Back to Journals » Journal of Inflammation Research » Volume 17
Reactive Oxygen Species Induced Upregulation of TRPV1 in Dorsal Root Ganglia Results in Low Back Pain in Rats
Authors Chen X, Chen Z , Ma G, Sha J, Zhao S, Liu Z, Chen N, Yang H
Received 11 November 2023
Accepted for publication 26 March 2024
Published 11 April 2024 Volume 2024:17 Pages 2245—2256
DOI https://doi.org/10.2147/JIR.S446841
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Xinyong Chen,1,2,* Zhe Chen,3,* Gongchang Ma,2 Jianjun Sha,2 Shan Zhao,2 Zuoqing Liu,2 Nong Chen,2 Huilin Yang1
1Department of Orthopedics, The First Affiliated Hospital of Soochow University, Suzhou, 215000, People’s Republic of China; 2Department of Orthopedics, Qingpu Branch of Zhongshan Hospital Affiliated to Fudan University, Shanghai, 200025, People’s Republic of China; 3Department of Orthopedics, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, 200025, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Nong Chen, Department of Orthopedics, Qingpu Branch of Zhongshan Hospital Affiliated to Fudan University, Shanghai, 200025, People’s Republic of China, Email [email protected] Huilin Yang, Department of Orthopedics, The First Affiliated Hospital of Soochow University, Suzhou, 215000, People’s Republic of China, Email [email protected]
Background: Dorsal root ganglia (DRGs) contain sensory neurons that innervate intervertebral discs (IVDs) and may play a critical role in mediating low-back pain (LBP), but the potential pathophysiological mechanism needs to be clarified.
Methods: A discogenic LBP model in rats was established by penetration of a lumbar IVD. The severity of LBP was evaluated through behavioral analysis, and the gene and protein expression levels of pro-algesic peptide substance P (SP) and calcitonin gene–related peptide (CGRP) in DRGs were quantified. The level of reactive oxygen species (ROS) in bilateral lumbar DRGs was also quantified using dihydroethidium staining. Subsequently, hydrogen peroxide solution or N-acetyl-L-cysteine was injected into DRGs to evaluate the change in LBP, and gene and protein expression levels of transient receptor potential vanilloid-1 (TRPV1) in DRGs were analyzed. Finally, an inhibitor or activator of TRPV1 was injected into DRGs to observe the change in LBP.
Results: The rats had remarkable LBP after disc puncture, manifesting as mechanical and cold allodynia and increased expression of the pro-algesic peptides SP and CGRP in DRGs. Furthermore, there was significant overexpression of ROS in bilateral lumbar DRGs, while manipulation of the level of ROS in DRGs attenuated or aggravated LBP in rats. In addition, excessive ROS in DRGs stimulated upregulation of TRPV1 in DRGs. Finally, activation or inhibition of TRPV1 in DRGs resulted in a significant increase or decrease of discogenic LBP, respectively, suggesting that ROS-induced TRPV1 has a strong correlation with discogenic LBP.
Conclusion: Increased ROS in DRGs play a primary pathological role in puncture-induced discogenic LBP, and excessive ROS–induced upregulation of TRPV1 in DRGs may be the underlying pathophysiological mechanism to cause nerve sensitization and discogenic LBP. Therapeutic targeting of ROS or TRPV1 in DRGs may provide a promising method for the treatment of discogenic LBP.
Keywords: lower back pain, reactive oxygen species, dorsal root ganglion, transient receptor potential vanilloid-1, substance p, calcitonin gene related peptide, intervertebral disc
Introduction
Low-back pain (LBP) impairs the physical and mental health of millions of people and is one of the most common chronic diseases of the musculoskeletal system. The 1-year incidence rate of first-onset LBP is estimated to be 15.4%.1 According to a meta-analysis, the prevalence of LBP is approximately 48–66%.2 Acute or chronic LBP affects the health of patients and causes an enormous impact on individuals and communities throughout the world.1 Therefore, it is important to understand the pathological and pathophysiological mechanisms underlying the prevention and treatment of LBP.
