Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Ravulizumab in Myasthenia Gravis: A Review of the Current Evidence
Authors Vu T, Wiendl H, Katsuno M, Reddel SW, Howard JF Jr
Received 8 June 2023
Accepted for publication 17 October 2023
Published 1 December 2023 Volume 2023:19 Pages 2639—2655
DOI https://doi.org/10.2147/NDT.S374694
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Richard J Porter
Tuan Vu,1 Heinz Wiendl,2 Masahisa Katsuno,3 Stephen W Reddel,4 James F Howard Jr5
1Department of Neurology, University of South Florida Morsani College of Medicine, Tampa, FL, USA; 2Department of Neurology with Institute of Translational Neurology, University Hospital Münster, Münster, Germany; 3Department of Neurology, Nagoya University Graduate School of Medicine, Nagoya, Japan; 4Department of Neurology, Concord Hospital, University of Sydney, Sydney, NSW, Australia; 5Department of Neurology, The University of North Carolina, Chapel Hill, NC, USA
Correspondence: Tuan Vu, Department of Neurology, University of South Florida Morsani College of Medicine, 13220 USF Laurel Drive, Tampa, FL, 33612, USA, Tel +1 813 396 2955, Email [email protected]
Abstract: The terminal complement C5 inhibitor ravulizumab was engineered from the humanized monoclonal antibody eculizumab to have an extended half-life and duration of action. It binds to human terminal complement protein C5, inhibiting its cleavage into C5a and C5b, thus preventing the cascade of events that lead to architectural destruction of the postsynaptic neuromuscular junction membrane by the membrane attack complex, and consequent muscle weakness in patients with anti-acetylcholine receptor (AChR) antibody-positive generalized myasthenia gravis (gMG). The 26-week randomized, placebo-controlled period (RCP) of the phase 3 CHAMPION MG study demonstrated the rapid efficacy of ravulizumab in reducing MG symptoms. Weight-based dosing of ravulizumab every 8 weeks provided sustained efficacy, in terms of patient-reported (Myasthenia Gravis–Activities of Daily Living) and clinician-reported (Quantitative Myasthenia Gravis) endpoints in patients with anti-AChR antibody-positive gMG. Pharmacokinetic and pharmacodynamic analyses showed therapeutic serum ravulizumab concentrations (> 175 μg/mL) were achieved immediately after the first dose and were maintained throughout 26 weeks, irrespective of patient body weight; inhibition of serum free C5 was immediate, complete (< 0.5 μg/mL), and sustained in all patients. Interim results from the open-label extension (OLE) showed that after 60 weeks, efficacy was maintained in patients continuing on ravulizumab. Rapid and sustained improvements in efficacy, similar to those seen in patients initiating ravulizumab in the RCP, were observed after initiation of ravulizumab treatment in patients who switched from placebo in the RCP to ravulizumab in the OLE. The findings from the RCP and OLE support ravulizumab’s favorable safety profile. In conclusion, ravulizumab has a simple weight-based administration and long dosing interval. Its targeted mechanism of action without generalized immunosuppression is reflected in its rapid onset of symptom improvement, sustained efficacy and good safety profile in the treatment of patients with anti-AChR antibody-positive gMG.
Plain Language Summary: Ravulizumab in anti-AChR antibody-positive gMGRavulizumab was engineered from eculizumab to have an extended half-life and longer duration of action. It binds to terminal complement protein C5 to inhibit anti-AChR antibody-mediated activation of terminal complement and destruction of the neuromuscular junction.Pharmacokinetic/pharmacodynamic analyses support a simple weight-based administration and long dosing interval and show that therapeutic ravulizumab concentrations and complete inhibition of C5 were achieved immediately after the first dose.The 26-week randomized placebo-controlled period (RCP) of the CHAMPION MG study in patients with anti-AChR antibody-positive gMG showed that ravulizumab has rapid and sustained efficacy with good safety and tolerability across a broad range of patients.Ravulizumab’s efficacy, safety, and tolerability were confirmed in interim analyses of the open-label extension study (including data for up to 60 weeks from the RCP baseline): efficacy was maintained in patients remaining on ravulizumab; rapid and sustained efficacy was established in patients switching from placebo to ravulizumab.
Keywords: acetylcholine receptor antibody, complement, membrane attack complex, monoclonal antibody, terminal complement complex
Introduction
Myasthenia Gravis
Myasthenia gravis (MG) is a chronic autoimmune disease that affects the neuromuscular junction (NMJ) and is characterized by exertional muscle fatigability and fluctuating muscle weakness.1–5 It most commonly presents initially as localized weakness in the peri-orbital/extraocular muscles. Overall, approximately 10–15% of patients have ocular symptoms only; the remainder have generalized MG (gMG), additionally involving muscles of the head, neck, trunk/thorax, and limbs, frequently within 2–3 years of disease onset.1–3,6 The fluctuating muscle weakness and fatigability lead to ptosis, diplopia, impairment of facial expression, exertion intolerance, dysarthria, dysphagia, and dyspnea that can progress to respiratory failure.1,3–5,7–10 Indeed, approximately 15–20% of patients with gMG experience myasthenic crisis during their disease course, requiring intensive care and respiratory support.11
The burden of MG is considerable. If not adequately controlled, MG symptoms may have a significant impact on patients’ daily personal and work activities, their emotional, social, and economic well-being, and their quality of life.7,12–17 The side effects of treatment for MG also have similar major impacts in these areas:7,15,18 most patients require treatment with corticosteroids or nonsteroidal immunosuppressive therapies with a known serious side-effect profile.13 Myasthenic exacerbations and crises impose a particularly high burden on patients, with substantial morbidity and mortality.19 Myasthenic crises are also associated with long-term physical and mental health consequences and substantial health care resource utilization.20,21
Epidemiology
MG is a rare disease, affecting men and women approximately equally overall, although age of onset peaks earlier in women (age 30–50 years) than in men (age over 60 years).1,22–26 The prevalence of MG is estimated to range from 150 to 250 cases per million and the annual incidence from 4 to 30 cases per million person-years.1,3 The estimated prevalence has been increasing over the last 50 years, partially as a result of improved recognition and diagnosis and a general increase in lifespan, but also possibly as part of a general increase observed in the prevalence of autoimmune diseases.1,3,27 MG may be associated with thymic tumors: approximately 30% of patients with a thymoma develop MG, and 10–20% of patients with MG have a thymoma.1,3
MG Autoantibodies and the Complement System
The majority (80–90%) of patients diagnosed with gMG have autoantibodies directed against the nicotinic acetylcholine receptor (AChR) on the postsynaptic NMJ membrane.3,28–31 The remaining patients may have autoantibodies against other antigens, including muscle-specific tyrosine kinase (MuSK) or low-density lipoprotein receptor-related protein 4 (LRP4), or no antibodies detectable with current assays.3,29,32
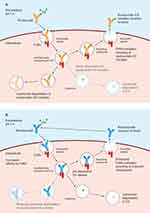
Anti-AChR antibodies are predominantly of the immunoglobulin (Ig) G1 and IgG3 subclasses, and among a range of possible mechanisms by which these antibodies give rise to gMG, there is good evidence that a significant factor is activation of the classical complement cascade on binding of anti-AChR antibodies to the AChR.4,5,29,30,32–34 Activation of the complement system leads to cleavage of complement C5 into C5a and C5b.4,29 C5a is a potent pro-inflammatory peptide. C5b combines with other complement proteins (C6, C7, C8, and multiple copies of C9) to form the terminal membrane attack complex (MAC; also known as the terminal complement complex) (Figure 1). Deposition of the MAC on the NMJ leads to destruction of the postsynaptic membrane, and ultimately a reduction in the number of functional AChRs, thereby reducing the neuromuscular transmission safety factor. Thus, impaired neuromuscular transmission and muscle weakness and fatigability ensue.1,3,4,29,32 Inhibition of terminal complement activation is therefore a rational approach to prevent formation of the MAC – and hence destruction of the NMJ – in patients with anti-AChR antibody-positive gMG.35
MG associated with anti-MuSK autoantibodies is less common, with a somewhat different clinical phenotype.36,37 Anti-MuSK antibodies are predominantly of the IgG4 subclass, which is not (or only weakly) complement activating,38–40 and complement deposition on endplates is detected in only a minority of anti-AChR antibody-negative gMG patients with anti-MuSK antibodies.41 Patients with anti-MuSK antibody-positive MG were not included in the pivotal complement inhibitor trials, and these therapies are unlikely to have significant benefit in most patients with anti-MuSK antibody-positive MG.
