Back to Journals » International Journal of General Medicine » Volume 16
ΔRDW Could Predict Major Adverse Cardiovascular Events in Patients with Heart Failure with Reduced Ejection Fraction After Sacubitril/Valsartan Treatment
Authors Wang J, Zhao J, Lin Q, Xu X, Jiang K, Li Y
Received 13 October 2023
Accepted for publication 9 December 2023
Published 19 December 2023 Volume 2023:16 Pages 5989—6003
DOI https://doi.org/10.2147/IJGM.S444585
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yuriy Sirenko
Jingsheng Wang,* Jian Zhao,* Quanqiang Lin,* Xiuxiu Xu, Ke Jiang, Yuanmin Li
Department of Cardiology, the Second Affiliated Hospital, Shandong First Medical University & Shandong Academy of Medical Sciences, Taian, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuanmin Li, Department of Cardiology, the Second Affiliated Hospital, Shandong First Medical University & Shandong Academy of Medical Sciences, No. 366 Taishan Street, Taian, 271000, People’s Republic of China, Tel +86-13583843518, Fax +86-538-6222036, Email [email protected]
Objective: This study aimed to evaluate the association between red blood cell distribution width (RDW) changes and major adverse cardiovascular event (MACE) occurrences during sacubitril/valsartan treatment in patients with heart failure with reduced ejection fraction (HFrEF).
Methods: This study retrospectively analyzed the medical records of patients with HFrEF hospitalized from April 2018 to February 2021. The patients were divided into two groups according to the inclusion of sacubitril/valsartan in the personal drug treatment regimen, the traditional and the sacubitril/valsartan group. RDW values before and after sacubitril/valsartan treatment were recorded respectively as RDW1 and RDW2. ΔRDW was defined as the difference between RDW2 and RDW1. The patients in the sacubitril/valsartan group were divided into two subgroups according to ΔRDW > 0 or ≤ 0. MACEs, such as readmission for HF, acute myocardial infarction, ischemic stroke, and malignant arrhythmia and death, were recorded during the 1-year follow-up period in each group.
Results: MACE development was lower in patients treated with sacubitril/valsartan than those treated with conventional therapy (log-rank, P< 0.001). The incidence of cardiac events during the follow-up period was greater in the group with ΔRDW > 0 than in the group with ΔRDW ≤ 0 (Breslow, P< 0.001). Increased RDW was associated with a higher likelihood of developing MACE than decreased RDW (odds ratio [OR] =2.055, 95% confidence interval [CI]:1.301– 3.246), and the risk of developing MACE increased by 22.1% for each unit increase in RDW (OR=1.221, 95% CI:1.074– 1.389).
Conclusion: Sacubitril/valsartan treatment is effective in reducing the risk of MACEs in HFrEF. Additionally, RDW changes are predictors of MACEs after sacubitril/valsartan treatment.
Keywords: red blood cell distribution width, heart failure, sacubitril/valsartan, major adverse cardiovascular events
Introduction
Heart failure (HF) is a complex clinical syndrome caused by ventricular overfilling, impaired ejection capacity, or both. HF has affected up to 23 million people worldwide, with HF with reduced ejection fraction (HFrEF) accounting for approximately 50%.1
Red blood cell distribution width (RDW) is a routine laboratory marker that reflects the degree of peripheral red blood cell volume heterogeneity. RDW abnormalities are seen in the nervous, respiratory, hematologic, and cardiovascular circulatory systems, and are elevated in tumors and novel coronavirus pneumonia.2 RDW has the same research value as N-terminal pro-b-type natriuretic peptide (NT-proBNP) in predicting early clinical HF outcomes.3 However, a single RDW measurement does not reflect dynamic changes in disease status, especially similar diseases to recurrent HF episodes, have a long disease duration, and are at high risk of death. A valid indicator is needed to reflect the treatment effect at different stages, and longitudinal RDW assessment over time may be a more effective indicator than baseline values.
A new concept, ΔRDW, has recently been proposed to reflect dynamic changes in RDW. It is used either to record the difference between RDW values at baseline and at various time points after clinical treatment or name unit changes in RDW in the study population by the investigator. ΔRDW has aroused the attention of some researchers in predicting the risk of infection in particular people,4 early postoperative morbidity,5 disease survival outcomes,6,7 and risk stratification of patients.8
The current study did not address dynamic RDW changes after sacubitril-valsartan treatment in patients with HF. Additionally, the association between RDW and major adverse cardiovascular events (MACEs)remained unknown.