LBP may originate from different sites, such as damaged muscles, injured ligaments, or degenerated facet joints. However, degenerated intervertebral discs (IVDs)—also known as discogenic LBP—are thought to be one of the most important sources of LBP.3 Anatomically, normal IVDs are poorly innervated organs because they are only innervated by sensory and sympathetic perivascular nerve fibers in the outer layer of the annulus fibrosus. However, the number of nociceptive nerve fibers increases, and the fibers even grow into the nucleus pulposus in degenerated IVDs, which is thought to be the primary pathophysiological factor for discogenic LBP.3,4
The dorsal root ganglia (DRGs) are one of the most important neurological tissues that are responsible for nociception, vibration, fine touch, and proprioception.5,6 In the process of neural signal transmission, gnostic sensory information is gathered in DRGs and projected into the spinal cord and cortex.5,6 A previous study showed that stimulation of the L2–L3 DRGs induced significant and effective pain relief in the patients’ low back.7 Therefore, further studies should focus on DRGs to reveal the pathological and pathophysiological mechanisms of discogenic LBP.
Reactive oxygen species (ROS) play complex pathophysiological roles when cells or tissues are damaged or stimulated. In the central and peripheral nervous systems, ROS also play vital roles in various pathological processes, such as cellular apoptosis and inflammatory reactions.8 The role of ROS in neuropathic pain has been validated, and previous studies have also demonstrated that increased ROS levels in IVDs have a strong relationship with LBP.9 Thus, ROS play important pathological and pathophysiological roles in various kinds of pain.
Considering that DRGs are the core system that mediates discogenic LBP, here we attempted to prove the potential pathological effect of ROS in DRGs in an animal model. In addition, we explored whether transient receptor potential vanilloid-1 (TRPV1)—an important ion channel in neurotransmission—participates in ROS-mediated pathophysiological changes in DRGs. Overall, this study provides insights into ROS and DRGs in discogenic LBP and provides a new strategy for treatment.
Methods
Establishment of an LBP Model and Intra-DRG Drug Delivery
Following a previous protocol, a rat model of discogenic LBP was established by puncturing lumbar IVDs at L4–5 and L5–6 levels.9,10 Briefly, 2.5% pentobarbital sodium was used to anesthetize 3-month-old male Sprague–Dawley rats (weighing approximately 250–300 g). Subsequently, the transabdominal median approach was used to reach the spinal column, and the targeted IVDs were penetrated using an 18G needle under radiographic guidance. After suturing the incision, the measures of keeping warm and injecting saline were used to promote rapid recovery of the rats; however, no drugs were used to avoid interfering with the results.
According to a previous study, lower IVDs are innervated predominantly by upper DRGs. Namely, retrograde tracing methods have suggested that a lower disc (L5–L6) is innervated predominantly by upper (L1 and L2) DRG neurons via the sympathetic trunk.10,11 Thus, we exposed DRGs L1–L3 for the study. With microscopic assistance, the dorsal surface of the spinal cord and the bilateral DRGs were exposed after the removal of the spinous process and vertebral laminae. A microsyringe was used for drug delivery to DRGs. The animals were assigned to the following groups: sham surgery; LBP; intra-DRG hydrogen peroxide (H2O2); LBP + intra-DRG N-acetyl-L-cysteine (NAC: inhibitor of ROS); LBP + intra-DRG AMG-9810 (inhibitor of TRPV1); and intra-DRG capsaicin (agonist of TRPV1). H2O2 solution was applied at a dosage of 1 µM per DRG. NAC (Cat No. S1623, Selleckchem, Houston, TX, USA) was administered at a concentration of 1 μM per DRG. AMG-9810 (Cat No. 2316, R&D Inc., Minneapolis, MN, USA) or capsaicin (Cat No. HY-10448, MedChemExpress, NJ, USA) was injected at a dose of 0.1 μg per DRG. The Animal Care and Use Committee of the First Affiliated Hospital of Soochow University granted permission for all animal studies, and the protocols of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978) were strictly adhered to.