A small subpopulation of patients have MG associated with anti-LRP4 autoantibodies.1,3 Anti-LRP4 antibodies are predominantly of the IgG1/IgG2 subclass and may therefore activate complement, although this remains to be demonstrated.1
Current Treatments
Current treatment for gMG includes oral acetylcholinesterase inhibitors, plasma exchange, intravenous immunoglobulin therapy (IVIg), and immunosuppression with established therapies such as corticosteroids and nonsteroidal immunosuppressants.42,43 However, most immunosuppressive therapies are nonspecific without directly targeting the complement system,38,44 and their long-term use can be associated with serious side effects.45,46 Furthermore, the long delay from drug initiation to onset of therapeutic effect with many of these drugs47 can be a significant source of dissatisfaction for patients. More targeted approaches to the treatment of gMG have therefore been developed, including inhibition of complement activation by humanized monoclonal antibodies such as eculizumab48 and ravulizumab49 or by the macrocyclic-peptide complement C5 inhibitor zilucoplan,50 inhibition of IgG recycling by neonatal Fc receptor (FcRn) antagonists such as efgartigimod and rozanolixizumab,51–53 and B-cell depletion by anti-CD20 antibodies such as rituximab.54–56
Complement Inhibition in MG
Eculizumab (Soliris®; Alexion, AstraZeneca Rare Disease) was the first human terminal complement C5 inhibitor to be approved for the treatment of gMG. Eculizumab is a humanized monoclonal antibody that binds specifically with high affinity to the human terminal complement protein C5, blocking its cleavage to C5a and C5b and thus preventing the cascade of events that leads to MAC-mediated architectural destruction of the NMJ postsynaptic membrane and consequent muscle weakness and fatigability. The REGAIN study (ClinicalTrials.gov ID: NCT01997229) and its open-label extension (OLE; ClinicalTrials.gov ID: NCT02301624) demonstrated the ability of eculizumab to improve clinical outcomes and to be well tolerated in patients with anti-AChR antibody-positive refractory gMG.48,57–59
Ravulizumab
Ravulizumab (Ultomiris®; Alexion, AstraZeneca Rare Disease), also a C5 inhibitor, was developed from eculizumab and engineered to have a longer half-life, maintaining therapeutic serum concentrations over an 8-week dosing interval.60 Four specific amino acid substitutions were incorporated: two that largely eliminate the target-mediated drug disposition (ie, the increased clearance of the drug following binding to the C5 target) seen with eculizumab and two that further extend the half-life by increasing the affinity for human FcRn, thus increasing the efficiency of FcRn-mediated recycling of unbound antibody (Figure 2). These modifications yielded an extended half-life and duration of action, delivering immediate, complete, and sustained inhibition of terminal complement activity.60 In contrast to FcRn inhibitors such as efgartigimod,51,52 ravulizumab does not block FcRn but binds long enough to be recycled through the FcRn pathway, rather than undergoing lysosomal degradation, and without changing general FcRn function.60,61 In a longitudinal analysis of serum concentrations of IgG and IgG subclasses regulated by FcRn in patients with paroxysmal nocturnal hemoglobinuria (PNH), endogenous IgG concentrations were shown to be unaffected by ravulizumab treatment.61
The phase 3 CHAMPION MG study showed that ravulizumab, administered every 8 weeks, provided rapid and sustained improvements of symptoms in adults with anti-AChR antibody-positive gMG, as determined by both patient-reported and clinician-rated outcomes, and was well tolerated.49 Ravulizumab was approved by the US Food and Drug Administration in April 2022 for the treatment of adults with anti-AChR antibody-positive gMG,62 and in Japan in August 2022 for the treatment of adults with anti-AChR antibody-positive gMG whose symptoms are difficult to control with high-dose IVIg or plasmapheresis. It was approved in the EU in September 2022 as an add-on to standard therapy for the treatment of adult patients with anti-AChR antibody-positive gMG. In addition, ravulizumab is approved for the treatment of patients with PNH and in those with atypical hemolytic uremic syndrome (aHUS) and is approved in the EU and Japan for the treatment of anti-aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder. These are all conditions associated with abnormal complement activation.
Phase 3 CHAMPION MG Study
The CHAMPION MG study was a phase 3 study of ravulizumab in patients with anti-AChR antibody-positive gMG (ClinicalTrials.gov ID: NCT03920293). The randomized placebo-controlled period (RCP) was 26 weeks in duration and was followed by an OLE of up to 4 years. The study enrolled adults (≥18 years) who had an MG diagnosis ≥6 months before study entry (screening visit). Other inclusion criteria were as follows: anti-AChR antibody-positive at screening, Myasthenia Gravis Foundation of America (MGFA) Disease Class II–IV disease at screening, Myasthenia Gravis–Activities of Daily Living (MG-ADL) total score ≥6 at screening and randomization, and no previous treatment with a complement inhibitor. The CHAMPION MG study population was not restricted to refractory patients, in contrast to the population in the REGAIN study of eculizumab in anti-AChR antibody-positive gMG (REGAIN defined patients with refractory gMG as those who had received treatment with two or more immunosuppressive therapies, or at least one immunosuppressive therapy with IVIg or plasma exchange given at least four times per year, for 12 months without symptom control). The CHAMPION MG study also included patients with a history of treated thymoma, whereas such patients were excluded from the study population in the REGAIN trial.
The ravulizumab dose regimen used in the CHAMPION MG study was based on the patient’s body weight,63,64 with initial loading doses of 2400 mg (body weight ≥40 kg to <60 kg), 2700 mg (body weight ≥60 kg to <100 kg), or 3000 mg (body weight ≥100 kg) at baseline (Day 1), and maintenance doses of 3000 mg (body weight ≥40 kg to <60 kg), 3300 mg (body weight ≥60 kg to <100 kg), or 3600 mg (body weight ≥100 kg) on Day 15 (Week 2) and then every 8 weeks.
Patients who completed the RCP of the CHAMPION MG study could enter the OLE, in which they would receive weight-based ravulizumab treatment every 8 weeks for up to 4 years while remaining blinded to their original treatment.
Ravulizumab Pharmacokinetics and Pharmacodynamics
The pharmacokinetics (PK), pharmacodynamics (PD), and potential immunogenicity of ravulizumab were analyzed in 86 adult patients with anti-AChR antibody-positive gMG who received ravulizumab (weight-based dosing) in the RCP of the CHAMPION MG study.65 Therapeutic serum ravulizumab concentrations (>175 µg/mL) were achieved immediately after the first ravulizumab dose and maintained throughout the 26-week RCP irrespective of patient body weight. No clinically significant differences were noted between weight categories.
The PK findings were consistent with ravulizumab’s mean elimination half-life of 56.6 days (8.1 weeks),62 which is substantially longer than that of eculizumab (18.2 days) in patients with anti-AChR antibody-positive gMG.66 With ravulizumab treatment, inhibition of serum free C5 was immediate (within 30 minutes of the end of infusion), complete (serum free C5 <0.5 μg/mL), and sustained throughout the 26-week RCP in 100% of patients at all time points,65 supporting ravulizumab’s mechanism of action. In comparison, eculizumab achieved complete inhibition of terminal complement activation at all time points in 92% of patients.66 No treatment-emergent anti-drug antibodies were detected, suggesting that the long-term efficacy of ravulizumab is unlikely to be diminished by anti-drug immunogenicity.