Therefore, this study aimed to investigate the use of RDW changes as predictors of risk stratification and long-term prognosis in patients with cardiovascular disease.
Materials and Methods
Participant Selection
Retrospective analyses were performed on the medical records of patients hospitalized for HF from April 2018 to February 2021. Eligible patients were aged≥18 years and diagnosed with HFrEF. This study has been approved by the Ethics Committee of the Second Affiliated Hospital of Shandong First Medical University. The investigation conformed to the principles outlined in the Declaration of Helsinki and the informed written consent was given prior to the inclusion of subjects in the study (See Figure 1).
 |
Figure 1 Study flow chart. |
General Clinical Data
The electronic medical record system of JiaheMeikang in Beijing was retrospectively reviewed. Additionally, sex, age, and history of hypertension, diabetes, previous smoking, alcohol consumption, and medication were collected. Specific medication history included: cardiac glycosides, β-blockers, thrombin inhibitors, lipid-lowering drugs, antiplatelet drugs, calcium channel blockers (CCBs), angiotensin-converting enzyme inhibitors (ACEIs), and angiotensin receptor blockers (ARBs). MACE was defined as readmission due to HF, acute myocardial infarction, malignant arrhythmia, ischemic stroke, and death.
Data Collection
Test results of platelet count (PLT), D-dimer, white blood cell count, low-density lipoprotein, hemoglobin, triglyceride, neutrophil percentage, RDW, fasting blood glucose, alanine aminotransferase, hematocrit, aspartate aminotransferase, blood urea nitrogen, serum potassium, total cholesterol, creatinine, and homocysteine were collected by the Remex laboratory management system. Left ventricular ejection fraction (LVEF) was obtained from a cardiac ultrasound report.
Methods of Measurement
RDW values of blood parameters measured at initial admission were recorded as RDW1 for patients in the sacubitril-valsartan treatment group. ΔRDW was defined as the difference between the last and first values (ie, ΔRDW = RDW2 − RDW1). The 1-year incidence of MACE was ascertained by examining medical records and by telephone after discharge.
Statistical Analysis
Data were expressed as means±standard deviations. Baseline characteristics were compared between the traditional and sacubitril/valsartan groups. Continuous variables were analyzed using Student’s t-test or Mann–Whitney U-test, and categorical variables were analyzed using the chi-square test. Multivariable Cox proportional hazards regression models were performed to identify independent risk factors associated with a 1-year incidence of MACE. Kaplan-Meier survival analysis was performed according to ΔRDW. All statistical analyses were performed using Statistical Package for the Social Sciences version 26 (IBM released in 2019; IBM SPSS Statistics for Windows, Version 26.0. Amenck, NY: IBM), and P-values of <0.05 was considered statistically significant.
Results
Comparison of MACE Between the Sacubitril/Valsartan and Traditional Groups
Baseline Characteristics
The sacubitril/valsartan group included 205 patients, ages 64.70±13.93 years, 76.1%were male, with a mean LVEF of 32.47% ±5.70%, and fewer patients (14.1%) had comorbid hypertension, as shown in Table 1. Significant differences were found in gender, LVEF values, systolic blood pressure, and diastolic blood pressure between the two groups, with statistical significance (P<0.05). RDW significantly decreased more in the sacubitril/valsartan than in the traditional groups (−0.40 [−1.45–0.70] vs 0.30 [−0.60–1.20], P<0.05). Both groups had a history of long-term smoking (51.2%vs 42.0%, P = 0.057) and alcohol consumption (52.2%vs 47.9%, P=0.382). NT-proBNP levels were above the normal range in both groups, with no significant difference (P>0.05).
 |
Table 1 Baseline Data of Sacubitril/Valsartan Group and Traditional Group |
Sacubitril/Valsartan versus Traditional Cox Analysis
The dependent variable was assigned (MACE=1, no MACE =0), and covariates were assigned a medical history of hypertension (yes=1, no=0), gender (male=1, female=0), cardiac glycosides (yes=1, no=0), beta drugs (yes=1, no=0), thrombin inhibitors (yes=1, no=0), and treatment regimen (sacubitril/valsartan treatment=1, traditional=0). Continuous numerical variables are not assigned values. The impact ofMACEs in both groups was only related to sacubitril treatment, as shown in Table 2. Pharmacological treatment with sacubitril/valsartan is a protective factor for MACE events in patients with HFrEF.