Evaluation of Discogenic LBP
For the assessment of LBP, the mechanical or cold allodynia threshold was the critical indicator according to our previous protocol.12,13 Briefly, after acclimation to the surrounding environment, the plantar surface of the rat hind limbs was stimulated with calibrated specific filaments (Stoelting, Wood Dale, IL, USA), and the presence of a positive reaction (defined as brisk movement of the hind limbs with/without mouthing or biting of toes) was recorded. If the rats showed a positive reaction, the next filament with the smallest diameter was applied. If the animals showed a negative reaction, a larger filament was used. When all the reactions were recorded, the mathematical model from Chaplan et al14 was used for the calculation. To evaluate cold allodynia, cold stimulation was induced when 0.1 mL of 100% acetone was applied around the plantar surface of the hind limbs of the rats. Similar to the mechanical stimulation, the percentage of brisk movement was recorded and calculated. Behavioral investigations were performed 3, 7, 11, and 14 days after surgery.
Gene Analysis of qRT-PCR
Following our previous protocol for real-time quantitative PCR,15 total RNA was extracted from the harvested tissues using TRIzol reagent (Invitrogen, Life Technologies Corp., CA, USA), and cDNA was synthesized using a reverse transcriptase kit (TaKaRa Inc., Shiga, Japan). qRT-PCR was performed using the SYBR Premix Ex Tag Kit (TakaRa, Shiga, Japan) and detected using an ABI 7500 Sequencing Detection System (Applied Biosystems, CA, USA). The cycling conditions were as follows: 40 cycles of denaturation at 95°C for 5 s, and amplification at 60°C for 24s. The primer sequences (Sangon Biotech, Shanghai, China) were as follows:
Rat GAPDH: forward 5’-ATGACTCTACCCACGGCAAG-3’ and reverse 5’-TACTCAGCACCAGCATCACC-3’;
Rat SUBSTANCE P: forward 5’-TGGTCAGATCTCTCACAAAGG-3’ and reverse 5’-TGCATTGCGCTTCTTTCATA-3’;
Rat calcitonin gene–related peptide (CGRP): forward 5’-TCTAGTGTCACTGCCCAGAAGAGA-3’ and reverse 5’-GGCACAAAGTTGTCCTTCACCACA-3’; and
Rat TRPV1: forward 5’-CAGCGAGTTCAAAGACCCAGAGAC-3’ and reverse 5’- GGAGCAGAGCGATGGTGTCATTC-3’.
The 2‒ΔΔCt method was used to calculate the normalized gene expression compared with that of GAPDH. All reactions were performed in triplicate.
Western Blot Analysis
To conduct Western blot analysis, the total proteins harvested from DRGs were separated using SDS-PAGE and then transferred onto polyvinylidene difluoride membranes (0.45 μm, Millipore, Bedford, MA, USA). Subsequently, the target proteins were incubated with primary antibodies against the CGRP of rats (dilution 1:600, Cat. No. AF6495; Beyotime Biotechnology Inc., Shanghai, China), substance P (SP) of rats (dilution 1:1000, Cat. No. abs110423; Absin Inc., Shanghai, China), TRPV-1 of rats (dilution 1:1000, Cat. No. AF8250; Beyotime Biotechnology, Shanghai, China), and β-actin at 4°C overnight. The proteins were then incubated with an HRP-conjugated secondary antibody at room temperature for 1 h, and the bands were visualized using chemiluminescence (Millipore, Bedford, MA, USA). Images were analyzed using Fusion FX7 (Vilber Lourmat, Marne-la-Vallée, France).
Histology and Immunohistochemistry
For hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC), immediately after harvesting, the DRGs were fixed with 4% paraformaldehyde for 24–48 hours. The IVD tissue was decalcified for 1 month. After routine embedding, sectioning, and deparaffinization, the tissue was stained using an H&E staining kit following the manufacturer’s protocol (Cat. No. G1004; Servicebio Inc., Wuhan, China).
