Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 13
Rational Dispensing of Oral Dosage Forms of Medicines to Children and Its Associated Factors in South West Ethiopia
Authors Ejeta F , Feyisa D , Aferu T , Siraj J , Melkam D, Ali A
Received 4 February 2022
Accepted for publication 29 March 2022
Published 8 April 2022 Volume 2022:13 Pages 103—113
DOI https://doi.org/10.2147/PHMT.S360383
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Fikadu Ejeta, Diriba Feyisa, Temesgen Aferu, Jafer Siraj, Demeke Melkam, Ahmed Ali
School of Pharmacy, College of Medicine and Health Sciences, Mizan-Tepi University, Mizan-Aman, Ethiopia
Correspondence: Fikadu Ejeta, Tel +251910136034, Email [email protected]
Introduction: Obstacles encountered when maintaining excellent dispensing practices for children include a lack of age-appropriate dosage forms, a shortage of medications in appropriate strengths for children, a lack of appetizing drugs, and a lack of competence in pediatric pharmacy. These difficulties contribute to ineffective dispensing procedures and an urgent need to study whether oral dose forms of medications are dispensed to children in a rational way.
Objective: The purpose of this study is to evaluate the rational dispensing of oral dosage forms of medicines to children, as well as the factors that influence this practice.
Methods: Using validated indicators, a cross-sectional study design was utilized to analyze the rational dispensing practice of oral dosage forms of medicines administered to children under the age of 12 years in seven pharmacies and two drug stores over a one-month period.
Results and discussions: Out of 810 medicines, 11.7% and 4% were irrationally manipulated, 5.8% and 1.8% needed manipulation, 3.7% and 0.2% were alternatively dispensed, 8.8% and 7.5% of the medicines had correct advice on their label and also 745 medicines were adequately labeled in the hospital and the selected drug stores. In this study, 92% of medicines were adequately labeled and had sufficient advice on their labels, 15.3% of medicines were irrationally manipulated and around 7.7% of the dosage forms needed manipulation during dispensing. The type of medicine retail outlet had a significant effect on the percentage of instances where alternative solid oral dosage forms were dispensed (p = 0.003), the percentage of dosage forms were adequately labeled (p = 0.008), and the percentage of dosage forms were irrationally manipulated before dispensing (p = 0.001).
Conclusion: The rational dispensing practice of oral dosage forms of medicines was relatively poor and there is room for improvement.
Keywords: rational dispensing, oral dosage forms, rational drug use, children
Introduction
Patients receive pharmaceuticals suited to their needs, in doses that match their own unique requirements, for an adequate duration of time, and at the lowest cost to them and their community.1
The key component in ensuring sensible use of medicine is good distribution procedures, the correct drug needs to be delivered to the correct patient in the correct way, in the correct amount and at the correct time, with clear instructions and in a way that preserves the drug’s potency.2
Safe, effective, and cost-efficient medication is delivered through rational drug use. Prescribers, dispensers, and drug users are all working together to achieve this. Adherence to treatment is ensured through rational prescribing, and drug consumers are protected from unwanted drug side effects. On the other side, rational dispensing encourages the safe, effective, and cost-efficient use of medications.3
Pharmacists encounter many challenges in dispensing medicine to children in resource-constrained settings; particularly when dispensing to children, pharmacists confront two primary obstacles every day: There are no readily available formulations of the pharmaceutical that are both appropriate in size and potent enough to be taken by a child.4 Because young children are unable to swallowsolid dosage forms designed for adults, these issues are magnified when dispensing oral dose forms (ODF) to them. Every day, pharmacists have to give pills or crushed tablets that parents must divide because not many medicines are available in appropriate sizes and dosage forms for young children. These altered adults dosage forms’ bioavailability, efficacy, purity, safety, taste, and acceptability are all in question, and they are becoming a severe stumbling block to proper dispensing practice and rational drug use.5,6
Inappropriate drug usage has health and financial ramifications. From clinical considerations, ineffective therapy can lead to unnecessary death and suffering, as well as immune-mediated sickness and hospitalizations, as well as increased antibiotic-resistant microbes. Inappropriate drug usage also lowers trust of the public in the health-care system and reduces the number of people who use therapeutic and preventive therapies. Inappropriate medication use wastes a wealth of materials and makes vital pharmaceuticals unavailable in other sectors where they are required.4