 |
Table 2 Multivariate COX Analysis of Two Treatment Regimens |
Kaplan-Meier Curve Analysis by Treatment Regimen
During the 1-year follow-up period, 98 and 154 MACEs occurred in the sacubitril/valsartan and traditional treatment groups, with 47.8% and 70.3% incidence of cardiac events, respectively (P<0.05). The mean time to MACE was compared between the two groups in Table 3. Mean event periods were lower in the conventional group than in the sacubitril/valsartan group. Kaplan-Meier analysis revealed a higher incidence of cardiac events during the observation period (log-rank, P<0.001), which occurred earlier in the conventional group than in the sacubitril/valsartan group (Figure 2).
 |
Table 3 Mean Time to Cardiac Event in Sacubitril/Valsartan versus Traditional Arm |
 |
Figure 2 Survival analysis of sacubitril-valsartan group versus traditional group. |
Comparison of Positive and Negative Changes in ΔRDW MACEs in the Sacubitril/Valsartan Group
General Data of Positive and Negative Change Grouping of ΔRDW
The effect of sacubitril/valsartan drugs on treatment outcomes was separately analyzed based only on considering the actual difference in RDW before and after sacubitril/valsartan treatment.
Of 205 patients treated with sacubitril/valsartan having ΔRDW of ≤0, 123 and 82 were classified as negative and positive ΔRDW, respectively. The baseline clinical characteristics of patients are shown in Table 4. No significant differences were found between the groups with ΔRDWof >0 and ≤0 in terms of age, gender, smoking history, drinking history, hypertension, and diabetes. Echocardiographic findings revealed no difference in LVEF between the two groups. Among the laboratory parameters, the group with ≤0 ΔRDW had lower leukocyte counts and higher urea and creatinine levels.
 |
Table 4 General Data of Positive and Negative Change Grouping of ΔRDW |
Correlation Between RDW and Multivariate
Correlation analysis in Table 5 shows a positively correlated baseline RDW and ΔRDW change rate, NT-proBNP, and urea (R=0.589, P<0.001; R=0.400, P<0.001; R=0.237, P=0.001, respectively).
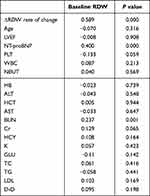 |
Table 5 Pearson Correlation Analysis Between RDW and Continuous Numerical Variables |
Kaplan-Meier Curve Analysis of RDW Change and the Occurrence of MACEs
The observation period revealed 98 (47.8%) cardiac events, including readmission for HF (n=92), acute myocardial infarction (n=1), ischemic stroke (n=1), malignant arrhythmia (n=3), and sudden cardiac death due to HF (n=1), as shown in Table 6. The incidence of cardiac events was 51.22% and 45.53% in the ΔRDW of >0 and ≤0 groups, respectively. Kaplan-Meier analysis of positive and negative RDW changes revealed higher cardiac event incidences during the observation period in the group with >0ΔRDWthan in the group with ≤0ΔRDW (Breslow, P<0.001) (Figure 3).
 |
Table 6 Distribution Table for MACE Events |
 |
Figure 3 Survival analysis Plot for Positive and Negative RDW Changes. |
The mean survival time in the group with ≤0 ΔRDWwas 9.981 months (95% confidence interval [CI]: 9.467–10.496), which was significantly higher than that in the group with >0 ΔRDW (mean=8.307, 95% CI: 7.297–9.316), as shown in Table 7.
 |
Table 7 Mean Time to MACE for Positive and Negative RDW Changes |
Multivariate Cox Risk Regression Analysis
Dependent variables were assigned (MACE =1, no MACE =0), covariates were assigned: ΔRDW group (group with >0 ΔRDW=1, group with ≤0 ΔRDW =0), history of hypertension (yes=1, no=0), history of diabetes (yes=1, no=0), history of smoking (yes=1, no=0), history of alcohol consumption (yes=1, no=0), sex (male=1, female=0), age (≥65 years=1, <65 years=0), cardiac glycosides (yes=1, no=0), beta drugs (yes=1, no=0), thrombin inhibitors (yes=1, no=0), lipid-lowering drugs (yes=1, no=0), antiplatelets (yes=1, no=0), CCB drugs (yes=1, no=0), ACEI drugs (yes=1, no=0), no=0), and ARB medications (yes=1, no=0). Continuous numerical variables are not assigned values.