For IHC analysis, the primary TRPV1 antibody (Cat. No. AF8250; Beyotime Biotechnology, Shanghai, China) was used for incubation, and a specific IHC kit (Bioworld Technology, MO, USA) was used for the entire process. Nuclei were counterstained with hemalum (FARCO Chemical Supplies, Hong Kong, China). All images were captured using a digital microscope (Axio; Carl Zeiss, Oberkochen, Germany).
Immunofluorescence
Following our previous protocol, the harvested bilateral DRGs were treated with 4% paraformaldehyde for 24 hours.15 The tissue was then frozen and sectioned into 5-μm-thick slices. The ROS level was detected using dihydroethidium (DHE, Cat. No. GDP1018; Servicebio, Wuhan, China) following the manufacturer’s instructions. For co-staining of myelin and TRPV1, the DRGs were longitudinally sectioned at 5 μm and incubated with primary anti-TRPV1 antibody (Cat No. AF8250; Beyotime Biotechnology Inc., Shanghai, China) at 4°C overnight. Alexa 488 fluorescent antibody conjugate (Thermo Fisher Scientific, MA, USA) was used for 1 hour. Subsequently, the axons were stained with FluoroMyelin™ Red Fluorescent Myelin Stain (Cat No. F34652; Thermo Fisher Scientific, MA, USA). Finally, the fluorescent images were captured using a microscope (Axio, Carl Zeiss, Oberkochen, Germany) and analyzed using ImageJ software.
Statistical Analyses
Data are expressed as mean ± standard deviation (SD), and a two-sided Student’s t-test was performed for comparison between two groups. For comparison between three groups, one-way ANOVA with post-hoc Tukey’s HSD test was conducted, and two-way ANOVA with post-hoc Tukey’s HSD test was performed for repeated measurements. Statistical significance was set at p < 0.05.
Results
Discogenic LBP in Rats with IVD Degeneration
To induce IVD degeneration, an 18-gauge needle was inserted into the lumbar IVDs at levels L4–5 and L5–6, as shown in Figure 1a. Fourteen days after disc puncture, the lumbar IVDs showed remarkable destruction and degeneration, with the disappearance of the bulging nucleus pulposus and disorganized annulus fibrosus compared with the normal IVDs, as shown in Figure 1b. With the destruction and degeneration of the lumbar IVDs, the rats exhibited significant discogenic LBP (Figure 1c), manifested as a decreased threshold of mechanical allodynia and an increased threshold of cold allodynia relative to the sham surgery group. Similar to the behavioral analysis, the pro-algesic peptides SP and CGRP were significantly upregulated, as shown by semiquantitative analysis of protein and gene expression, further validating the presence of discogenic LBP in rats (Figure 1d and e).
To detect the ROS level in DRGs, we harvested bilateral DRGs on day 14 and found that the expression of ROS was significantly increased in DRGs, as indicated by immunofluorescence (IF) and DHE staining (Figure 1f and g). Thus, we can conclude that IVD puncture caused significant discogenic LBP and excessive ROS expression in DRGs.
Overexpressed ROS in DRGs of Rats with Discogenic LBP
Previous studies have suggested that DRGs play a critical role in mediating discogenic LBP because they are the primary afferent nodes. After the verification of overexpressed ROS in DRGs, we attempted to determine whether ROS are stimuli for discogenic LBP in DRGs. After exposure of the bilateral DRGs in rats from the LBP group after disc penetration (Figure 2a), we performed intra-DRG injection of NAC with a microsyringe, and the results showed that discogenic LBP significantly decreased since day 7 compared with the puncture-induced LBP group, as depicted in Figure 2b. Meanwhile, the protein and gene expression levels of SP and CGRP also decreased, as shown in Figure 2c and d.
In contrast, if we directly injected H2O2 into DRGs of normal rats, the animals showed a similar severity of discogenic LBP compared with the puncture-induced LBP group (Figure 2e). The intra-DRG injection of H2O2 also caused a significant increase in SP and CGRP levels, as shown in Figure 2f and g. These data indicate that the upregulated ROS in DRGs contributed to discogenic LBP in rats.
