Thomas M. Effect of a concomitant diagnosis of allergic rhinitis on asthma-related health care use by adults. Clin Exp Allergy. 2005;35(3):282–287. | ||
Demoly P, Bousquet PJ, Mesbah K, Bousquet J, Devillier P. Visual analogue scale in patients treated for allergic rhinitis: an observational prospective study in primary care: asthma and rhinitis. Clin Exp Allergy. 2013;43(8):881–888. | ||
Davies JM. Grass pollen allergens globally: the contribution of subtropical grasses to burden of allergic respiratory diseases. Clin Exp Allergy. 2014;44(6):790–801. | ||
Ciprandi G, Cirillo I. Monosensitization and polysensitization in allergic rhinitis. Eur J Intern Med. 2011;22(6):e75–e79. | ||
Settipane RA. Other causes of rhinitis: mixed rhinitis, rhinitis medicamentosa, hormonal rhinitis, rhinitis of the elderly, and gustatory rhinitis. Immunol Allergy Clin North Am. 2011;31(3):457–467. | ||
Smith P, Price D, Carney AS, et al. Oral antihistamine prescription as a proxy marker for rhinitis in the UK. 2015. Presented at: Respiratory Effectiveness Group Summit; January 22–24; 2015; Rotterdam, The Netherlands. | ||
Leonardi A, Castegnaro A, Valerio AL, Lazzarini D. Epidemiology of allergic conjunctivitis: clinical apperance and treatment patterns in a population-based study. Curr Opin Allergy Clin Immunol. 2015;15(5):482–488. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.