This study performed univariate and multivariate Cox proportional hazards model analyses to investigate the relationship between forward ΔRDW and MACEs. Univariate analysis showed a significant association between forward ΔRDW levels and cardiac events, with a hazard ratio of 2.061 (95% CI: 1.312–3.253, P<0.05) (Table 8). A multivariate model adjusted for possible confounders (ie, smoking, thrombin inhibitors, PLT, RDW, and diabetes) revealed a significant association between the forward ΔRDW level and cardiac events, with a hazard ratio of 2.055 (95% CI: 1.301–3.246, P=0.002). Smokers and patients with diabetes were 1.919 (95% CI: 1.270–2.901, P=0.002) and 1.825 (95% CI: 1.136–2.931, P =0.013) times more likely to have a MACE, respectively, and each unit increase in RDW was associated with a 22.1% increased risk of a MACE (95% CI: 1.074–1.389, P=0.002), as shown in Table 9.
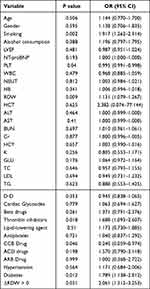 |
Table 8 ΔRDW versus MACE Event Univariate Cox Proportional Hazard Regression Model |
 |
Table 9 ΔRDW versus MACE Event Multivariate Cox Proportional Hazard Regression Model |
Discussion
This study revealed several significant findings. First, the risk of cardiac events in patients with HFrEF varies between treatment regimens. The sacubitril/valsartan drug regimen is a protective factor for MACEdevelopment, with RDW showing a more pronounced decrease in this group. Second, the Kaplan-Meier analysis for the sacubitril-valsartan group revealed more frequent cardiac events in the positive ΔRDW group (ΔRDW of >0) compared to the negative ΔRDW group (ΔRDW of ≤0). These results suggest that RDWchanges are associated with prognosis in patients with HFrEF treated with sacubitril-valsartan drugs.
Ventricular remodeling is involved in the disease progression of HF and is an essential process of its pathological changes. Renin, angiotensin, and aldosterone system hyperexcitability is an essential physiological mechanism that leads to ventricular structural changes and disease progression.9 At present, angiotensin receptor-neprilysin inhibitor is often used as the drug of choice in the clinical treatment of HF. Enkephalinase is a necessary endonuclease to hydrolyze several endogenous vasoactive peptides, including vasodilators, such as natriuretic peptide and bradykinin, and vasoconstrictors, such as angiotensin.10 The natriuretic peptide level increases correspondingly as neprilysin is inhibited, but the degradation of vasoconstrictors, such as angiotensin, simultaneously decreases. Enkephalin inhibitors alone may be less effective in treating HF because vasodilation and vasoconstriction are mutually antagonistic. Concomitant use with angiotensin antagonists may compensate for the disadvantages of antagonism between relaxation and contraction.11 Sacubitril and valsartan sodium tablets can selectively act on angiotensin II type 1 receptor (AT1R), thereby blocking angiotensin II to achieve the treatment purpose,12 and can inhibit myocardial fibrosis and myocardial hypertrophy, thereby improving hemodynamics and cardiac function in patients with cardiomyopathy. It inhibits vascular factors and norepinephrine, increases renal blood flow, and reduces aldosterone secretion, thereby lowering blood pressure and reducing the burden on the patient’s heart. It can have a long-term stable therapeutic effect and is more suitable for long-term application.13
Related studies revealed that sacubitril-valsartan reduces the risk of HF rehospitalization by 21% compared with traditional treatment modalities.14 Notably, both groups significantly revealed that older males are at high risk for HF.
RDW independently predicted 1-year mortality in patients with acute and chronic HF.15 This study revealed that each unit increase in RDW was associated with a 22.1% (95% CI: 1.074–1.389, P = 0.002) increased risk of a MACE. The mechanism of RDW abnormalities in cardiovascular disease is not precisely explained. Chronic inflammation, oxidative stress, and neurohormonal activation play an essential role among many potential factors. Previous studies revealed that inflammation causes bone marrow suppression, decreases renal erythropoietin synthesis, and triggers apoptosis of erythroid precursors in the bone marrow, resulting in differences in RBC volume and size, with increased RDW values in laboratory results.16 This study revealed that RDW showed some negative relationship with hemoglobin and a positive relationship with NT-proBNP.