Excessive Expression of TRPV1 in DRGs with ROS Stimulation
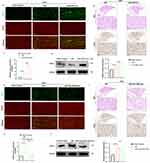
To investigate the underlying mechanism of ROS-induced LBP in DRGs, we focused on TRPV1—an important pain-related ion channel in the nervous system—because the expression of TRPV1 is strongly correlated with afferent pain, according to a previous study. In rats with LBP, we outlined DRGs and neuron afferent axons with myelin staining (red) and showed that the TRPV1 protein (green) had a significant increase in the punctured-induced LBP rats and a much higher expression in the intra-DRG H2O2 rats, as shown in Figure 3a. Meanwhile, in the transected DRG tissue (Figure 3b) with IHC staining, the expression of TRPV1 had a more significant increase than that in the LBP rats. Semiquantitative gene and protein analyses also revealed an increase in TRPV1 in the punctured-induced LBP rats and intra-DRG H2O2 rats, as shown in Figure 3c and d.
Next, we attempted to neutralize ROS with NAC in DRGs, and longitudinal IF and transected IHC staining revealed the significantly decreased expression of TRPV1, as shown in Figure 3e and f. Furthermore, the intra-DRG injection of NAC significantly suppressed the overexpressed gene and protein of TRPV1, as shown in Figure 3g and h. Thus, the accumulated data suggested that excessive ROS stimulated the expression of TRPV1 in DRGs.
Upregulated TRPV1 in DRGs is Responsible for Discogenic LBP
It has been proven that ROS induce the overexpression of TRPV1 in DRGs. Thus, we sought to investigate whether blocking the function of TRPV1 would alleviate discogenic LBP. After an intra-DRG injection of AMG-9810—an inhibitor of TRPV1—the severity of discogenic LBP was significantly ameliorated, as suggested by decreased mechanical and cold allodynia (Figure 4a). SP and CGRP levels in DRGs also decreased after intra-DRG treatment with AMG-9810, as shown in Figure 4b and c. In contrast, activation of TRPV1 in DRGs with capsaicin in normal rats significantly increased mechanical and cold allodynia and upregulated the expression of SP and CGRP genes and proteins in DRGs, as shown in Figure 4d–f. Thus, we may conclude that ROS-induced TRPV1 upregulation is responsible for discogenic LBP, and a schematic illustration of the potential mechanism is shown in Figure 5.
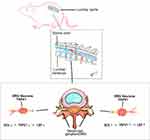 |
Figure 5 Illustration of ROS-induced upregulation of TRPV1 in DRGs of rats. Schematic illustration of DRG anatomy and potential mechanism. |
Discussion
Our data suggest that the ROS-induced upregulation of TRPV1 plays a pivotal role in mediating LBP in rats. In the discogenic LBP rat model, bilateral DRGs at L1–L3 showed a remarkable increase in ROS levels, and when excessive ROS were neutralized, discogenic LBP was ameliorated. Additionally, excessive ROS stimulated the upregulation of TRPV1 in DRGs, and the TRPV1 channel played a critical role in mediating DRGs. Thus, we may conclude that the upregulation of ROS in DRGs causes discogenic LBP by stimulating the upregulation of TRPV1 in DRGs.
There is no doubt that DRGs are critical relay stations that connect the peripheral and central nervous systems. Afferent nerve fibers from various spinal structures, such as facet joints, spinous processes, back muscles, and sacroiliac joints, are organized in DRGs and maintained within the dorsal horn.5,11 The afferent nerve fibers of IVDs are also organized in DRGs and project to the central nervous system. The lower IVDs are innervated predominantly by upper DRGs; namely, retrograde tracing methods have suggested that a lower IVD (L5–L6) is innervated predominantly by upper (L1 and L2) DRG neurons via the sympathetic trunk, as demonstrated by neurotracer Fluoro-Gold.10 When DRGs are damaged by physical or chemical stimulation, animals show obvious peripheral pain.16 In contrast, in an LBP model, different kinds of pain-related peptides, metabolites, or proteins increase in DRG neurons.17 Therefore, the role of DRGs in disc-related pain is critical.
