Back to Journals » Journal of Pain Research » Volume 9
Real-life efficacy of pregabalin for the treatment of peripheral neuropathic pain in daily clinical practice in Denmark: the NEP-TUNE study
Authors Crawford M, Poulsen PB , Sciøttz-Christensen B, Habicht A, Strand M, Bach F
Received 18 December 2015
Accepted for publication 26 January 2016
Published 20 May 2016 Volume 2016:9 Pages 293—302
DOI https://doi.org/10.2147/JPR.S102744
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael Schatman
Article video Abstract Caption: Video abstract presented Flemming W Bach.
Views: 4756
Michael E Crawford,1 Peter Bo Poulsen,2 Berit Schiøttz-Christensen,3 Andreas Habicht,4 Mette Strand,2 Flemming W Bach5
1Copenhagen City Pain Clinic, Copenhagen K, 2Pfizer Denmark ApS, Ballerup, 3Spine Center Southern Denmark, Lillebælt Hospital, Middelfart, 4Signifikans ApS, Vedbæk, 5Department of Neurology, Aalborg University Hospital, Aalborg, Denmark
Objective: The aim of this study was to provide evidence regarding the real-life efficacy of pregabalin in the treatment of peripheral neuropathic pain (NeP) in Denmark.
Methods: In this prospective, observational, noninterventional study, pregabalin (Lyrica®) was prescribed following usual clinical practice. Compared with baseline, the primary study end points after 3 months of observation were changes in 1) the average level of pain during the past week, 2) the worst level of pain during the past week, and 3) the least level of pain during the past week. The Wilcoxon signed-rank test was used to perform paired analyses, and a multivariate regression analysis investigated factors driving change in pain.
Results: A total of 86 of the 128 patients included were regarded as efficacy evaluable (those completing 3 months of pregabalin treatment). Patients (59 years) were long-time sufferers of peripheral NeP, and 38% of them had comorbidities. The majority had previously been treated with tricyclic antidepressants or gabapentin. The average dose of pregabalin was 81.5 mg/d at baseline and 240 mg/d after 3 months. A clinically and statistically significant improvement of 2.2 points in the average level of pain intensity was found after 3 months. The higher the pain intensity at baseline, the higher was the reduction of the pain score. Positive results were also found for pain-related sleep interference, patients’ global impression of change, quality of life, and work and productivity impairment. Twenty-one patients reported 28 adverse events.
Conclusion: This real-life study indicates that for some patients (two-thirds), addition of pregabalin for peripheral NeP helps to reduce their pain intensity significantly.
Keywords: noninterventional study, pain intensity, usual clinical practice, sleep interference and quality of life
Introduction
Peripheral neuropathic pain (NeP) includes pain conditions such as postherpetic neuralgia and painful diabetic neuropathy (DNP). The European guidelines for the pharmacological treatment of NeP issued by the European Federation of Neurological Societies recommend pregabalin (Lyrica®; Pfizer, Inc., New York, NY, USA) as the first-line treatment for the most common NeP conditions. Other drugs also recommended as first-line treatment are tricyclic antidepressants (TCA), gabapentin, and the serotonin–norepinephrine reuptake inhibitors duloxetine and venlafaxine for DNP.1 These drugs are also recommended for the treatment of NeP in Denmark. However, because the Danish reimbursement policy places pregabalin as a third-line treatment option after TCA and gabapentin, pregabalin can be reimbursed only if lack of efficacy or tolerability has been documented for first- and second-line treatment drugs.
Pregabalin has been studied in a large number of randomized, placebo-controlled clinical trials in different NeP conditions, including peripheral NeP. These clinical trials have shown that pregabalin is effective and that the numbers needed to treat with pregabalin are between 3.4 and 4.2 for DNP and 3.4 and 5.6 for postherpetic neuralgia.2 Only a few studies have investigated the real-life use and efficacy of pregabalin in daily clinical practice in a nonrandomized clinical trial setting, for example, studies by Anastassiou et al,3 Patel et al,4 and Happich et al.5 Evidence from observational, noninterventional studies in real-life daily practice is important when determining whether the effectiveness of pregabalin in daily clinical practice is comparable to that observed in randomized clinical trials; real-life, noninterventional studies complement the randomized clinical trials.