The role of RDW in HF continues to be explored, and this observation is based on a single RDW measure; however, no studies were reported on the effect of RDW changes before and after HF treatment on assessing the prognosis of patients due to the lack of attention and in-depth studies. A new concept, ΔRDW, has recently been proposed to reflect dynamic changes in RDW. It can be used either to record the difference between RDW values at baseline and at various time points after clinical treatment or to name unit changes in RDW in the study population by the investigator. Turcato et al17 revealed that ΔRDW was low and stable in patients who survived within 30 days, and negative changes in RDW better reflected the treatment effect. Our study followed the MACE occurrence in patients with HFrEF treated with sacubitril/valsartan over 1 year. Kaplan-Meier analysis showed that the increased ΔRDW group was more likely to have a significant adverse cardiac event than the decreased ΔRDW group during the observation period. Univariate analysis showed a significant association between forward ΔRDW levels and cardiac events with a hazard ratio of 2.061 (95% CI: 1.312–3.253, P<0.05). A multivariate model adjusted for possible confounders (ie, smoking, thrombin inhibitors, PLT, RDW, diabetes) revealed a significant association between the forward ΔRDW level and cardiac events with a hazard ratio of 2.055 (95% CI: 1.301–3.246, P=0.002). Deaths occurred in the negative ΔRDW group in this study although they had a better prognosis than the positive ΔRDW group. Retrospective data revealed that this case had ΔRDW of −6.0, 10weeks reexamination period. The time to patient death was 3 months; thus, it was proposed that the incidence of cardiac events increased when considerably RDW changed, whether increased or decreased, and further validation was required.
Patients in the traditional group had significantly higher blood pressure than those in the sacubitril/valsartan group, which was related to the fact that patients also produced some antihypertensive effect during the sacubitril/valsartan treatment for HF in this study. The association between hypertension and HF is well-known and left ventricular hypertrophy, arteriosclerosis, and renal impairment are all likely to contribute to the development of this syndrome.18 In terms of treatment, diuretics are often required to control sodium and water retention in HF, making them first-line therapy for comorbid hypertension.
Blood pressure control remained significantly lower than expected in patients in the traditional group, and blood pressure was not well controlled in more than one-third of patients although three different classes of blood pressure lowering drugs, including ARBs or ACEIs, CCBs, and diuretics, were used during treatment. Enkephalin inhibition reduced the number of patients with hypertension and blood pressure in patients who still had hypertension in the sacubitril/valsartan group. Table 1 shows that sacubitril/valsartan is not used in a large and combined manner in antihypertensive drugs because patients have reached their maximum personal tolerance when treated with sacubitril/valsartan, and increasing the dose can cause hypotensive discomfort, affected by blood pressure. Elderly patients are less likely to receive target doses or HF drug combinations. A related analysis of the PIONEER-HF trial19 revealed that the therapeutic efficacy and safety of sacubitril/valsartan were generally consistent across dose levels. The results broadly support the continued use of sacubitril/valsartan at the start of the first admission and after discharge. Additionally, they include patients who may not tolerate up-titration to the target dose and maximum dose in the early stages.
Enkephalin inhibition may be an effective and safe antihypertensive therapy in HFrEF and is a valuable supplement to antihypertensive treatment for patients.
The laboratory parameters urea and creatinine slightly increased within the normal range in the sacubitril/valsartan group without significantly declining renal function compared with the conventional group, which is particularly important in patients simultaneously taking ARBs and MRAs, thereby reducing the risk of renal function decline and hyperkalemia.20 Relevant studies have confirmed that sacubitril/valsartan treatment is feasible, safe, and beneficial in patients with severe HF receiving hemodialysis.21 Sacubitril-valsartan, in addition to more effective blood pressure lowering drugs, reduces the degree of arteriosclerosis and left ventricular mass more than ARBs,22 and switching from ACEIs or ARBs to sacubitril/valsartan may provide a new, safe, and valuable method for controlling refractory hypertension in patients with HFrEF.
This study revealed that patients with long-standing diabetes and inadequate glycemic control are 1.789 times more likely to have cardiac events (95% CI: 1.138–2.812, P=0.012), and the hazard ratio increased to 1.825 (95% CI: 1.136–2.931, P=0.013) considering the combined effect of ΔRDW. Therefore, the prevalence of diabetes should also be considered when evaluating the prognosis of patients with HFrEF.