Previous studies have revealed that excessive ROS strongly correlate with LBP and IVD degeneration,9 but there have been no reports on how degenerated IVDs induce the overexpression of ROS in DRGs. It has been suggested that injured discs may produce various neural factors, such as nerve growth factor, brain-derived neurotrophic factor, or serious proinflammatory factors.18,19 Considering that DRGs innervate the IVD with many nerve fibers and additional fibers would grow into the IVD after degeneration, we speculated that increased neural factors or proinflammatory factors may cause overexpression of ROS via retrograde transportation in axons.
ROS are involved in many nervous system activities, especially in neuropathic pain,20 and the elimination of excessive ROS significantly reduces neuroexcitability and restricts pain-related pathological pain signal transmission.21 For example, Xu et al demonstrated that upregulated ROS levels resulted in the plasma membrane translocation of PKCε in rat DRG neurons during neuropathic pain.22 It has also been suggested that ROS could induce neurite outgrowth to remodel nerve sensitivity.23,24 Our results are in accordance with the first mechanism that the upregulation of ROS induces the overexpression of TRPV1 and that the neutralization of excessive ROS inhibits the expression of TRPV1, thereby resulting in chronic nerve sensitivity.
TRPV1 is a nonselective cation channel expressed in nociceptors, and it is involved in a variety of physiological and pathophysiological neural processes.25,26 Previous studies have verified that inhibition of the function or expression of TRPV1 in DRGs significantly attenuates injury-induced neuropathic pain,27,28 and clinical trials on TRPV1 antagonists for pain treatment have progressed remarkably.26 TRPV1-induced hypersensitivity may induce the upregulation of a series of neural peptides or enhance synaptic transportation.29 In our study, inhibition of TRPV1 with AMG-9810 also resulted in a decrease in SP and CGRP levels, which is in accordance with a previous explanation. Thus, TRPV1 may represent an alternative target for LBP relief.
Treatment of DRGs is an important strategy for alleviating chronic and refractory LBP. In clinical practice, DRG stimulation has progressed significantly in patients with chronic LBP.30,31 The potential mechanism for DRG stimulation is that electric fields may have effects on immune modulation, which inhibits the expression of proinflammatory cytokines.32 In addition, in the study by Richardson et al, the bilateral L1 and L2 DRGs of 12 patients were blocked using drug cocktail therapy (methylprednisolone 80 mg, clonidine 75 μg, and 0.5% bupivacaine 4 mL), but the therapeutic effect was controversial.33 Our present results suggest that neutralizing ROS or blocking TRPV1 has a significant analgesic effect, but further exploratory preclinical research is needed to verify this hypothesis.
However, this study has some limitations. Further cellular investigations are needed to demonstrate how ROS induce TRPV1 upregulation. In addition, we proved that TRPV1 is a downstream effector protein of ROS in DRGs. The pathological effects of other potentially important proteins, such as NaV1.7 or Piezo-1, may also contribute to discogenic LBP. Finally, the long-term pharmacological effectiveness of this novel treatment requires further clinical and research evidence.
In conclusion, increased ROS in DRGs play a primary pathological role in puncture-induced discogenic LBP, and excessive ROS–induced upregulation of TRPV1 in DRGs is the underlying pathophysiological mechanism that causes nerve sensitization and discogenic LBP. Therapeutic targeting of ROS or TRPV1 may be a promising method for discogenic LBP treatment.
Abbreviations
LBP, low back pain; IVD, intervertebral disc; DRG, dorsal root ganglion; ROS, reactive oxygen species; TRPV1, transient receptor potential vanilloid-1; SP, substance P; CGRP, calcitonin gene related peptide.
Data Sharing Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
This work was supported by a grant from the Science and Technology Development Fund Project (Grant No. QKY2023-19) of Qingpu District, and the Health Commission research project (Grant No. QWJ2023-06) in Qingpu District. We would appreciate the contribution from Mrs djt.C for the illustration of Figure 5. We thank LetPub (Accdon LLC) for its linguistic assistance during the preparation of this manuscript.
Disclosure
The authors declare no conflicts of interest regarding the publication of this paper.