The aim of this study was to provide additional evidence regarding the real-life efficacy of pregabalin and to collect data about how pregabalin is used for the treatment of peripheral NeP in daily practice within primary and secondary care in Denmark.
Methods
The study was designed as a prospective, multicenter, observational, noninterventional study and was conducted in both primary and secondary care settings in Denmark. Patients had been diagnosed with peripheral NeP by their general practitioner (GP) or a specialist and were treated as per usual clinical practice. It was beyond the scope of the study to investigate how the patients had been diagnosed with NeP. When a patient, independent of the study and before consideration for observation in the study, was prescribed pregabalin for the treatment of peripheral NeP, the patient was informed about the study by the GP or specialist. The patient had to give informed consent before the first dose of pregabalin was taken in order to be enrolled in the study by the GP or specialist. Pregabalin was prescribed at doses and durations at the discretion of the prescribing physicians providing patient care, as per usual clinical practice. Being a noninterventional study following daily practice, the payment of pregabalin followed the Danish rules of national reimbursement. This resulted in some patients having pregabalin reimbursed, with only a small co-payment to be paid by the patient, while other patients paid for the medication fully themselves. Reimbursement for pregabalin was not an inclusion/exclusion criterion. The observational period was 3 months during which the patients were followed in accordance with regular clinical practice. Due to the nature of the study, there were no protocol-scheduled study visits to the clinic, only visits due to daily clinical practice. At the end of the 3-month observation period, all patients received a telephone follow-up by the prescribing physician, unless they had dropped out before the end of the 3-month observation period.
End points
The primary study end points were 1) the average level of pain during the past week compared with baseline, 2) the worst level of pain during the past week compared with baseline, and 3) the least level of pain during the past week compared with baseline. “Past week” refers to the week leading up to the 3-month telephone follow-up. To evaluate efficacy, the primary end points were measured using the numeric rating scale (0–10 Numeric Rating Scale-Pain Intensity, also referred to as the 11-point Likert scale). This was done to measure the pain intensity at each clinic visit, including the baseline visit and telephone follow-up at the end of the 3-month observation period. An improvement in pain for the group of at least 2.0 points on the 11-point Likert scale was regarded as a clinically relevant and important improvement, in accordance with Farrar et al.6 A number of secondary end points were also examined. The secondary end point pain-related sleep interference during the past week compared with baseline was measured using the 11-point Likert scale. An improvement in this outcome from baseline to end of follow-up of 30% or more on the 11-point Likert scale measuring pain-related sleep interference was regarded as both a clinically meaningful and a sustained response, as is the case in other studies.7 The patient’s global impression of change (PGIC – 7-point scale) was used to measure the clinical significance of the treatment for the patient. PGIC values of 6 and 7, that is, much improved and very much improved, indicated that the patient had experienced an actual change at follow-up.8 The level of impairment/disability from work due to disease was measured using the work productivity and activity impairment (WPAI) questionnaire,9,10 which has previously been used for patients with NeP and chronic pain.11–13 Finally, the patients’ health-related quality of life was measured using the standardized and generic EQ-5D instrument.14,15 The end points were measured at all clinic visits during the 3-month observation period, including the baseline visit and the telephone follow-up after 3 months.
Patient selection
All patients enrolled in the study had to meet the usual prescribing criteria for pregabalin as per the local product information and were entered into the study at the prescribing physicians’ discretion. Patients included in the study had to meet the following six inclusion criteria: 1) aged 18 years or above; 2) diagnosed with peripheral NeP; 3) had not previously been prescribed pregabalin for the treatment of peripheral NeP (“first prescription patients”) or were prescribed pregabalin again (“retreatment patients”) but had not used pregabalin within the previous 6 months; 4) had not taken the first dose of the prescribed pregabalin at enrollment; 5) able to read and understand Danish and fill in patient questionnaires; and 6) signed a dated informed consent document. Exclusion criteria were 1) patients younger than 18 years, 2) patients who did not consent to participate, and 3) patients who at study inclusion (baseline) had already been prescribed pregabalin for the treatment of generalized anxiety disorder or epilepsy.