HF and type 2 diabetes mellitus (T2DM) frequently co-exist, and T2DM is prevalent in approximately 30–50% of patients with HF.23 Glucose and lipid metabolism dysregulation in T2DM triggers oxidative stress, activates various inflammatory pathways, and disrupts the normal regulation of cardiomyocyte proliferation and hypertrophy, leading to pathological myocardial remodeling, reflected on the one hand by progressive left ventricular dilation, and on the other hand, by reduced systolic function. Cardiac remodeling is a crucial mechanism in HF development and progression in patients with T2DM and is associated with mortality and HF hospitalization.24
Our findings suggest that reverse cardiac remodeling is similar in patients with HFrEF with or without T2DM following sacubitril/valsartan use. This can be explained by several factors. First, the mechanism of action of sacubitril/valsartan sodium tablets is to inhibit neprilysin, which is responsible for various vasoactive peptide degradation.25 Thus, sacubitril/valsartan increases vasoactive peptide concentrations, such as glucagon-like peptide 1, which is an integral part of insulin sensitivity and metabolism. This contributes to better glycemic control and enhances lipid mobilization from adipose tissue, muscle oxidative capacity, and adiponectin release, all of which improve pathological cardiac remodeling and play a significant role in delaying diabetic cardiomyopathy progression.26 The PARADIGM-HF study revealed14 that sacubitril-valsartan could improve insulin resistance by regulating the endocrine system in patients with diabetes and positively affect patients with HF with DM. Second, sacubitril/valsartan increases cyclic guanosine monophosphate levels, which is essential to prevent the loss of the protective effect of protein kinase G on cardiomyocyte stiffness and hypertrophy.24 Additionally, aldosterone levels may be elevated in T2DM, which inhibits nitric oxide synthase, promotes inflammation and fibrosis, and have direct deleterious effects on cardiomyocytes.27
This study revealed that regular sacubitril/valsartan sodium tablet use could effectively improve cardiac function and reduce readmission rate in patients with HFrEF and DM compared with traditional treatment regimens, with 98 and 154 readmissions in the sacubitril/valsartan and traditional groups, respectively.
Epidemiological studies have identified several risk factors associated with the development of cardiac events in HF. Smoking, dyslipidemia, etc., have also been implicated as potential factors for the risk of cardiac events, in addition to age and gender.28,29
The human heart has a sustained high energy demand. However, the failing heart does not consistently generate sufficient energy requirements. Lipids, among the underlying molecular mechanisms, are critical for cardiac metabolic flexibility, and lipotoxic compounds may be critical factors linking metabolic stress to sustained myocardial tissue injury.30 Plasma lipidomics profiles reflect alterations in cardiac lipid metabolism predisposing to HFin animal models.31 Additionally, lipid metabolism disorders are indirectly associated with HF through common comorbidities, including coronary artery disease (CAD)32 and T2DM.33 This study revealed no correlation between age, gender, blood lipid levels, and cardiac events. The results may be related to the small sample size or deviations caused by the use of drugs and diet control in patients during long-term follow-up.
Smoking may be another critical factor independent of traditional risk factors for HF.34 Smoking may have other effects that lead to cardiac dysfunction and HFalthough smoking increases the risk of CAD, the leading cause of HF. For example, smoking acutely increases systolic and diastolic blood pressure, total systemic vascular resistance, pulmonary arterial pressure, and pulmonary vascular resistance, which are known risk factors for HF.35 Additionally, smoking is associated with carbon monoxide exposure, which has been reported to increase oxidative stress and lead to impaired mitochondrial function, inflammation, endothelial function, and renal function deterioration, all of which are implicated in HF pathophysiology.29 This study revealed that MACE incidence was 1.917 times higher in smokers than in nonsmokers (95% CI: 1.262–2.914, P = 0.002) by univariate analysis, and the hazard ratio was 1.919 (95% CI: 1.270–2.901, P = 0.002) after accounting for RDW, ΔRDW, and diabetes. Therefore, smoking should also be included in assessing risk factors for MACEs in patients with HFrEF treated with sacubitril-valsartan.
Limitations
The results showed that RDW changes were significantly correlated with MACEs before and after sacubitril-valsartan treatment in patients with HFrEF. The decrease in RDW was a protective factor for MACEs. The specificity of this indicator was unsatisfactory although stable changes in RDW were found beneficial to patients and a cut-off value was calculated. Additionally, further verification of the validity of this value is needed. Two consecutive laboratory test reports should be used in selecting participants because this is a retrospective study. The review time range is defined as 3–6 months (according to the literature, the red blood cell life span of patients with HF is lower than that of the average population); thus, most patients are excluded, resulting in a small sample size. Therefore, we look forward to a more extensive study to further verify the relationship between the two and more fully understand the relationship between RDW changes and the long-term prognosis and risk stratification of patients with HFrEF.
Conclusions
Sacubitril/valsartan treatment is effective in reducing the risk of MACEs in HFrEF. Additionally, RDW changes are predictors of MACEs after sacubitril/valsartan treatment.