References
1. Hoy D, Brooks P, Blyth F, Buchbinder R. The epidemiology of low back pain. Best Pract Res. 2010;24(6):769–781. doi:10.1016/j.berh.2010.10.002
2. Shetty GM, Jain S, Thakur H, Khanna K. Prevalence of low back pain in India: a systematic review and meta-analysis. Work. 2022;73(2):429–452. doi:10.3233/wor-205300
3. García-Cosamalón J, Del Valle ME, Calavia MG, et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J Anatomy. 2010;217:1–15. doi:10.1111/j.1469-7580.2010.01227.x
4. Ohtori S, Miyagi M, Inoue G. Sensory nerve ingrowth, cytokines, and instability of discogenic low back pain: a review. Spine Surg Related Res. 2018;2(1):11–17. doi:10.22603/ssrr.2016-0018
5. Chapman KB, Groenen PS, Vissers KC, van Helmond N, Stanton-Hicks MD. The pathways and processes underlying spinal transmission of low back pain: observations from dorsal root ganglion stimulation treatment. Neuromodulation. 2021;24(4):610–621. doi:10.1111/ner.13150
6. Krames ES. The role of the dorsal root ganglion in the development of neuropathic pain. Pain Med. 2014;15(10):1669–1685. doi:10.1111/pme.12413
7. Huygen F, Liem L, Cusack W, Kramer J. Stimulation of the L2–L3 dorsal root ganglia induces effective pain relief in the low back. Pain Pract. 2018;18(2):205–213. doi:10.1111/papr.12591
8. Malcangio M. Role of the immune system in neuropathic pain. Scand J Pain. 2019;20:33–37. doi:10.1515/sjpain-2019-0138
9. Zheng J, Zhang J, Zhang X, Guo Z, Wu W. Reactive oxygen species mediate low back pain by upregulating substance p in intervertebral disc degeneration. Oxid Med Cell Longevy. 2021;2021:6681815. doi:10.1155/2021/6681815
10. Orita S, Eguchi Y, Kamoda H, et al. Brain-derived neurotrophic factor inhibition at the punctured intervertebral disc downregulates the production of calcitonin gene-related peptide in dorsal root ganglia in rats. Spine. 2011;36(21):1737–1743. doi:10.1097/BRS.0b013e31821d7b9f
11. Aoki Y, Takahashi Y, Ohtori S, Moriya H, Takahashi K. Distribution and immunocytochemical characterization of dorsal root ganglion neurons innervating the lumbar intervertebral disc in rats: a review. Life Sci. 2004;74(21):2627–2642. doi:10.1016/j.lfs.2004.01.008
12. Jiao Y, Yuan Y, Lin Y, et al. Propionibacterium acnes induces discogenic low back pain via stimulating nucleus pulposus cells to secrete pro-algesic factor of IL-8/CINC-1 through TLR2-NF-kappaB p65 pathway. J Mol Med. 2019;97:25–35. doi:10.1007/s00109-018-1712-z
13. van Heeswijk VM, Thambyah A, Robertson PA, Broom ND. Does an annular puncture influence the herniation path?: an in vitro mechanical and structural investigation. Spine. 2018;43(7):467–476. doi:10.1097/BRS.0000000000002336
14. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi:10.1016/0165-0270(94)90144-9
15. Jiao Y, Lin Y, Zheng J, et al. Propionibacterium acnes contributes to low back pain via upregulation of NGF in TLR2-NF-κB/JNK or ROS pathway. Microbes Infect. 2022;24(6–7):104980. doi:10.1016/j.micinf.2022.104980
16. Lin XY, Yang J, Li HM, Hu SJ, Xing JL. Dorsal root ganglion compression as an animal model of sciatica and low back pain. Neurosci Bulletin. 2012;28:618–630. doi:10.1007/s12264-012-1276-9
17. Sadamasu A, Sakuma Y, Suzuki M, et al. Upregulation of NaV1.7 in dorsal root ganglia after intervertebral disc injury in rats. Spine. 2014;39:E421–E426. doi:10.1097/brs.0000000000000229