Data recorded
Data were collected and recorded using electronic case report forms at the baseline visit and at all subsequent visits, including the telephone follow-up. The following information was collected: demographic and socioeconomic data (age and sex), data regarding the patient’s pain history (time with NeP pain condition, etiology, other pain types, comorbidities, and pain drug history), treatment (prebaseline treatment, pregabalin treatment and dose, postbaseline treatment), treatment end points (pain intensity, pain-related sleep inference, WPAI, PGIC, and quality of life), and finally any reported adverse events. Standard criteria were applied in the study to all observed or volunteered adverse events, regardless of suspected causal relationship to pregabalin, which were recorded on the adverse event pages of the case report form, including criteria for classification as a serious adverse event. Data transformation of WPAI and EQ-5D followed the official guidelines issued for these instruments.9,10
Study size
The study was designed to have 80% power to show an improvement of at least 0.75 points on the 11-point Likert pain scale using a 95% confidence level and assuming a true improvement of 1.25 points and standard deviation of 1.91. Using these assumptions, 115 evaluable patients were needed. Patients who were evaluable for efficacy were defined as patients with data on pregabalin use at baseline and at the 3-month telephone follow-up.
Statistical analysis
The study is primarily descriptive and exploratory. No hypotheses are presented. Descriptive analyses were performed for all socioeconomic data, pain history data, and pregabalin treatment data. All primary and secondary end points were analyzed for all patients who continued their pregabalin treatment throughout the study and had data collected both at baseline and at the telephone follow-up after 3 months. Last observation carried forward was used; however, no other interpretation of missing data was performed.
A clinically relevant and important improvement on the 11-point Likert pain scale was regarded as an improvement of at least 2.0 points, following Farrar et al.6 To determine whether improvements were statistically significant, statistical tests and confidence intervals (CIs) in the study were performed and assessed from a 5% significance level (two-sided).
The Wilcoxon signed-rank test was used to perform paired analysis of the data (baseline vs follow-up after 3 months).
The factors that drive the change in pain (least, average, and worst) were evaluated using a multivariate regression model (proc mixed model using SAS statistical programming – normality checked via residual plots). Factors investigated were age, pregabalin dose change from baseline, pain intensity at baseline, comorbidity (yes/no), pain history of >12 months at baseline (yes/no), and concomitant pain medication used at baseline (yes/no). For continuous factors (age, pregabalin dose, pain intensity), negative estimates indicated that higher factors resulted in improvement of pain, whereas for binomial factors (yes/no), negative estimates indicated that a “yes” resulted in improvement of pain. The regression analysis was performed for the group of patients who received the 3-month follow-up and were still using pregabalin at that time.
Finally, patients who dropped out, were lost to follow-up, or discontinued pregabalin were analyzed in a dropout analysis. The patients from this analysis were compared with the patients who were fully evaluable, that is, continued their pregabalin treatment throughout the study.
Data protection and ethics committee
The study and its data collection were approved by the Danish Data Agency. Furthermore, the Danish Medicines Agency and the National Committee on Health Research Ethics both confirmed that the study was a noninterventional observational study. According to the Danish Medicines Act, the obligation to apply for authorization does not apply to noninterventional studies; hence, approvals by either of these bodies are not required.
Results
From December 2012 to March 2014, a total of 128 patients were screened and included in the study by 28 GPs and four pain specialists (86 and 42 patients, respectively). One hundred of these patients were classified as completers because they had at least a full baseline visit and a telephone follow-up after 3 months. The remaining 28 patients were classified as noncompleters because they were lost to follow-up or had dropped out before the end of the 3-month observation period. Eighty-six patients were still on pregabalin treatment at the time of the telephone follow-up. This population with both a pre- and a postobservation was regarded as the patients who were evaluable for efficacy. It is this group that is the focus of this study of pregabalin.