Data Sharing Statement
Data and material are transparent, and the corresponding author can be contacted if requested.
Ethical Approval
All the patient investigations conformed to the principles outlined in the Declaration of Helsinki, and the study was approved by the ethical committee of the Second Affiliated Hospital of Shandong First Medical University, Shandong, China. This article does not contain any studies with animals performed by any of the authors.
Consent to Participate
All the patients were informed about the purposes of the study and have signed their “consent of the patient”.
Consent for Publication
Written informed consent for the use of any clinical data in research was obtained for all patients. All individuals were informed about the purposes of the study and have signed their consent for publishing the data.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Murphy SP, Ibrahim NE, Januzzi JL. Heart failure with reduced ejection fraction: a review. JAMA. 2020;324(5):488–504. doi:10.1001/jama.2020.10262
2. Wang J, Xiao Q, Li Y. ΔRDW: a novel indicator with predictive value for the diagnosis and treatment of multiple diseases. Int J Gene Med. 2021;14:8667. doi:10.2147/IJGM.S339945
3. He W, Jia J, Chen J, et al. Comparison of prognostic value of red cell distribution width and NT-proBNP for short-term clinical outcomes in acute heart failure patients. Int Heart J. 2014;56(1):13–172. doi:10.1536/ihj.14-136
4. Guo B-F, Sun S-Z. Diagnostic accuracy of a dynamically increased red blood cell distribution width in very low birth weight infants with serious bacterial infection. Ital J Pediatr. 2021;47(1):1–7. doi:10.1186/s13052-021-00994-w
5. Lee SI, Lee SY, Choi CH, et al. Relation between changes in red blood cell distribution width after coronary artery bypass grafting and early postoperative morbidity. J Thoracic Dis. 2018;10(7):4244. doi:10.21037/jtd.2018.06.108
6. Ebata S, Yoshizaki A, Fukasawa T, et al. Increased red blood cell distribution width in the first year after diagnosis predicts worsening of systemic sclerosis-associated interstitial lung disease at 5 years: a pilot study. Diagnostics. 2021;11(12):2274. doi:10.3390/diagnostics11122274
7. Habibpour H, Torabi M, Mirzaee M. The value of red cell distribution width (RDW) and trauma-associated severe hemorrhage (TASH) in predicting hospital mortality in multiple trauma patients. Bull Emerg Trauma. 2019;7(1):55. doi:10.29252/beat-070108
8. Ferreira JP, Girerd N, Arrigo M, et al. Enlarging red blood cell distribution width during hospitalization identifies a very high-risk subset of acutely decompensated heart failure patients and adds valuable prognostic information on top of hemoconcentration. Medicine. 2016;95(14):e3307. doi:10.1097/MD.0000000000003307
9. Xu M, Yan L, Xu J, et al. Predictors and prognosis for incident in-hospital heart failure in patients with preserved ejection fraction after first acute myocardial infarction: an observational study. Medicine. 2018;16(1):97. doi:10.1186/s12916-018-1080-0
10. Chang P-C, Lin S-F, Chu Y, et al. LCZ696 therapy reduces ventricular tachyarrhythmia inducibility in a myocardial infarction-induced heart failure rat model. Cardiovasc Ther. 2019;2019:1–9. doi:10.1155/2019/6032631
11. Mochel JP, Teng CH, Peyrou M, et al. Sacubitril/valsartan (LCZ696) significantly reduces aldosterone and increases cGMP circulating levels in a canine model of RAAS activation. Eur J Pharm Sci. 2019;128:103–111. doi:10.1016/j.ejps.2018.11.037
12. Wadell D, Jensen J, Englund E, et al. Triple therapy after PCI–Warfarin treatment quality and bleeding risk. PLoS One. 2018;13(12):e0209187. doi:10.1371/journal.pone.0209187
13. Zheng X, Wu L. The value of cytochrome P4502C19 gene assay for anti-platelet therapy after PCI in stable angina patients with left main coronary artery lesions. J Southern Med Univ. 2020;40(2):274–278. doi:10.12122/j.issn.1673-4254.2020.02.21
14. McMurray JJ, Packer M, Desai AS, et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin‐converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM‐HF). Eur J Heart Fail. 2013;15(9):1062–1073. doi:10.1093/eurjhf/hft052