Concomitant administration of plasma exchange, plasmapheresis or IVIg treatment has been shown to reduce serum ravulizumab concentrations; therefore, a supplemental dose of ravulizumab is required to compensate for the intervention.62
The PK/PD evidence thus supports the use of ravulizumab every 8 weeks for immediate (by the end of the initial infusion), complete, and sustained inhibition of terminal complement in treating patients with anti-AChR antibody-positive gMG. The ability of ravulizumab to provide consistent suppression of complement activation over periods of at least 8 weeks is of significance for the clinical management of gMG given the chronic but fluctuating nature of the disease and its propensity for unpredictable exacerbations and crises if inadequately controlled.
Ravulizumab Efficacy Data
CHAMPION MG Randomized Controlled Period
Primary and Secondary Outcomes
The 26-week RCP of the phase 3 CHAMPION MG study established the efficacy and favorable safety and tolerability profile of ravulizumab in patients with anti-AChR antibody-positive gMG.49 To address multiplicity, the primary endpoint (change from baseline in MG-ADL total score at 26 weeks) in the CHAMPION MG study was statistically tested first, followed by the secondary endpoints in a fixed-sequence hierarchical testing procedure.49 The first secondary endpoint was the change from baseline in Quantitative Myasthenia Gravis (QMG) total score at 26 weeks, followed in the testing hierarchy by responder analysis of the QMG total score (improvement from baseline of 5 points or greater), change from baseline in revised 15-item Myasthenia Gravis Quality of Life (MG-QOL15r) questionnaire score at 26 weeks, change from baseline in Neurological Quality of Life (Neuro-QoL) Fatigue subscale score at 26 weeks, and responder analysis of the MG-ADL total score (improvement from baseline of 3 points or more). If a given endpoint did not reach statistical significance, the procedure deemed that endpoints below it in the testing hierarchy should not be regarded as being statistically significant.
Ravulizumab treatment was associated with significantly greater mean improvements from baseline to Week 26 in MG-ADL total score compared with placebo (−3.1 vs −1.4; p = 0.0009; the primary endpoint).49 The mean improvement in MG-ADL total score with ravulizumab treatment was greater than the minimum clinically important difference (MCID) for MG-ADL total score, reported to be 2 points.67 Improvement was observed within 1 week (first observation point) of ravulizumab treatment initiation and was sustained through Week 26 (Figure 3).
Ravulizumab was also associated with significantly greater mean improvements from baseline to Week 26 in QMG total score versus placebo (−2.8 vs −0.8; p = 0.0009; the first secondary endpoint).49 Similar to the rapid onset and durability of improvement seen with MG-ADL total score, the QMG total score improved within 1 week of the start of ravulizumab treatment and was maintained throughout the 26-week RCP.
 |
Figure 3 Change from baseline in (A) MG-ADL and (B) QMG total scores in the randomized, placebo-controlled period of the CHAMPION MG study. From NEJM Evid. Vu T, Meisel A, Mantegazza R, et al. Terminal complement inhibitor ravulizumab in generalized myasthenia gravis. 1(5):EVIDoa2100066; Copyright © (2022) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.49 Abbreviations: MG-ADL, Myasthenia Gravis–Activities of Daily Living; QMG, Quantitative Myasthenia Gravis. |
With regard to the health-related quality-of-life (HR-QoL) measures, although there were numerically greater changes from baseline to Week 26 in the ravulizumab group versus the placebo group for both MG-QOL15r total and Neuro-QoL Fatigue subscale scores, neither between-group difference reached statistical significance.49 It is worth noting that the CHAMPION MG study was conducted in part during the COVID-19 pandemic, and deterioration of HR-QoL in patients with MG during the pandemic has been reported.68,69 When the analysis of changes in MG-QOL15r score in the RCP was repeated, excluding patients affected by COVID-19, the difference between ravulizumab and placebo in terms of improvement in MG-QOL15r score was statistically significant (p = 0.0424),49 suggesting that COVID-19 may have been a confounding factor in the study and may have masked the true beneficial impact of ravulizumab treatment on HR-QoL.
Compared with the MCIDs for MG-ADL and QMG total scores (2 points67 and 3 points,70 respectively), the thresholds used in the CHAMPION MG study to determine MG-ADL and QMG “response” were conservative (greater improvement required; a 5-point or greater improvement in QMG total score or a 3-point or greater improvement in MG-ADL total score). QMG total scores improved by 5 points or more in a significantly greater proportion of ravulizumab-treated patients than of those receiving placebo (30.0% vs 11.3%; p = 0.0052) (Figure 4).49 The proportion of patients in whom MG-ADL total score improved by 3 points or more was higher in the ravulizumab group than in those receiving placebo (56.7% vs 34.1%; p = 0.0049); however, this last secondary endpoint was subjected to hierarchical statistical testing and as previous endpoints in the hierarchy (changes from baseline in MG-QOL15r score and in Neuro-QoL Fatigue score) did not reach statistical significance, this p-value should be regarded as nominal.49
 |
Figure 4 Pyramid plots showing minimum point reductions from baseline in (A) MG-ADL and (B) QMG total score at Week 26 in the randomized, placebo-controlled period of the CHAMPION MG study. From NEJM Evid. Vu T, Meisel A, Mantegazza R, et al. Terminal complement inhibitor ravulizumab in generalized myasthenia gravis. 1(5):EVIDoa2100066; Copyright © (2022) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.49 Abbreviations: MG-ADL, Myasthenia Gravis–Activities of Daily Living; QMG, Quantitative Myasthenia Gravis. |
Subgroup Analyses
In the RCP of the CHAMPION MG study, ravulizumab was shown to be effective and well tolerated across a broad age range of male and female patients.49 Further analysis showed that ravulizumab provided generally consistent therapeutic benefits in terms of changes from baseline in MG-ADL and QMG total scores across all subgroups defined by sex (prespecified analysis) and age at diagnosis (<50 vs ≥50 years; threshold defined post hoc).71 Changes from baseline in MG-ADL total score were statistically significantly greater in patients receiving ravulizumab compared with those receiving placebo, both in patients aged <50 years and in those aged ≥50 years at diagnosis. For QMG, change from baseline was significantly greater in the ravulizumab group than in those receiving placebo in patients aged <50 years at diagnosis. In patients aged ≥50 years at diagnosis, the difference favored ravulizumab but did not reach statistical significance.
A post hoc analysis was also conducted based on the time from MG diagnosis to entry into the study: a threshold of 2 years since diagnosis was used as the cut-off (≤2 vs >2 years at study entry).72 Regardless of when patients began ravulizumab treatment after their MG diagnosis, there was a significantly greater improvement in MG-ADL total score from baseline to Week 26 for ravulizumab versus placebo. Overall, a trend was observed toward greater reduction in MG-ADL total scores in patients who initiated ravulizumab earlier after MG diagnosis compared with later.
Although further studies are needed to confirm the findings, the results from this post hoc analysis suggest that treatment with ravulizumab earlier in the course of the disease may result in greater therapeutic benefit for patients.
CHAMPION MG Open-Label Extension
In total, 161 of the 162 patients who completed the RCP entered the OLE; 78 had received ravulizumab in the RCP (RAV–RAV) and 83 had received placebo (PBO–RAV). An interim analysis was performed of data collected up to 60 weeks from the RCP initiation.73–75 Although not all patients had reached Week 60 by the cut-off date, all patients had entered the study at least 52 weeks before data cut-off.