The mean age of the patients included was 59 years (range 26–89 years, 95% CI 55.8–63.0), and 61% were females (N=52). Table 1 shows the patients’ baseline pain characteristics, including pain treatment prescribed before the initiation of treatment with pregabalin (baseline), and comorbidities. Data are presented for all screened and enrolled patients (N=128), for the group of patients who were evaluable for efficacy after 3 months of observation (N=86), and for the group of patients who were not evaluable for efficacy due to dropping out, lost to follow-up, or discontinued pregabalin treatment (N=42).
  | Table 1 Patients’ baseline pain characteristics, origin, and comorbidities |
The table shows that the majority of the patients who were evaluable for efficacy had suffered from peripheral NeP for quite a long time; nearly 80% had experienced NeP for >12 months. More than half of the patients (51%–52%) had concomitant nociceptive or visceral pain conditions. Many of the patients who were prescribed pregabalin also had other concomitant pain conditions in addition to their NeP diagnosis, such as musculoskeletal pain, postoperative pain, and posttraumatic pain. Around 38% of the patients had comorbidities, with pain-related sleep interference being the most frequent, followed by depression and anxiety. Despite a lower rate of comorbidity, the patients who were evaluable for efficacy had similar baseline pain characteristics as all screened and included patients.
Due to the Danish reimbursement-driven treatment algorithm, most of the patients had previously been treated with TCA (35%), gabapentin (54%), or duloxetine (6%) for their peripheral NeP. For 36% of the patients, pregabalin was the first drug for the treatment of peripheral NeP.
At initiation, the pregabalin dose varied from 25 mg to 600 mg; the average dose was 81.5 mg (Table 2).
At the 3-month follow-up, the average dose of pregabalin was increased to 241 mg/d (range 25–900 mg), which is below the recommended daily defined dose of 300 mg/d.16 Dose increases were recorded infrequently during the 3-month observation period because pain was not always the patient’s primary reason for returning to the GP or specialist.
At the baseline visit, 96.5% of the patients used pain medication for their peripheral NeP. Once treatment with pregabalin was initiated, only 77.7% of the patients used more than one pain medication. The major reason for this drop was that most of the patients who had previously used gabapentin stopped using this drug from 23.1%–5.4% after initiating use of pregabalin. The remaining group expected to finalize treatment with gabapentin soon after initiating the treatment with pregabalin. There were also slightly fewer patients who received weak opioids from 24.5%–21.4% and nonsteroidal anti-inflammatory drugs from 17.5%–14.3% after the initiation of pregabalin.
Primary efficacy end point
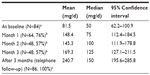
Figure 1 shows the reduction of experienced peripheral NeP (average, worst, and least pain) recorded at the end of the 3-month observation period compared with baseline (prior to initiation of the pregabalin treatment).
Figure 1 shows that the average level of experienced pain was reduced by 2.2 points on the 11-point Likert pain scale after 3 months of treatment compared with baseline levels. The pain intensity was reduced from a baseline score of 6.7%–4.5% on the scale (minimum–maximum: 3–10; P<0.001). For all three primary end points, the pain reduction was statistically significant, and for the average and worst pain intensity, the results at 3 months also show clinically important improvements (changes ≥2.0).
A multivariate regression analysis was performed to determine which factors (age, pregabalin dose at baseline, pain intensity at baseline, comorbidity, pain history, and concomitant pain medication) drove the change in pain intensity during the 3-month observation period. Results showed that for all three levels of pain intensity (least, average, and worst), a high pain intensity at baseline resulted in a significant improvement of the pain condition. Furthermore, for the worst pain experienced, a pain history of >12 months at baseline resulted in significantly (P=0.027) less improved pain. All other factors were nonsignificant.
Secondary end points
A number of secondary end points were reported in the study, that is, pain-related sleep interference during the past week, PGIC, patients’ WPAI, and finally patients’ health-related quality of life.
Figure 2 shows the pain-related sleep interference after 3 months of observation of pregabalin treatment compared with baseline.
As shown in Figure 2, pain-related sleep interference was on average reduced by 3 points (57%) on the scale during the 3-month treatment period. This is both clinically relevant (≥30% improvement from baseline) and statistically significant. After 3 months, only minor pain-related sleep interference was present with a value of 2.3 on the Likert scale. Median pain-related sleep interference was reduced by 4 points (from 6 points at baseline to 2 points after 3 months).