To be deemed rational drug users, individuals need to acquire medicines that are suitable for particular healthcare needs, in quantities that meet their specific needs, for an appropriate duration, and also at the lowest cost possible to all of them and society.7 Drug use that is rational improves high-quality treatment and is cost-effective treatment. It ensures that pharmaceuticals are only used when absolutely necessary, and that people will understand what they are for and what to utilize them for.7,8 Over half of all drugs in the world are prescribed, given, or marketed wrongly, and half of the population does not take their medications as prescribed.9 Furthermore, around one-third of the world’s population lacks access to basic medicines.9,10 Different studies have found that a lack of comprehension of drugs leads to non-adherence in Ethiopia.7,11–13
In developing countries, irrational dispensing practices such as dispensing prescription-only drugs at partial doses or even without a prescription, poor labeling of dispensed drugs, a lack of patient counseling, incomplete compiling and recording of prescriptions, and charging patients an unreasonably high price for dispensed items are common.14 Because dispensing is such an important element of procedure for using drugs, improper practices during dispensing can have a negative impact on the health-care delivery system. Information presented during dispensing has a significant impact on patient compliance with prescription instructions. Mostly in impoverished countries, medication dispensing is typically performed by unskilled personnel. Risk of contamination is an issue that occurs when using a dispensing device to measure liquids or count tablets/capsules. Inadequate identification of supply containers can lead to incorrect preparation choices and raise the risk of accidents. Choosing a supply container during the dispensing procedure based on color or position while actively looking it up has serious consequences for the patient.15 The manner in which pharmaceuticals are administered and the kind of information given to patients during the procedure have an influence on the efficiency and effectiveness of dispensing practices. The pharmacist has the final decision about whether or not to issue the medication.16 The study’s findings could be used to educate planning officials, health practitioners, medical training centers, and other health-related NGOs about the flaws in Aman’s dispensing practices at health facilities so that health-care practitioners can improve their methods by correcting flaws and malpractices. The study’s goal is to enhance dispensing practices at various health-care facilities in the Aman town area. It provides pharmacists with information to help them identify primary causes of dispensing errors. It also serves as a baseline for future research because there is currently no study on good dispensing procedures in Aman and creates a chance for others to study about rational dispensing in Aman.
The goal of this study is to assess the rational dispensing of oral dose forms of medicines to children and assess the dispensing practice as well as the factors that influence this practice.
Methods
Study Area and Period
The study was conducted in MTUTH and selected drug stores in Aman town from September, 2021 to October, 2021 G.C. Aman town is located 583 km from the capital city of Ethiopia in the south-west. It contains a teaching hospital which provides service to a catchment population of 178,886. This hospital has five major wards, i.e. emergency, inpatient, pediatric, surgical and gynecology and obstetrics wards and each ward has their own pharmacy as a dispensing unit. The hospital delivers its services to the society under inpatient and outpatient case teams with different health-care professionals.
Study Design
The rational dispensing practice of oral dose forms of medications in the MTUTH and selected drugstores was investigated in a cross-sectional study design.
Population
Source of population
All children <12 years old who visited the hospital during data collection.
Study Population
All children who took oral dosage forms during the study period.
Inclusion and Exclusion
Inclusion Criteria
All children <12 years old who visited the health facilities during the study period.
Exclusion Criteria
- Children greater than 12 years old.
- Children who take dosage forms other than oral dosage forms.
Sample Size and Sampling Technique
At least 600 prescription interactions should be evaluated for evaluation of dispensing practice in a given health-care environment, according to the WHO guideline for assessing rational use of medications in outpatient departments of medical facilities.7,17 There is only one hospital in the area and the drug stores were selected randomly.
Every other child was chosen through systematic sampling, but the first child was chosen at random, considering that a prescription contained at least one oral dosage form. Starting from the first child all the dispensing units data were collected accordingly.
Study Variables
The variables to be included in the study are categorized as dependent and independent variables.
Independent Variables
- Age
- Experience
- Monthly income
- Marital status
- Sex
- Types of dosage forms
Dependent Variables
- Rational dispensing, dispensing practice.
Data Quality Control Measure
The pre-test was done to check the feasibility of the study and suitability of data collection tool. The data collection tool was pre-tested to check whether it could enable an appropriate data collection process. It was done on seven prescriptions on a dispensary unit.
Data Collection Instruments
Semi-structured questionnaires and structured observation checklist were used to collect the necessary information.