Primary and Secondary Outcomes
The sustained efficacy of ravulizumab was confirmed in patients continuing ravulizumab treatment (RAV–RAV group). Improvements in MG-ADL score were maintained in the OLE (least-squares mean change [95% confidence interval] from RCP baseline at Week 60: −4.0 [−4.8, −3.1]; p < 0.0001). Improvements were also maintained in QMG total, MG-QOL15r total, and Neuro-QoL Fatigue scores through Week 60.73–76 Rapid and sustained improvements, similar to those seen in patients initiating ravulizumab in the RCP, were observed in all efficacy endpoints after initiation of ravulizumab treatment in patients who switched from placebo in the RCP to ravulizumab in the OLE (PBO–RAV). The improvement in MG-ADL score on switching to ravulizumab (least-squares mean change [95% confidence interval] from OLE baseline at OLE Week 2: −1.7 [−2.4, −1.0]; p < 0.0001) was sustained through OLE Week 34 (−1.7 [−2.7, −0.8]; p = 0.0007).
Overall, the interim analysis of data from the CHAMPION MG study OLE supports the sustained clinical effectiveness of ravulizumab.
Timing of Response
The timing of first response to ravulizumab in patients was assessed post hoc by analyzing data from the RCP and OLE of the CHAMPION MG study.77 “Response” was defined as ≥3-point improvement in MG-ADL total score. Patients were included in the analysis if they had an MG-ADL total score of 6 or greater at ravulizumab initiation (this included all patients in the RAV–RAV group, as an MG-ADL score ≥6 was required for study entry, and PBO–RAV patients who had an OLE baseline MG-ADL score of ≥6).
Median time to MG-ADL first response, as determined by ≥3-point improvement in MG-ADL score, was 29.0 (interquartile range 170.0) days (4.1 weeks).77 The cumulative response rates indicate that the first MG-ADL response was achieved by 45.3% of patients after one ravulizumab infusion (ie, by Week 2). It is interesting to note in this regard that, in the REGAIN study, although most patients with gMG that was defined as refractory achieved clinical response by 12 weeks of treatment with eculizumab, some patients took longer to respond.78
As in the prespecified analyses in the RCP, more rigorous definitions of MG-ADL and QMG response than the MCIDs were applied; therefore, the results of this analysis should be considered conservative. It would be interesting to explore further whether there are patient and/or disease characteristics that may predict early or late response to ravulizumab, and whether it is possible to use the data to determine how long a drug trial should be continued in an individual patient. With regard to stopping treatment with C5 inhibitors, there is currently no documented evidence of any rebound symptoms.
Clinical Deterioration
Ravulizumab treatment was associated with fewer clinical deteriorations (exacerbations and crises) compared with placebo during the RCP.49,79,80 In the 1-year period before the start of the study, the clinical deterioration event rate (adjusted for treatment exposure) in patients who entered the study was 44.4 per 100 patient-years. This increased to 61.6 per 100 patient-years in patients receiving placebo during the RCP but decreased to 17.8 per 100 patient-years in patients receiving ravulizumab in the RCP and/or OLE.80 Ravulizumab treatment was associated with a reduction in the exposure-adjusted clinical deterioration event rate per 100 patient-years of 59.8% versus the pre-study rate (p = 0.0019) and a reduction of 71.1% versus placebo (p = 0.0011).80
Post-Intervention Status
A prespecified exploratory analysis examined whether ravulizumab helped patients achieve improved MG-related clinical status and reach the goal of minimal manifestations (MM), as evaluated by a modified version of the MGFA Post-intervention Status (MGFA-PIS) classification.81 The proportions of patients with each modified MGFA-PIS classification (improved and achieved MM, improved without MM, unchanged, worsened) were assessed at Week 26 in those who completed the RCP and had Week 26 MGFA-PIS data and at Week 60 in those who received ≥1 ravulizumab dose during the OLE and had Week 60 MGFA-PIS data. Ravulizumab-treated patients were significantly more likely than those receiving placebo to have achieved MGFA-PIS of improved, with or without MM at Week 26 (treatment effect across the four MGFA-PIS classifications: p = 0.01; adjusted odds ratio, 2.24 [95% confidence interval: 1.21, 4.13]). At Week 60, improved status, with/without MM, was achieved by 34%/32%, respectively, of patients who continued ravulizumab treatment, and by 32%/42%, respectively, of patients who had switched to ravulizumab after receiving placebo for 26 weeks in the RCP.
Ravulizumab Safety Data
The findings of the CHAMPION MG study RCP demonstrate that ravulizumab was well tolerated over the 26-week course, with no notable differences in adverse events being observed between the ravulizumab and placebo groups.49 The proportions of patients who experienced adverse events, or adverse events that were considered by the investigator to be related to trial drug, were similar between the ravulizumab and placebo groups. The most frequent adverse event in the RCP was headache, experienced by 19% of patients receiving ravulizumab and 26% of those receiving placebo; the next most frequent adverse events were diarrhea (ravulizumab group: 15% of patients; placebo group: 12% of patients) and nausea (10% of patients in each group). During the RCP, serious adverse events were reported for 23% of patients (35 events in 20 patients) in the ravulizumab group and 16% in the placebo group (16 events in 14 patients). The most frequent were related to worsening of MG (one with ravulizumab, three with placebo) and COVID-19 (two with ravulizumab, one with placebo). Two serious adverse events in two patients in the ravulizumab group and four serious adverse events in four patients in the placebo group in the RCP were classified by the investigators as being related to study treatment.49 Longer-term treatment in the OLE did not reveal any new safety concerns.73–7576 No patients who were receiving ravulizumab withdrew from the study due to adverse events related to study treatment in the RCP or OLE (up to the Week 60 data cut-off). During the RCP and OLE, four deaths occurred in patients treated with ravulizumab, none of which was considered by investigators as related to ravulizumab treatment: three were due to COVID-19, one to spontaneous cerebral hemorrhage.73 There were no deaths in patients receiving placebo. A recent analysis82 showed a high death rate in patients with MG who contracted COVID-19, with a mortality rate that was three times higher in patients with MG than in those without MG (10.6% vs 3.0%); this was not statistically significant after adjusting for covariates. Whether the mortality rate in such cases is influenced by recent treatment such as higher corticosteroid doses (as it is in more common diseases such as rheumatoid arthritis or multiple sclerosis) is currently undetermined.
It should be noted that, as ravulizumab inhibits terminal complement activation, patients may have an increased susceptibility to infections with encapsulated bacteria, including serious meningococcal infections that may potentially result in septicemia and/or meningitis. Meningococcal vaccination before initiation of ravulizumab treatment – with antibacterial drug prophylaxis if necessary – should be instigated according to local prescribing information. All patients should be closely monitored for early signs and symptoms of meningococcal infection. No cases of meningococcal infection have been reported, either in the RCP or the OLE of the CHAMPION MG trial.
Ravulizumab’s safety and tolerability profile in patients with anti-AChR antibody-positive gMG is consistent with that observed in previous phase 3 studies in PNH63 and aHUS64 and with that of eculizumab in patients with MG defined as refractory.48,58 The findings from the OLE support ravulizumab’s long-term safety and confirm the safety profile observed in the RCP of the CHAMPION MG study.49
Discussion
The efficacy, safety, and tolerability of ravulizumab for the treatment of gMG have been established in the RCP of the CHAMPION MG study, with rapid and sustained improvements in MG-ADL and QMG scores and a favorable safety profile; interim analysis of data from the OLE provides reassurance of longer-term maintenance of these benefits. Ravulizumab’s longer half-life, with dosing every 8 weeks, lowers the burden of administration relative to eculizumab. As data accumulate from real-world practice, it will be interesting to see whether this translates into a patient preference.
The PK/PD data show that in all patients treated with ravulizumab in the RCP, 8-weekly dosing delivered therapeutic concentrations of ravulizumab throughout the 26 weeks of the RCP, with complete terminal complement inhibition observed in all patients throughout the study. This consistent long-term suppression of complement activity is important in a chronic but fluctuating disease subject to exacerbations and crises.