Similar to the primary end point of reduction in pain intensity and the reduction in pain-related sleep interference, the results of the patient’s own assessment, as recorded on the PGIC, showed a clinically significant improvement after 3 months of treatment; 62% (N=53) of the participating patients were (very) much improved (Figure 3). Furthermore, 30% (N=26) felt minor improvements caused by the treatment. Only seven patients did not experience any improvement.
  | Figure 3 Patients’ global impression of change (PGIC; N=86). |
The patients’ expressed quality of life also improved during the 3-month observation period. The mean quality-adjusted life year (QALY) for the study patients at baseline was 0.47 (EQ-5D). This figure increased to 0.63 after 3 months of treatment, corresponding to a statistically significant 0.16 QALY improvement on the 0–1 QALY scale. This result was based on 64 patients who filled out the patient questionnaires both at baseline and after 3 months. Similar significant improvement was found on the visual analog scale of the EQ-5D instrument (0.15).
Because 80% of the patients included in the study were not active in the workforce due to retirement (65+ age and early retirement due to disability), only 25 (20%) of the included patients with pain were working. For these working subjects, the number of their working hours affected over the past 7 days due to their NeP condition (~28–30 hours on average), as recorded on the WPAI questionnaire, was comparable at baseline and after 3 months of treatment. However, their work productivity was statistically significantly improved (P=0.0081) because they were less impaired by their NeP condition after 3 months of treatment. Similarly, there was a statistically significant improvement in the ability of the patients with NeP to perform regular daily activities other than work (P=0.0005), and less hours in general were affected due to their pain condition (P=0.001) after 3 months of treatment.
Adverse events
No serious adverse events were reported. However, 21 (16%) of the 128 patients included in the study reported 28 adverse events. Of the 28 adverse events, 27 were assessed by the investigator to be related to the use of pregabalin. The most frequent of these were dizziness (5), sedation (4), nausea (3), vertigo (3), and increase in weight (3).
Half of the 28 patients who dropped out of the study (N=14) reported adverse events as the reason for their dropout. Four patients indicated lack of effect of the pregabalin treatment as a reason for their discontinuation. The rest of the patients indicated other discontinuation reasons, such as a positive effect of treatment with no perceived need to continue taking medicine, cost of drug, confidentiality concerns, and moving to another city.
Patients who were not evaluable for efficacy
A total of 42 of the 128 included patients were not evaluable for efficacy and were, therefore, not included in the analysis. This group of patients had dropped out, was lost to follow-up, or had discontinued pregabalin at the 3-month telephone follow-up. The average age of the nonevaluable group was 57 years, and 74% were females. Compared with the 86 patients who were evaluable for efficacy, the average age was nearly the same in the two groups, but the percentage of females was lower in the group evaluable for efficacy (61%). As shown in Table 1, the group of nonevaluable patients tended to have a slightly longer history of NeP (86% with >12 months), and nearly one-fourth had a history of NeP of >10 years as compared with 7% among the efficacy evaluable patients. Differences were less pronounced with respect to pain type and etiological background between the two groups (Table 1). However, a higher share of patients had comorbidities among the 42 nonevaluable (50%) than the 86 evaluable patients (39%); the former reporting more frequent pain-related sleep interference, memory impairment, and concentration difficulties. The initiating average daily dose of pregabalin at the baseline visit was also slightly higher for the group of patients who were not evaluable for efficacy (93.5 mg, 95% CI 56–131) compared with the 86 patients evaluable for efficacy (81.5 mg, 95% CI 62.2–100.9).
Using the method for last observation on pregabalin therapy carried forward, change in pain (least, average, and worst) was further evaluated among the 28 dropout patients. Results were compared with the patients who were evaluable for efficacy (N=86). This comparison showed that the magnitude of the average change in pain found in the dropout group was lower than the average change in pain found among the patients who were evaluable for efficacy; the magnitude of the pain reduction for the dropout group was less than half of that found for patients who were evaluable for efficacy. However, this difference was not evaluated statistically due to lack of statistical power.
Discussion
The objective of this study was to assess the use and efficacy of pregabalin in the treatment of patients with peripheral NeP in real-life daily clinical practice. The patient group investigated was a difficult-to-treat group of patients with the majority having a long (>12 months) history of NeP. Moreover, a large proportion of the patients had previously been treated with TCA and/or gabapentin.