Data Analysis and Interpretation
The data were collected, counted; Statistical Package for Social Sciences was used to classify and analyze data (SPSS version 20). A structured, pre-tested observation checklist and a semi-structured questionnaire were used to obtain the relevant data through observation. The association between the variables was investigated using the Chi square test and the one-way ANOVA post-hoc test. SPSS version 20 was used to analyze the data. It was considered to be significant if the p-value was less than 0.05. These results were interpreted and presented using tables.
Ethical Clearance and Informed Consent
Mizan-Tepi University’s School of Pharmacy, College of Medicine and Health Sciences provided ethical approval. Subsequently the formal letter with the reference number CP/562/2013 was received on July 26 and was given to MTUTH health administrative office in order to get permission to conduct the study collection in accordance with the Declaration of Helsinki. The data were collected after obtaining written informed consent from parents/guardians of children. Starting with the design of the data collection instrument, the confidentiality of the information acquired was guaranteed. The collected data were kept secure.
Definition and Calculations of Indicators
Indicator 1: Instances where pharmaceutical alternatives were required; and it was calculated by dividing the total cases where replacement was done to the total number of prescribed medicines.
Indicator 2: This is defined under operational definition and the calculation was similar for all five indicators.
Indicator 3: Irrational manipulation of solid dosage forms occurs when patients do not receive pharmaceuticals that are appropriate to their clinical needs, in doses that satisfy their own specific requirements, for an adequate period of time, and at the lowest cost to them and their community.9
Indicator 4: Solid dosage forms which need manipulation before being taken by the patients with the aim of obtaining the required dose.
Indicator 5: Appropriate storage instructions of medicine is information delivered to patients on how they can appropriately store their medications.
Operational Definition
- The age groups: have been derived mainly from physiological and pharmacokinetic differences from birth to adulthood:
- Adequate labeling and packaging: as per WHO it is “drug packages containing at least patient name, drug name and when the drug should be taken”.7
- Rational dispensing: Patients receive pharmaceuticals suited to their needs, in doses that match their own unique requirements, for an adequate duration of time, and at the lowest cost to them and their community, according to the WHO definition.7
- Proper labeling (Indicator 2): finished drug products bearing at least the following information: name of the drug, list of the active ingredients, the batch number, the expiry date and the name and address of the manufacturer.20
Results
Socio-Demographic Characteristics of Pharmacy Professionals
A total of 22 dispensers participated in the study giving response rate of 100%. Of these 20 (90.90%) of respondents were from MTUTH and 2 (9.10%) were from DS in Aman town. Majority of the respondents were males 13 (2.2%) and within age group of 31−40 years. About 16 (72.7%), 2(9.1%), 4(18.2%), of respondents were pharmacist, pharmacy technician, druggist respectively. Meanwhile 14 (63.6%) and 8 (36.4%) were married and single respectively. Concerning training of rational dispensing, 14 (63.6%) dispensers had taken training of rational dispensing, 8 (36.4%) had not taken training on rational dispensing and also 20 (90.9%) had not dispensed drugs without prescription but 2 (9.1%) had dispensed drugs without prescription, as shown in Tables 1 and 2.
 |
Table 1 Socio-Demographic Characteristics of Pharmacy Professionals |
 |
Table 2 Questions Related to Practice of Rational Dispensing by Pharmacy Professionals |
From the total of 600 patients, 12.3%, 26.5%, 30% and 31.2% were new-term, infant and toddler, preschool age and school age who had taken oral dosage forms of medicines respectively both in MTUTH and DS. Solid dosage forms accounted for 31.2% and 10.3%, liquid forms accounted for 34% and 14.7%, and other forms accounted for 5.5% and 4.3% in MTUTH and DS, respectively as shown in Tables 3 and 4.
 |
Table 3 Socio-Demographic Characteristics of Patients |
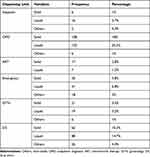 |
Table 4 Types of Oral Dosage Forms Dispensed to Pediatric Population |
Indicator 1: Percentage of cases where ODF replacements have been dispensed: The dosage forms details in the prescription were available for all 600 prescriptions from the ODF information obtained by the pharmacy and drug retailers. Alternative ODFs were dispensed only 22 (3.7%) and 1 (0.2%) of the instances at MTUTH and drug stores, respectively as shown in Table 5.
 |
Table 5 Summary of Indicators Measurement in Different Medicine Retail Outlets |
Indicator 2: The percentage of oral dose forms that are properly labeled.