As body weight is a clinically significant covariate affecting ravulizumab PK,62,83 a weight-based dosing regimen was developed for use in phase 3 studies of ravulizumab in PNH and aHUS.63,64 This was then implemented in the CHAMPION MG study.49 Without dosing based on weight, higher body-weight patients may experience lower exposures to ravulizumab than those with lower body weight; thus, body weight-based dosing limited inter-patient variability in exposure across a wide range of patient body weights.
Outstanding Questions in Clinical Practice
Long-Term Efficacy and Safety
The OLE of the CHAMPION MG trial and real-world evidence will provide further long-term data on the impact of ravulizumab on exacerbations and crises, response durability, use of concomitant medication, and safety. Longer-term treatment effects may become evident, as was seen in the OLE of the REGAIN study of eculizumab, in which 88% of patients achieved an MGFA post-intervention status of “improved” and 57% of patients achieved a post-intervention status of “minimal manifestations” after 130 weeks’ treatment.84
Comparison with Eculizumab and Transition from Eculizumab to Ravulizumab
Although there have been no direct head-to-head clinical-trial comparisons between ravulizumab and eculizumab in MG, the PD analysis of CHAMPION MG data showed some potential advantages for ravulizumab. Ravulizumab inhibited terminal complement completely in all patients at all time points;65 in comparison, an earlier PD analysis showed that eculizumab achieved complete inhibition of terminal complement at all time points in 92% of patients.66 Comparisons of ravulizumab and eculizumab in patients with PNH have shown that (in patients who were naïve to complement inhibitor therapy) ravulizumab treatment given every 8 weeks was noninferior to eculizumab (administered every 2 weeks) for all efficacy endpoints studied, with a similar safety and tolerability profile.63,85 In a further study, ravulizumab administered every 8 weeks was also noninferior to eculizumab administered every 2 weeks in patients with PNH who were clinically stable during previous eculizumab therapy, with the investigators concluding that patients with PNH could be safely and effectively switched from eculizumab to ravulizumab.85 A follow-up study showed consistent efficacy of ravulizumab treatment in patients who had originally been treated with eculizumab, with patients who had experienced incomplete complement C5 inhibition on eculizumab all showing complete C5 inhibition and improved suppression versus eculizumab after switching to ravulizumab.86
The PK of both eculizumab66 and ravulizumab65 are affected by body weight; as mentioned above, the weight-based dosing recommended for ravulizumab mitigates the potential inter-patient variability in exposure.
Combination with Other Drugs
Although an interaction with IVIg is noted in the ravulizumab prescribing information62,87 (the combination may lead to a reduction in serum ravulizumab concentrations and require administration of a supplemental dose of ravulizumab), data on use in combination with other therapies are needed to optimize use of ravulizumab. It is not known whether ravulizumab could be administered concomitantly with drugs with a different mode of action such as FcRn inhibitors; the current US prescribing information advises that FcRn inhibitors may interfere with the endosomal FcRn recycling mechanism of monoclonal antibodies and that co-administration with ravulizumab may lower systemic exposures and reduce effectiveness of ravulizumab.62
Pregnancy
gMG affects women of child-bearing age but does not affect fertility; therefore, pregnancy can be expected in women with gMG. Data on the use of ravulizumab during pregnancy in women with gMG are not yet available, and only sparse data on C5 inhibition have been published. A recent case report of successful pregnancy in a patient with refractory MG treated with eculizumab throughout the course of her pregnancy suggests a potential role for complement inhibition during pregnancy in women with gMG.88 The use of eculizumab in pregnant patients for the management of PNH showed maternal benefits throughout pregnancy with a low rate of maternal complications, largely good pregnancy outcomes, and a high rate of fetal survival.89,90
Biomarkers to Predict Response
Further research into biomarkers or other predictive factors could be useful in determining the likelihood of optimal response in an individual patient and support tailoring of ravulizumab and other anti-complement therapies for improved treatment of MG, or selection of patients who may best benefit from ravulizumab treatment. A recent study has revealed more details of the complement biomarker profile (reduced C2 and C5, increased C3, C3b, C5a) in patients with anti-AChR antibody-positive MG.32 Furthermore, the currently used biomarker (AChR autoantibody titer) does not necessarily correlate with disease severity, and it has been suggested that there is synergistic interaction between antibodies targeting different subunits of the AChR.34 Assays that specifically evaluate AChR autoantibody-mediated complement activity, rather than measuring autoantibody binding as do current clinical assays used to diagnose patients with MG, may provide a better understanding of AChR autoantibody mechanisms and the opportunity to better predict treatment response.33,91 Ideally, such an assay would provide rapid results to allow monitoring of treatment response and would be readily commercially available.
Place in Treatment Algorithm/Future of Ravulizumab in gMG
The time-from-diagnosis analysis conducted on data from CHAMPION MG suggests that treatment with ravulizumab earlier in the course of the disease may result in greater therapeutic benefit for patients.72 However, the optimal place of ravulizumab in the treatment algorithm for gMG remains to be determined, requiring data on health outcomes and costs – not only of treatment but of administration, adverse events, and societal costs such as reducing the impact of MG on employment. As real-world evidence accumulates in a broader population of patients, this will supplement the clinical trial data. The future place of ravulizumab in the treatment of MG also depends on the relative effectiveness, safety, and tolerability of other novel approaches such as the use of FcRn antagonists to reduce IgG levels by blockade of FcRn-mediated recycling of IgG.92
As therapeutic serum concentrations of ravulizumab (>175 µg/mL) were achieved immediately after the first dose,65 the use of ravulizumab in acute treatment of MG is also of potential interest. Although there have been a few case reports and anecdotal reports of eculizumab used in myasthenic crisis with acceptable outcomes,93–95 there are as yet no data for ravulizumab.
Conclusions
In contrast to many immunosuppressive therapies, ravulizumab has a rapid onset of action, with treatment benefit demonstrated within 1 week as measured by clinically meaningful endpoints. The targeted mechanism of action without general immunosuppressive properties is reflected in ravulizumab’s significant efficacy in the treatment of patients with anti-AChR antibody-positive gMG with a good safety profile. Ravulizumab’s weight-based administration and long redosing interval are favorable for outpatient treatment including newly diagnosed patients. Further data from the OLE are eagerly awaited.
Acknowledgments
Editorial assistance in the development of this review article was provided by Dr Duncan Porter of Piper Medical Communications, funded by Alexion, AstraZeneca Rare Disease. Alexion, AstraZeneca Rare Disease provided a courtesy medical-accuracy review of the final draft. The authors thank Dr Paul Tamburini and Dr Bruce Andrien (both Alexion, AstraZeneca Rare Disease) for advice on the schematic diagrams depicting the complement cascade and the mechanism of ravulizumab’s extended duration of action.
Disclosures
Tuan Vu: USF Site Principal Investigator for MG clinical trials sponsored by Alexion, AstraZeneca Rare Disease, argenx, Ra/UCB Pharma, Horizon/Viela Bio, Janssen/Momenta, Regeneron, Immunovant, Dianthus, and Cartesian Therapeutics; speaker and/or consultant honoraria from Alexion, AstraZeneca Rare Disease, UCB, and argenx.
Heinz Wiendl: honoraria for serving on advisory boards for Janssen, Merck, and Novartis; speaker honoraria and travel support from Alexion, AstraZeneca Rare Disease, Amicus Therapeutics, Biogen, Biologix, Bristol Myers Squibb, Cognomed, F. Hoffmann-La Roche, Gemeinnützige Hertie-Stiftung, Medison, Merck, Novartis, Roche Pharma AG, Genzyme, TEVA, and WebMD Global. Acts as a paid consultant for AbbVie, Actelion, Amicus Therapeutics, argenx, Beckton Dickinson, Biogen, Bristol Myers Squibb, EMD Serono, EPG Health, Fondazione Cariplo, Gossamer Bio, Idorsia, Immunic, Immunovant, Janssen, Merck, Neurodiem, NexGen, Novartis, Ology, Roche, Sandoz, Sangamo, Sanofi, the Swiss Multiple Sclerosis Society, Syneos Health Germany GmbH, TEVA, Toleranzia, WebMD Global, Worldwide Clinical Trials, Viatris, and UCB. Research funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgesellschaft (DFG), Deutsche Myasthenie Gesellschaft e.V., European Union, Alexion, AstraZeneca Rare Disease, Amicus Therapeutics, argenx, Biogen, CSL Behring, F. Hoffmann-La Roche, Genzyme, Merck KGaA, Novartis Pharma, Roche Pharma, and UCB Biopharma.