Registration of pregabalin treatment throughout the study showed that dose escalation occurred in the primary and secondary care as expected. However, the dosages used (average dose of 241 mg/d after 3 months of treatment) were generally lower than the recommended daily defined dose of 300 mg.16 They were also lower than the dosages typically used in pregabalin randomized controlled trials, including trials where patients were allowed to titrate.17 One explanation for the lower average dose of pregabalin in this study is that some clinics enrolled many elderly people, primarily women, for whom uptitration of the pregabalin dosage started from a lower level and only slowly increased. A further argument could be that the period of observation was only 3 months, whereas a longer observation period, for example, 6 months, would have contributed to a higher dosage level of pregabalin. However, a previous study with a 6-month observation period has not been able to confirm this.5
Use of low doses of pregabalin after a period of treatment has also been found in a number of other studies focusing on real-life efficacy of pregabalin. Johannessen Landmark et al18 called in real-life settings upon individualization of pregabalin therapy and doses regardless of treatment indication. In Anastassiou et al,3 the most common dose was 150 mg/d at the last follow-up after 62 days. In Happich et al,5 the mean daily dosage over a 6-month treatment period with pregabalin was reported as low as 53.9 mg/d, and in a study by Blanco Tarrio et al,19 the average dose per day of 1,670 patients treated with pregabalin was 202 mg/d. Similar low dosages down to 125 mg/d have been found in market research studies of pregabalin. In comparison to these studies, the average dosage of 241 mg/d after 3 months of pregabalin treatment in this study is neither low nor unusual. Yang et al20 reported poor patient compliance due to low treatment dosage levels. However, this did not seem to have been the case in the present study, and we claim that, in contrast, too high dosage levels may lead to poor patient compliance as a consequence of early undesired side effects. Twenty-eight patients did drop out of the study, and half of them due to adverse events. Given that it is difficult to treat a group of patients who have tried other pharmacological treatments without adequate success before, this dropout rate is not regarded as being high.
The patients included in this study did experience a significant and clinically relevant improvement in their average level of pain intensity, with a reduction of 2 points on the 11-point Likert pain scale following 3 months of treatment with pregabalin. The results of the secondary end points support the effectiveness of pregabalin in the treatment of peripheral NeP. A significant and clinically relevant improvement was found with respect to pain-related sleep interference and the patients’ subjective assessment of pain as measured by the PGIC questionnaire. After the pain-treatment period of 3 months, the work productivity was significantly improved for the subset of patients who were still in the workforce. Furthermore, quality of life of the patients was also significantly improved.
Similar results were obtained in a noninterventional, multicenter, postmarketing study conducted in Greece and published in 2011.3 This study, however, showed a lower effect size in pain reduction than the study conducted in Greece.
Study limitations
The study was a prospective, multicenter, observational, noninterventional drug study. The main limitation of this noninterventional study was within its study design. The open-label nature of the design of this study imposes limitations on the conclusion that can be drawn concerning the efficacy and tolerability of pregabalin. These include a lack of placebo-treated control patients and the fact that patients and prescribing physicians were not blinded to treatment. Because of these limitations, the efficacy and safety profile of pregabalin may be influenced by both the patients and the prescribing physician’s expectations. This, coupled with the absence of a placebo-control group, may make it difficult to firmly and confidently conclude that pregabalin is effective in real-life clinical practice.
The statistical analysis was performed without adjustment for multiplicity of the three pain end points. However, the P-value of the primary end point, as well as the P-values of the other two pain measures, was low and would also have been statistically significant when adjustment for multiplicity in the three pain end points was applied, using the Bonferroni correction method.
Eighty-six patients of the 115 patients needed, as defined by the sample size calculations, completed the study. This may call for a careful interpretation of the results as the patients completing the 3 months of pregabalin treatment were from a selected patient population who had responded to or tolerated the treatment with pregabalin. However, when comparing the baseline data for the 86 patients who were evaluable for efficacy with the data for the 42 patients who were not evaluable for efficacy, no large differences were found. The comparison did, however, imply that the 42 patients who were not evaluable for efficacy had a longer history of NeP and were more diseased in terms of comorbidities; both of these conditions cannot be excluded as reasons for dropout or lost to follow-up. The proportion of patients using concomitant pain medication decreased in this study, following the initiation of pregabalin treatment.