18. Kartha S, Zeeman ME, Baig HA, Guarino BB, Winkelstein BA. Upregulation of BDNF and NGF in cervical intervertebral discs exposed to painful whole-body vibration. Spine. 2014;39:1542–1548. doi:10.1097/brs.0000000000000457
19. Nakawaki M, Uchida K, Miyagi M, et al. Changes in nerve growth factor expression and macrophage phenotype following intervertebral disc injury in mice. J Orthopaedic Res. 2019;37:1798–1804. doi:10.1002/jor.24308
20. Calvo M, Dawes JM, Bennett DL. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012;11(7):629–642. doi:10.1016/s1474-4422(12)70134-5
21. Grace PM, Gaudet AD, Staikopoulos V, et al. Nitroxidative signaling mechanisms in pathological pain. Trends Neurosci. 2016;39(12):862–879. doi:10.1016/j.tins.2016.10.003
22. Xu J, Wu S, Wang J, et al. Oxidative stress induced by NOX2 contributes to neuropathic pain via plasma membrane translocation of PKCε in rat dorsal root ganglion neurons. J Neuroinflammation. 2021;18(1):106. doi:10.1186/s12974-021-02155-6
23. Kotake-Nara E, Saida K. Characterization of CoCl2-induced reactive oxygen species (ROS): inductions of neurite outgrowth and endothelin-2/vasoactive intestinal contractor in PC12 cells by CoCl2 are ROS dependent, but those by MnCl2 are not. Neurosci Lett. 2007;422(3):223–227. doi:10.1016/j.neulet.2007.06.026
24. He W, Cui L, Zhang C, et al. Sonic hedgehog promotes neurite outgrowth of cortical neurons under oxidative stress: involving of mitochondria and energy metabolism. Exp Cell Res. 2017;350(1):83–90. doi:10.1016/j.yexcr.2016.11.008
25. Li F, Wang F. TRPV1 in pain and itch. Adv Exp Med Biol. 2021;1349:249–273. doi:10.1007/978-981-16-4254-8_12
26. Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: recent advances and setbacks. Brain Res Rev. 2009;60(1):267–277. doi:10.1016/j.brainresrev.2008.12.006
27. Wang Z, Ling D, Wu C, Han J, Zhao Y. Baicalin prevents the up-regulation of TRPV1 in dorsal root ganglion and attenuates chronic neuropathic pain. Vet Med Sci. 2020;6(4):1034–1040. doi:10.1002/vms3.318
28. Ma W, St‐Jacques B, Rudakou U, Kim YN. Stimulating TRPV 1 externalization and synthesis in dorsal root ganglion neurons contributes to PGE 2 potentiation of TRPV 1 activity and nociceptor sensitization. Eur J Pain. 2017;21(4):575–593. doi:10.1002/ejp.959
29. Gunthorpe MJ, Szallasi A. Peripheral TRPV1 receptors as targets for drug development: new molecules and mechanisms. Curr Pharm Des. 2008;14:32–41. doi:10.2174/138161208783330754
30. Chapman KB, van Roosendaal BK, Yousef TA, Vissers KC, van Helmond N. Dorsal root ganglion stimulation normalizes measures of pain processing in patients with chronic low-back pain: a prospective pilot study using quantitative sensory testing. Pain Pract. 2021;21(5):568–577. doi:10.1111/papr.12992
31. Chapman KB, Groenen PS, Patel KV, Vissers KC, van Helmond N. T12 dorsal root ganglion stimulation to treat chronic low back pain: a case series. Neuromodulation. 2020;23(2):203–212. doi:10.1111/ner.13047
32. Sluijter ME, Teixeira A, Serra V, Balogh S, Schianchi P. Intra-articular application of pulsed radiofrequency for arthrogenic pain--report of six cases. Pain Pract. 2008;8:57–61. doi:10.1111/j.1533-2500.2007.00172.x
33. Richardson J, Collinghan N, Scally AJ, Gupta S. Bilateral L1 and L2 dorsal root ganglion blocks for discogenic low-back pain. Br J Anaesth. 2009;103(3):416–419. doi:10.1093/bja/aep166
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.