Masahisa Katsuno: Speaker honoraria from Biogen Japan, Chugai, and Eisai; and financial research support from Alexion, AstraZeneca Rare Disease, argenx, Eisai, and Mitsubishi-Tanabe.
Stephen W Reddel: Funds over the last 5 years including but not limited to travel support, honoraria, trial payments, research and clinical support to the neurology department or academic projects of which he is a member have been received from bodies and charities: NHMRC, MRFF, NBA, Myasthenia Alliance Australia, Lambert Initiative, Beeren foundation, anonymous donors; and from pharmaceutical/biological companies: Alexion, Biogen, CSL, Genzyme, Grifols, Merck, Novartis, Roche, Sanofi, UCB. Additional interests and potential conflicts of interest include: Co-founder/shareholder of RxPx health; National IVIG Governance Advisory Council & Specialist Working Group Australia (Neurology) (paid); Australian Medical Services Advisory Committee ad-hoc sub-committee on IVIG (paid); Australian Technical Advisory Group on Immunisation Varicella Zoster working party (unpaid); public salary as a staff specialist neurologist from Concord Hospital Sydney Local Health District (paid); private billings from patients and medicare Australia reimbursement as a private practice neurologist (paid); medical advisor (unpaid) to various patient and advocacy groups.
James F Howard Jr: Research funding (paid to institution): Alexion, AstraZeneca Rare Disease, argenx, Cartesian Therapeutics, Centers for Disease Control and Prevention, MGFA, Muscular Dystrophy Association, NIH, PCORI, Ra Pharmaceuticals, Takeda Pharmaceuticals, and UCB; honoraria from AcademicCME, Alexion, AstraZeneca Rare Disease, argenx, Biologix Pharma, F. Hoffmann-LaRoche Ltd, Horizon Therapeutics plc, Medscape CME, Merck EMB Serono, NMD Pharma, Novartis Pharma, PeerView CME, Regeneron Pharmaceuticals, Sanofi US, and Zai Labs; non-financial support from Alexion, AstraZeneca Rare Disease, argenx, Toleranzia AB, and Zai Labs.
References
1. Dresser L, Wlodarski R, Rezania K, Soliven B. Myasthenia gravis: epidemiology, pathophysiology and clinical manifestations. J Clin Med. 2021;10(11):2235. doi:10.3390/jcm10112235
2. Gilhus NE. Myasthenia gravis. N Engl J Med. 2016;375(26):2570–2581. doi:10.1056/nejmra1602678
3. Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren JJGM. Myasthenia gravis. Nat Rev Dis Primers. 2019;5(1):30. doi:10.1038/s41572-019-0079-y
4. Howard JF
5. Melzer N, Ruck T, Fuhr P, et al. Clinical features, pathogenesis, and treatment of myasthenia gravis: a supplement to the Guidelines of the German Neurological Society. J Neurol. 2016;263(8):1473–1494. doi:10.1007/s00415-016-8045-z
6. Evoli A, Tonali P, Bartoccioni E, Lo Monaco M. Ocular myasthenia: diagnostic and therapeutic problems. Acta Neurol Scand. 1988;77(1):31–35. doi:10.1111/j.1600-0404.1988.tb06970.x
7. Jackson K, Parthan A, Lauher-Charest M, Broderick L, Law N, Barnett C. Understanding the symptom burden and impact of myasthenia gravis from the patient’s perspective: a qualitative study. Neurol Ther. 2023;12:107–128. doi:10.1007/s40120-022-00408-x
8. Hoffmann S, Ramm J, Grittner U, Kohler S, Siedler J, Meisel A. Fatigue in myasthenia gravis: risk factors and impact on quality of life. Brain Behav. 2016;6(10):e00538. doi:10.1002/brb3.538
9. Andersen H, Mantegazza R, Wang JJ, O’Brien F, Patra K, Howard JF
10. Tran C, Bril V, Katzberg HD, Barnett C. Fatigue is a relevant outcome in patients with myasthenia gravis. Muscle Nerve. 2018;58(2):197–203. doi:10.1002/mus.26069
11. Claytor B, Cho SM, Li Y. Myasthenic crisis. Muscle Nerve. 2023;68(1):8–19. doi:10.1002/mus.27832
12. Barnett C, Bril V, Bayoumi AM. EQ-5D-5L and SF-6D health utility index scores in patients with myasthenia gravis. Eur J Neurol. 2019;26(3):452–459. doi:10.1111/ene.13836
13. Cutter G, Xin H, Aban I, et al. Cross-sectional analysis of the myasthenia gravis patient registry: disability and treatment. Muscle Nerve. 2019;60(6):707–715. doi:10.1002/mus.26695
14. Frost A, Svendsen ML, Rahbek J, Stapelfeldt CM, Nielsen CV, Lund T. Labour market participation and sick leave among patients diagnosed with myasthenia gravis in Denmark 1997–2011: a Danish nationwide cohort study. BMC Neurol. 2016;16(1):224. doi:10.1186/s12883-016-0757-2
15. Nagane Y, Murai H, Imai T, et al. Social disadvantages associated with myasthenia gravis and its treatment: a multicentre cross-sectional study. BMJ Open. 2017;7(2):e013278. doi:10.1136/bmjopen-2016-013278
16. Twork S, Wiesmeth S, Klewer J, Pöhlau D, Kugler J. Quality of life and life circumstances in German myasthenia gravis patients. Health Qual Life Outcomes. 2010;8:129. doi:10.1186/1477-7525-8-129
17. Marbin D, Piper SK, Lehnerer S, Harms U, Meisel A. Mental health in myasthenia gravis patients and its impact on caregiver burden. Sci Rep. 2022;12(1):19275. doi:10.1038/s41598-022-22078-3
18. Bacci ED, Coyne KS, Poon JL, Harris L, Boscoe AN. Understanding side effects of therapy for myasthenia gravis and their impact on daily life. BMC Neurol. 2019;19(1):335. doi:10.1186/s12883-019-1573-2
19. Nelke C, Stascheit F, Eckert C, et al. Independent risk factors for myasthenic crisis and disease exacerbation in a retrospective cohort of myasthenia gravis patients. J Neuroinflammation. 2022;19(1):89. doi:10.1186/s12974-022-02448-4
20. Heatwole C, Johnson N, Holloway R, Noyes K. Plasma exchange versus intravenous immunoglobulin for myasthenia gravis crisis: an acute hospital cost comparison study. J Clin Neuromuscul Dis. 2011;13(2):85–94. doi:10.1097/cnd.0b013e31822c34dd
21. Liu C, Li T, Wang Q, Xu A, Wu B. Post-traumatic stress disorder symptoms after respiratory insufficiency in patients with myasthenia gravis. Psychol Health Med. 2021;26(2):221–227. doi:10.1080/13548506.2020.1807577
22. García Estévez DA, López Díaz LM, Pardo Parrado M, et al. Epidemiology of myasthenia gravis in the province of Ourense (Galicia, Spain). Neurologia. 2020. doi:10.1016/j.nrl.2020.06.011
23. Martinka I, Fulova M, Spalekova M, Spalek P. Epidemiology of myasthenia gravis in Slovakia in the years 1977–2015. Neuroepidemiology. 2018;50(3–4):153–159. doi:10.1159/000487886
24. Sanders DB, Raja SM, Guptill JT, Hobson-Webb LD, Juel VC, Massey JM. The Duke myasthenia gravis clinic registry: I. Description and demographics. Muscle Nerve. 2021;63(2):209–216. doi:10.1002/mus.27120