15. Wołowiec Ł, Rogowicz D, Banach J, et al. Prognostic significance of red cell distribution width and other red cell parameters in patients with chronic heart failure during two years of follow-up. Kardiol Pol. 2016;74(7):657–664. doi:10.5603/KP.a2016.0004
16. Förhécz Z, Gombos T, Borgulya G, et al. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009;158(4):659–666. doi:10.1016/j.ahj.2009.07.024
17. Turcato G, Zorzi E, Prati D, et al. Early in-hospital variation of red blood cell distribution width predicts mortality in patients with acute heart failure. Int J Cardiol. 2017;243:306–310. doi:10.1016/j.ijcard.2017.05.023
18. Slivnick J, Lampert BC. Hypertension and heart failure. Heart Fail Clin. 2019;15(4):531–541. doi:10.1016/j.hfc.2019.06.007
19. Berg DD, Braunwald E, DeVore AD, et al. Efficacy and safety of sacubitril/valsartan by dose level achieved in the PIONEER-HF trial. Heart Failure. 2020;8(10):834–843. doi:10.1016/j.jchf.2020.06.008
20. Damman K, Gori M, Claggett B, et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC. 2018;6(6):489–498. doi:10.1016/j.jchf.2018.02.004
21. Heyse A, Manhaeghe L, Mahieu E, et al. Sacubitril/valsartan in heart failure and end‐stage renal insufficiency. ESC Heart Fail. 2019;6(6):1331–1333. doi:10.1002/ehf2.12544
22. Neeland IJ, Winders BR, Ayers CR, et al. Higher natriuretic peptide levels associate with a favorable adipose tissue distribution profile. Journal of the American College of Cardiology. 2013;62(8):752–760. doi:10.1016/j.jacc.2013.03.038
23. Sharma A, Zhao X, Hammill BG, et al. Trends in noncardiovascular comorbidities among patients hospitalized for heart failure: insights from the get with the guidelines–heart failure registry. Circulation. 2018;11(6):e004646. doi:10.1161/CIRCHEARTFAILURE.117.004646
24. Tan Y, Zhang Z, Zheng C, et al. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat Rev Cardiol. 2020;17(9):585–607. doi:10.1038/s41569-020-0339-2
25. Kobalava Z, Kotovskaya Y, Averkov O, et al. Pharmacodynamic and pharmacokinetic profiles of sacubitril/valsartan (LCZ 696) in patients with heart failure and reduced ejection fraction. Cardiovasc. Ther. 2016;34(4):191–198. doi:10.1111/1755-5922.12183
26. Giamouzis G, Butler J. Glycaemic control in heart failure: a PARADIGM shift for patients with concomitant diabetes? The lancet. Diabetes Endocrinol. 2017;5:314–315.
27. Fredersdorf S, Endemann D, Luchner A, et al. Increased aldosterone levels in a model of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2009;117(01):15–20. doi:10.1055/s-2008-1073128
28. Wittenbecher C, Eichelmann F, Toledo E, et al. Lipid profiles and heart failure risk: results from two prospective studies. Circ Res. 2021;128(3):309–320. doi:10.1161/CIRCRESAHA.120.317883
29. Lu Y, Xu Z, Georgakis MK, et al. Smoking and heart failure: a Mendelian randomization and mediation analysis. SC Heart Fail. 2021;8(3):1954–1965. doi:10.1002/ehf2.13248
30. Goldberg IJ, Trent CM, Schulze PC. Lipid metabolism and toxicity in the heart. Cell Metab. 2012;15(6):805–812. doi:10.1016/j.cmet.2012.04.006
31. Halade GV, Kain V, Tourki B, et al. Lipoxygenase drives lipidomic and metabolic reprogramming in ischemic heart failure. Metabolism. 2019;96:22–32. doi:10.1016/j.metabol.2019.04.011
32. Ruiz‐Canela M, Hruby A, Clish CB, et al. Comprehensive metabolomic profiling and incident cardiovascular disease: a systematic review. J Am Heart Assoc. 2017;6:e005705.
33. Guasch-Ferré M, Hruby A, Toledo E, et al. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care. 2016;39(5):833–846. doi:10.2337/dc15-2251
34. Ahmed AA, Patel K, Nyaku MA, et al. Risk of heart failure and death after prolonged smoking cessation: role of amount and duration of prior smoking. Circulation. 2015;8(4):694–701. doi:10.1161/CIRCHEARTFAILURE.114.001885
35. Nicolozakes AW, Binkley PF, Leier CV. Hemodynamic effects of smoking in congestive heart failure. Am J Med Sci. 1988;296(6):377–380. doi:10.1097/00000441-198812000-00002
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