Another limitation could be the lack of consistent evaluation or diagnosis criteria across the patient cohort in the study because the patients were diagnosed and followed up using the individual GP or specialist practice’s criteria. This is a consequence and potential weakness of a noninterventional study following daily clinical practice.
Despite the limitations in the study design, the study has a high external validity, that is, it shows the efficacy of pain treatment in clinical practice in a group of difficult-to-treat patients who have received pregabalin for a 3-month period. The external validity is probably higher than in randomized controlled trial studies with strict inclusion and exclusion criteria and criteria for study conduct.
Comparing with other studies in the literature
Similar to other studies in the literature, this study found improvement in the pain intensity following initiation of pregabalin treatment for peripheral NeP. Anastassiou et al3 assessed the impact of pregabalin for the treatment of NeP under real-life conditions and concluded that pregabalin led to significant 4-point reductions in pain (4.16 on a 11-point numerical rating scale) and in pain-related sleep interference. Patel et al4 and Happich et al5 evaluated the real-life efficacy of pregabalin for the treatment of diabetic peripheral neuropathy and found similar reductions in the mean pain intensity, pain-related sleep, and PGIC. Focusing on pain-related sleep interferences in patients with spinal cord injury and NeP, Cardenas et al7 demonstrated a significant improvement of sleep quality following pregabalin treatment. In terms of tolerability, the 78% pregabalin continuation rate at the end of study found in this study is comparable to the 76% found in the study by Anastassiou et al.3 Discontinuation due to lack of efficacy occurred in 3% of the patients in this study compared with 0.7% in Anastassiou et al.3 The explanation for this may be that the group of patients in this study was a harder-to-treat group of patients with a long history of peripheral NeP and use of pain-related medicine. Finally, the number of adverse events found in this study was not high. As an example, more than half of the patients (54%) experienced all-cause adverse events in the study by Anastassiou et al,3 whereas this was the case only for 22% in this study.
Conclusion
Although the recruitment target was not met, this study of real-life pain management in Denmark indicates a positive effect in terms of pain relief on those patients with peripheral NeP completing 3 months of pregabalin treatment (86 of 128 included). The addition of pregabalin treatment helps to significantly reduce pain intensity and improve well-being in these patients with difficult-to-treat peripheral NeP who had tried many types of treatment, for example, with TCA and/or gabapentin (67%), without satisfactory pain relief. The higher pain intensity the patient had at baseline, the higher was the reduction in the pain score experienced by the patient after 3 months of treatment. Fewer patients used other concomitant pain medications when treatment with pregabalin was initiated. Patients not completing the 3 months of pregabalin medication had typically a longer history of NeP, more frequently had comorbidities, and had higher individual doses of pregabalin (faster titration). This study protocol based on daily practice may prove to be useful as a platform for individual patient treatment with regard to titration of pregabalin dosage. The tolerability of pregabalin treatment seems to be comparable to that found in other studies, with similar rates of discontinuation. Therefore, the positive benefit from pregabalin in the treatment of NeP as shown in the randomized clinical trials is confirmed in this real-life study.
Acknowledgments
Each of the specialists and GPs participating in the NEP-TUNE study are acknowledged for their participation. The investigators were paid a minor fee for each patient included in the study as compensation for the registration of data in the study. The size of the fee was approved by the Danish Medicines Agency prior to the start of the study. This study was sponsored by Pfizer Inc., including fees to investigators.
Author contributions
MEC collected data for the study, contributed to the data analysis, participated in the drafting and revision of the manuscript. PBP conceived of the study, participated in its design, contributed to the data analysis, and drafted and revised the manuscript. BSC and FWB participated in the design of the study, contributed to the data analysis, participated in the drafting and revision of the manuscript. AH carried out the data manipulation, statistical analyses, and participated in the drafting and revision of the manuscript. MS conceived of the study, participated in its design, contributed to the data analysis and participated in the drafting and revision of the manuscript. All authors have read and approved the final version of the manuscript to be published and are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
MC was paid by Pfizer Inc. for his work as investigator in the study. BSC and FB were paid by Pfizer Inc. for their work as members of the study steering committee. AH was paid as a contractor to Pfizer Inc. for his work with the statistical analysis and study report. PBP and MS are full-time employees of Pfizer Inc. and are both members of the study steering committee. The authors report no other conflicts of interest in this work.