All ODFs supplied in drug stores and pharmacies followed the label’s frequency and duration recommendations. Adequate labeling was 94.7%. But 5.3% of dispensed drugs were inadequately labeled.
Indicator 3: Percentage of solid oral dosage forms (SODFs) irrationally manipulated by the pharmacist before dispensing.
Results showed that 68 (11.7%) and 24 (4%) of the oral dosage forms had been manipulated inappropriately during dispensing by MTUTH pharmacy professionals and in drug stores, respectively. These manipulations consisted of broken down into pieces, powdered, and packaged in different packs.
Indicator 4: The proportion of solid dose units which must be manipulated for providing a single amount. In around 35 (5.8%) and 11 (1.8%) solid ODFs that were dispensed by MTUTH pharmacies and drug stores, respectively, when delivering a single entity, manipulating was required.
Indicator 5: The percent of times oral medication formulations are supplied with appropriate storage instructions. Only 256 (42.7%) and 112 (18.7%) ODFs were given appropriate storing instructions in MTUTH and drug stores in Aman town as shown in Table 5.
The type of medicine retail outlets had significant effect on percentage of instances where alternative ODFs were dispensed (p = 0.003), percentage of ODFs adequately labeled (p = 0.008), percentage SODFs irrationally manipulated before dispensing (p = 0.001), percentage of SODFs which need manipulation before dispensing (p = 0.004) and percentage of ODFs dispensed with correct advice (p = 0.001).
The results of the one-way ANOVA test showed that being a druggist had significance on indicator 3 (p = 0.049) and indicator 4 (p = 0.03*); type of oral dosage forms as both solid and liquid dosage forms had significant association on indicator 1 (p = 0.001), indicator 3 (p = 0.001) and indicator 5 (p = 0.003); and semi-solid dosage forms on indicator 1 (p = 0.002). There was no association between independent variables (age, monthly income and experience) and indicators of rational dispensing, as shown in Table 6.
 |
Table 6 The Association Between Sociodemographic of Pharmacy Professionals and Indicators of Rational Dispensing |
Discussion
In order to improve the effectiveness of the dispensing process, which is critical for clinical outcomes, the rational dispensing process of pharmaceuticals inside a health-care facility must be assessed.
A study conducted in pediatric hospital of Sri Lanka in 2020 indicated that an average number of 2.54 ODFs per children was reported.3
In our study, 515 children received 810 drugs, among these 690 ODFs were dispensed in MTUTH and also 120 medicines were dispensed to 85 at the drug stores in Aman town with an average number of 1.34 and 1.41 ODFs per child, respectively. This could be due to the low number of patients per day and could also be attributed to the difference in sample size.
In our study only 22 (3.7) and 1 (0.2%) different oral dose formulations other than those prescribed were provided to the children in both MTUTH and drug stores, respectively in Aman town.
In another study, alternative ODF dispensed were only 0.9% of the instances. While 85% of the ODFs contained the dose forms in the prescriptions issued by the practitioners, alternate ODFS were actually given for only 0.7% of cases.3
This might be due to unavailability of ODFs in the prescribed form in the study area.
In our study 68 (11.7%) and 24 (4%) ODFs were irrationally manipulated in MTUTH and DS, respectively. In another study, none of the oral dosage forms were modified inappropriately by the pharmacy professionals when the parents come to purchase a drug, while 2.8% were irrationally manipulated by clinical pharmacy. These oral dosage forms were broken, powdered, and packaged in different packages such as envelopes and given to parents/guardians for administration to children under the age of two years most frequently.3,10 This might be justified as some professionals did not take training on rational dispensing in our case.
In our study 35 (5.8%) and 11 (1.8%) solid ODFs needed manipulation before administration in MTUTH and drug stores, respectively. In another study the amount of oral dosage forms which needed to be manipulated for administration as single entity was lower compared with our study.3,11 This might be due to the fact that manipulation does affect therapeutic goals.