25. Santos E, Coutinho E, Moreira I, et al. Epidemiology of myasthenia gravis in Northern Portugal: frequency estimates and clinical epidemiological distribution of cases. Muscle Nerve. 2016;54(3):413–421. doi:10.1002/mus.25068
26. Sobieszczuk E, Napiórkowski Ł, Szczudlik P, Kostera-Pruszczyk A. Myasthenia gravis in Poland: national healthcare database epidemiological study. Neuroepidemiology. 2021;55:62–69. doi:10.1159/000512973
27. Dinse GE, Parks CG, Weinberg CR, et al. Increasing prevalence of antinuclear antibodies in the United States. Arthritis Rheumatol. 2022;74(12):2032–2041. doi:10.1002/art.42330
28. Ha JC, Richman DP. Myasthenia gravis and related disorders: pathology and molecular pathogenesis. Biochim Biophys Acta. 2015;1852(4):651–657. doi:10.1016/j.bbadis.2014.11.022
29. Mantegazza R, Vanoli F, Frangiamore R, Cavalcante P. Complement inhibition for the treatment of myasthenia gravis. Immunotargets Ther. 2020;9:317–331. doi:10.2147/itt.s261414
30. Phillips WD, Vincent A. Pathogenesis of myasthenia gravis: update on disease types, models, and mechanisms. F1000Res. 2016;5:1513. doi:10.12688/f1000research.8206.1
31. Vincent A, Huda S, Cao M, et al. Serological and experimental studies in different forms of myasthenia gravis. Ann N Y Acad Sci. 2018;1413(1):143–153. doi:10.1111/nyas.13592
32. Iacomino N, Vanoli F, Frangiamore R, et al. Complement activation profile in myasthenia gravis patients: perspectives for tailoring anti-complement therapy. Biomedicines. 2022;10(6):1360. doi:10.3390/biomedicines10061360
33. Obaid AH, Zografou C, Vadysirisack DD, et al. Heterogeneity of acetylcholine receptor autoantibody-mediated complement activity in patients with myasthenia gravis. Neurol Neuroimmunol Neuroinflamm. 2022;9(4):e1169. doi:10.1212/nxi.0000000000001169
34. Rose N, Holdermann S, Callegari I, et al. Receptor clustering and pathogenic complement activation in myasthenia gravis depend on synergy between antibodies with multiple subunit specificities. Acta Neuropathol. 2022;144(5):1005–1025. doi:10.1007/s00401-022-02493-6
35. Albazli K, Kaminski HJ, Howard JF
36. Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7(3):365–368. doi:10.1038/85520
37. Evoli A, Bianchi MR, Riso R, et al. Response to therapy in myasthenia gravis with anti-MuSK antibodies. Ann N Y Acad Sci. 2008;1132:76–83. doi:10.1196/annals.1405.012
38. Mantegazza R, Antozzi C. From traditional to targeted immunotherapy in myasthenia gravis: prospects for research. Front Neurol. 2020;11:981. doi:10.3389/fneur.2020.00981
39. McConville J, Farrugia ME, Beeson D, et al. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann Neurol. 2004;55(4):580–584. doi:10.1002/ana.20061
40. Oskam N, Damelang T, Streutker M, et al. Factors affecting IgG4-mediated complement activation. Front Immunol. 2023;14:1087532. doi:10.3389/fimmu.2023.1087532
41. Shiraishi H, Motomura M, Yoshimura T, et al. Acetylcholine receptors loss and postsynaptic damage in MuSK antibody-positive myasthenia gravis. Ann Neurol. 2005;57(2):289–293. doi:10.1002/ana.20341
42. Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016;87(4):419–425. doi:10.1212/wnl.0000000000002790
43. Narayanaswami P, Sanders DB, Wolfe G, et al. International consensus guidance for management of myasthenia gravis: 2020 update. Neurology. 2021;96(3):114–122. doi:10.1212/wnl.0000000000011124
44. Menon D, Barnett C, Bril V. Novel treatments in myasthenia gravis. Front Neurol. 2020;11:538. doi:10.3389/fneur.2020.00538
45. Lascano AM, Lalive PH. Update in immunosuppressive therapy of myasthenia gravis. Autoimmun Rev. 2021;20(1):102712. doi:10.1016/j.autrev.2020.102712
46. Schneider-Gold C, Hagenacker T, Melzer N, Ruck T. Understanding the burden of refractory myasthenia gravis. Ther Adv Neurol Disord. 2019;12:1756286419832242. doi:10.1177/1756286419832242
47. Guptill JT, Soni M, Meriggioli MN. Current treatment, emerging translational therapies, and new therapeutic targets for autoimmune myasthenia gravis. Neurotherapeutics. 2016;13(1):118–131. doi:10.1007/s13311-015-0398-y
48. Howard JF
49. Vu T, Meisel A, Mantegazza R, et al. Terminal complement inhibitor ravulizumab in generalized myasthenia gravis. NEJM Evid. 2022;1(5):EVIDoa2100066. doi:10.1056/EVIDoa2100066
50. Howard JF
51. Heo YA. Efgartigimod: first approval. Drugs. 2022;82(3):341–348. doi:10.1007/s40265-022-01678-3
52. Howard JF
53. Bril V, Drużdż A, Grosskreutz J, et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023;22(5):383–394. doi:10.1016/s1474-4422(23)00077-7
54. Di Stefano V, Lupica A, Rispoli MG, Di Muzio A, Brighina F, Rodolico C. Rituximab in AChR subtype of myasthenia gravis: systematic review. J Neurol Neurosurg Psychiatry. 2020;91(4):392–395. doi:10.1136/jnnp-2019-322606
55. Bastakoti S, Kunwar S, Poudel S, et al. Rituximab in the management of refractory myasthenia gravis and variability of its efficacy in anti-MuSK positive and anti-AChR positive myasthenia gravis. Cureus. 2021;13(11):e19416. doi:10.7759/cureus.19416
56. Nowak RJ, Coffey CS, Goldstein JM, et al. Phase 2 trial of rituximab in acetylcholine receptor antibody-positive generalized myasthenia gravis: the BeatMG study. Neurology. 2021;98(4):e376–e389. doi:10.1212/wnl.0000000000013121
57. Howard JF
58. Muppidi S, Utsugisawa K, Benatar M, et al. Long-term safety and efficacy of eculizumab in generalized myasthenia gravis. Muscle Nerve. 2019;60(1):14–24. doi:10.1002/mus.26447
59. Vissing J, Jacob S, Fujita KP, O’Brien F, Howard JF. ‘Minimal symptom expression’ in patients with acetylcholine receptor antibody-positive refractory generalized myasthenia gravis treated with eculizumab. J Neurol. 2020;267(7):1991–2001. doi:10.1007/s00415-020-09770-y
60. Sheridan D, Yu ZX, Zhang Y, et al. Design and preclinical characterization of ALXN1210: a novel anti-C5 antibody with extended duration of action. PLoS One. 2018;13(4):e0195909. doi:10.1371/journal.pone.0195909
61. Alashkar F, Rottinghaus S, Vance C, et al. No evidence for hypogammaglobulinemia in patients with paroxysmal nocturnal hemoglobinuria (PNH) chronically treated with ravulizumab. PLoS One. 2020;15(3):e0230869. doi:10.1371/journal.pone.0230869
62. Alexion Pharmaceuticals Inc. ULTOMIRIS® (ravulizumab-cwvz) injection prescribing information; 2022. Available from: https://alexion.com/Documents/ultomiris_uspi.