References
Attal N, Gruccu G, Baron R, et al; European Federation of Neurological Societies. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113–1123. | |
Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–581. | |
Anastassiou E, Iatrou CA, Vlaikidis N, et al; ATLAS Investigators. Impact of pregabalin treatment on pain, pain-related sleep interference and general well-being in patients with neuropathic pain: a non-interventional, multicentre, post-marketing study. Clin Drug Investig. 2011;31:417–426. | |
Patel N, Mishra V, Patel P, Dikshit RK. A study of the use of carbamazepine, pregabalin and alpha lipoic acid in patients of diabetic neuropathy. J Diabetes Metab Disord. 2014;13:62. | |
Happich M, Schneider E, Boess FG, et al. Effectiveness of duloxetine compared with pregabalin and gabapentin in diabetic peripheral neuropathic pain: results from a German observational study. Clin J Pain. 2014;30:875–885. | |
Farrar JT, Pritchett YL, Robinson M, Prakash A, Chappell A. The clinical importance of changes in the 0 to 10 numeric rating scale for worst, least and average pain intensity: analysis of data from clinical trials of duloxetine in pain disorders. J Pain. 2010;11:109–118. | |
Cardenas DD, Emir B, Parsons B. Examining the time to therapeutic effect of pregabalin in spinal cord injury patients with neuropathic pain. Clin Ther. 2015;37:1081–1090. | |
Amirfez R, Pentlow A, Foote J, Leslie I. Assessing the clinical significance of change scores following carpal tunnel surgery. Int Orthop. 2009;33:181–185. | |
Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353–365. | |
Work Productivity and Activity Impairment Questionnaire [homepage on the Internet]. Reilly Associates. 2011. Available from: http://www.reillyassociates.net. Accessed September 29, 2011. | |
Kronborg C, Handberg G, Axelsen F. Health care costs, work productivity and activity impairment in non-malignant chronic pain patients. Eur J Health Econ. 2009;10:5–13. | |
Langley PC, Van Litsenburg C, Cappelleri JC, Carroll D. The burden associated with neuropathic pain in Western Europe. J Med Econ. 2013;16:85–95. | |
Skljarevski V, Zhang S, Desaiah D, et al. Duloxetine versus placebo in patients with chronic low back pain: a 12-week, fixed-dose, randomized, double-blind trial. J Pain. 2010;11:1282–1290. | |
EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. | |
Van Reenan M, Oppe M. EQ-5D-3L User Guide. Basic Information on How to Use the EQ-5D-3L Instrument. [Webpage in Internet]. Version 5.1. Rotterdam: EuroQol Group; April 2015. Available from: http://www.euroqol.org/fileadmin/user_upload/Documenten/PDF/Folders_Flyers/EQ-5D-3L_UserGuide_2015.pdf. Accessed February 17th 2016. | |
WHOCC [webpage on the Internet]. ATC/DDD Index. 2015. Available from: http://www.whocc.no/atc_ddd_index/?code=N03AX16. Accessed December 17, 2015. | |
van Seventer R, Bach FW, Toth CC, et al. Pregabalin in the treatment of post-traumatic peripheral neuropathic pain: a randomized double-blind trial. Eur J Neurol. 2010;17:1082–1089. | |
Johannessen Landmark C, Beiske G, Baftiu A, Burns ML, Johannessen SI. Experience from therapeutic drug monitoring and gender aspects of gabapentin and pregabalin in clinical practice. Seizure. 2015;28:88–91. | |
Blanco Tarrio E, Gálvez Mateos R, Zamorano Bayarri E, López Gómez V, Pérez Páramo M. Effectiveness of pregabalin as monotherapy or combination therapy for neuropathic pain in patients unresponsive to previous treatments in a Spanish primary care setting. Clin Drug Investig. 2013;33:633–645. | |
Yang M, Qian C, Liu Y. Suboptimal treatment of diabetic peripheral neuropathic pain in the United States. Pain Med. 2015;16(11):2075–2083. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.