63. Lee JW, Sicre de Fontbrune F, Wong Lee Lee L, et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study. Blood. 2019;133(6):530–539. doi:10.1182/blood-2018-09-876136
64. Rondeau E, Scully M, Ariceta G, et al. The long-acting C5 inhibitor, ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment. Kidney Int. 2020;97(6):1287–1296. doi:10.1016/j.kint.2020.01.035
65. Vu T, Ortiz S, Katsuno M, et al. Ravulizumab pharmacokinetics and pharmacodynamics in patients with generalized myasthenia gravis. J Neurol. 2023;270:3129–3137. doi:10.1007/s00415-023-11617-1
66. Monteleone JPR, Gao X, Kleijn HJ, Bellanti F, Pelto R. Eculizumab pharmacokinetics and pharmacodynamics in patients with generalized myasthenia gravis. Front Neurol. 2021;12:696385. doi:10.3389/fneur.2021.696385
67. Muppidi S. The myasthenia gravis-specific activities of daily living profile. Ann N Y Acad Sci. 2012;1274:114–119. doi:10.1111/j.1749-6632.2012.06817.x
68. Kalita J, Tripathi A, Dongre N, Misra UK. Impact of COVID-19 pandemic and lockdown in a cohort of myasthenia gravis patients in India. Clin Neurol Neurosurg. 2021;202:106488. doi:10.1016/j.clineuro.2021.106488
69. Stojanov A, Stojanov J, Milosevic V, et al. The impact of the coronavirus disease-2019 pandemic on the psychological status and quality of life of myasthenia gravis patients. Ann Indian Acad Neurol. 2020;23(4):510–514. doi:10.4103/aian.AIAN_551_20
70. Katzberg HD, Barnett C, Merkies IS, Bril V. Minimal clinically important difference in myasthenia gravis: outcomes from a randomized trial. Muscle Nerve. 2014;49(5):661–665. doi:10.1002/mus.23988
71. Uzawa A, Juel V, Vu T, et al. Efficacy of ravulizumab across sex and age subgroups of patients with generalized myasthenia gravis: a post hoc analysis of the CHAMPION MG study. 34th Annual Meeting of the Japanese Society for Neuroimmunology, October 20, 2022. Clin Exp Neuroimmunol. 2023;14:72. doi:10.1111/cen3.12738
72. Suzuki S, Howard JF
73. Meisel A, Annane D, Vu T, et al. Long-term efficacy and safety of ravulizumab in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis: results from the phase 3 CHAMPION MG open-label extension. J Neurol. 2023;270:3862–3875. doi:10.1007/s00415-023-11699-x
74. Vu T, Meisel A, Mantegazza R, et al. Long-term efficacy and safety of ravulizumab, a long-acting terminal complement inhibitor, in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis: results from the phase 3 CHAMPION MG open-label extension. 14th Myasthenia Gravis Foundation of America International Conference; 2022; Miami, FL, USA. Muscle Nerve. 2022;65: S2–S3. doi:10.1002/mus.27540
75. Vu T, Meisel A, Mantegazza R, et al. Long-term efficacy and safety of ravulizumab in generalized myasthenia gravis: phase 3 CHAMPION MG study open-label extension. 34th Annual Meeting of the Japanese Society for Neuroimmunology, October 20, 2022. Clin Exp Neuroimmunol. 2023;14:73. doi:10.1111/cen3.12738
76. AstraZeneca. Ultomiris demonstrated sustained improvements in functional activities and quality of life in adults with generalised myasthenia gravis through 60 weeks [press release]; 2022. Available from: https://www.astrazeneca.com/media-centre/press-releases/2022/ultomiris-demonstrated-sustained-improvements-functional-activities-quality-life-adults-generalised-myasthenia-gravis-60-weeks.html.
77. Habib AA, Benatar M, Vu T, et al. Ravulizumab for the treatment of generalized myasthenia gravis: timing of response.
78. Howard JF
79. Mantegazza R, Meisel A, Vu T, et al. Ravulizumab reduces clinical deteriorations in patients with generalised myasthenia gravis [Oral presentation OPR-020]. 8th Congress of the European Academy of Neurology, June 25–28, 2022, Vienna, Austria. Eur J Neurol. 2022;29(1):61.
80. Mantegazza R, Meisel A, Vu T, et al. Ravulizumab reduces clinical deteriorations in patients with generalized myasthenia gravis: results from the CHAMPION MG study [Poster no. 148].
81. Muppidi S, Narayanaswami P, Meisel A, et al. Achievement of improved post-intervention status in patients with generalized myasthenia gravis treated with ravulizumab during the CHAMPION MG study (S5.008). Neurology. 2023;100(17 Suppl 2):1852. doi:10.1212/WNL.0000000000202156
82. Kim Y, Li X, Huang Y, et al. COVID-19 outcomes in myasthenia gravis patients: analysis from electronic health records in the United States. Front Neurol. 2022;13:802559. doi:10.3389/fneur.2022.802559
83. Peffault de Latour R, Brodsky RA, Ortiz S, et al. Pharmacokinetic and pharmacodynamic effects of ravulizumab and eculizumab on complement component 5 in adults with paroxysmal nocturnal haemoglobinuria: results of two phase 3 randomised, multicentre studies. Br J Haematol. 2020;191(3):476–485. doi:10.1111/bjh.16711
84. Mantegazza R, Wolfe GI, Muppidi S, et al. Post-intervention status in patients with refractory myasthenia gravis treated with eculizumab during REGAIN and its open-label extension. Neurology. 2021;96:e610–e618. doi:10.1212/wnl.0000000000011207
85. Kulasekararaj AG, Hill A, Rottinghaus ST, et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: the 302 study. Blood. 2019;133(6):540–549. doi:10.1182/blood-2018-09-876805
86. Kulasekararaj AG, Hill A, Langemeijer S, et al. One-year outcomes from a phase 3 randomized trial of ravulizumab in adults with paroxysmal nocturnal hemoglobinuria who received prior eculizumab. Eur J Haematol. 2021;106(3):389–397. doi:10.1111/ejh.13564
87. Alexion Europe SAS. Ultomiris (ravulizumab) summary of product characteristics; 2023. Available from: https://www.ema.europa.eu/en/documents/product-information/ultomiris-epar-product-information_en.pdf.
88. Vu T, Harvey B, Suresh N, Farias J, Gooch C. Eculizumab during pregnancy in a patient with treatment-refractory myasthenia gravis: a case report. Case Rep Neurol. 2021;13(1):65–72. doi:10.1159/000511957
89. Kelly RJ, Höchsmann B, Szer J, et al. Eculizumab in pregnant patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2015;373(11):1032–1039. doi:10.1056/nejmoa1502950
90. Manning JE, Anderson RM, Hill A, Zeidan D, Ciantar E. Pregnancy outcomes in women receiving eculizumab for the management of paroxysmal nocturnal haemoglobinuria. Obstet Med. 2022;15(1):45–49. doi:10.1177/1753495x211019899
91. Masi G, O’Connor KC. Novel pathophysiological insights in autoimmune myasthenia gravis. Curr Opin Neurol. 2022;35(5):586–596. doi:10.1097/wco.0000000000001088
92. Wolfe GI, Ward ES, de Haard H, et al. IgG regulation through FcRn blocking: a novel mechanism for the treatment of myasthenia gravis. J Neurol Sci. 2021;430:118074. doi:10.1016/j.jns.2021.118074
93. Strano CMM, Sorrenti B, Bosco L, Falzone YM, Fazio R, Filippi M. Eculizumab as a fast-acting rescue therapy in a refractory myasthenic crisis: a case report. J Neurol. 2022;269(11):6152–6154. doi:10.1007/s00415-022-11222-8
94. Hofstadt-van Oy U, Stankovic S, Kelbel C, et al. Complement inhibition initiated recovery of a severe myasthenic crisis with COVID-19. J Neurol. 2021;268(9):3125–3128. doi:10.1007/s00415-021-10428-6
95. Vinciguerra C, Bevilacqua L, Toriello A, et al. Starting eculizumab as rescue therapy in refractory myasthenic crisis. Neurol Sci. 2023;44:3707–3709. doi:10.1007/s10072-023-06900-y
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.