In our study 66.7% and 28% ODFs were adequately labeled in MTUTH and DS, respectively. A study was done in Ethiopia, to assess duration of labeling by dispensers,21 to investigate the way information on drug use is communicated to patients and to evaluate the effectiveness of the information. The checklist on the dispensed packages revealed that 100% of the labels did not include the name of the patient while the name of the drug (product) was indicated on all of them. The strength of the preparations was on 92% and 84% of labels; the frequency of administration was given on 60% of the labels issued by hospital I (Black Lion) and Hospital II (St Paulo’s) respectively.10 This could be due to experience of pharmacy professionals i.e. governmental health facilities conform to the WHO standard compared with the private medicine retail outlets.
In our study 256 (42.7%) and 112 (18.7%) ODFs were dispensed with correct advice in MTUTH and DS in Aman town, respectively. This figure is higher compared with a study conducted in Sri Lanka.3 This might be attributed to patient overload, absence of counseling area and a shortage of qualified staff available to inform the patient about their prescriptions.14
Associated factors were also identified in this study, as the level of dispenser certification was found to be substantially linked to indices of rational dispensing. The likelihood of rational dispensing was highest among druggists who dispensed irrationally manipulated solid oral dose forms in drug stores before dispensing, when before giving a single unit, ODFs must be manipulated. This could be because druggists’ training programs (core competencies) place a greater emphasis on dispensing services. This research supports the findings of a previous study conducted in Ethiopia.22 Besides types of dosage forms were significantly associated with rational dispensing indicators as indicator 1, indicator 3 and indicator 5 showed a significant relationship. This research supports the findings of a previous study in Sri Lanka.3
Limitation of the Study
The study’s main weakness was the risk of observer bias. The research team paid unannounced inspections, and no survey members of the team were there throughout counseling or dispensing, which reduced biases.
Conclusion and Recommendation
Conclusion
The rational dispensing practice of oral dosage forms for children in Mizan Tepi University teaching hospital and drug stores was low. As a result, pharmacy practitioners must receive ongoing professional development in pediatric pharmacy skills such as anticipating needs, labeling, storage, communicating with parents, and giving details. The low rate of rational dispensing shows there is need of more professional developments among the pharmacists. This shows there is still a gap to be improved by the medicine retail outlets from a rational dispensing point of view.
Recommendation
Based on the study’s findings, the following recommendations have been forwarded to the appropriate bodies. To ensure rational dispensing, pharmacy employees should have relevant and up-to-date drug information, and the drug should be given with the written indication, dose, strength, and instructions. Pharmacists should be trained in rational dispensing by health departments.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. The rational use of drugs: report of the conference of experts, Nairobi, 25–29 November 1985 [Internet]. Geneva PP - Geneva: World Health Organization; 1985. Available from: https://apps.who.int/iris/handle/10665/37174.
2. USAID. Rational drug use: prescribing, dispensing, dispensing, counseling and adherence in ART programs; 1985: 21. Available from: https://www.who.int/hiv/amds/capacity/ken_msh_rational.pdf.
3. Nadeshkumar A, Sathiadas G, Ranganathan SS. Rational dispensing of oral dosage forms of medicines to children at a teaching hospital in Sri Lanka. BMC Health Serv Res. 2020;20:1–6. doi:10.1186/s12913-020-05246-x
4. Yoseph E, Bekele A. Assessment of dispensing practices and quality of pharmaceutical services given at Shenen Gibe Hospital and Mendera Kochi Health Center in Jimma Town South West Ethiopia. Glob J Pharm Pharm Sci. 2018;5(4):84–91.
5. Quinzler R, Gasse C, Schneider A, Kaufmann-Kolle P, Szecsenyi J, Haefeli WE. The frequency of inappropriate tablet splitting in primary care. Eur J Clin Pharmacol. 2006;62(12):1065–1073. doi:10.1007/s00228-006-0202-3
6. Cleary JD, Evans PC, Hikal AH, Chapman SW. Administration of crushed extended-release pentoxifylline tablets: bioavailability and adverse effects. Am J Heal Pharm AJHP off J Am Soc Heal Pharm. 1999;56(15):1529–1534.
7. World Health Organization. Promoting rational use of medicines: core components. In: WHO policy perspectives on medicines No. 5. Geneva: World Health Organization; 2002.
8. Eftimova B, Lazarova B. Drug use at clinical hospital stip-evaluation of rational/irrational use Biljana Eftimova Clinical Hospital - Stip, Ljuben Ivanov bb, [email protected] Vrnjacka Banja, Serbia, March, 2018. Knowl Int J. 2018;22(5):2011–2013.
9. World Health Organization. Promoting rational use of medicines: core components; 2002: 1–6. Available from: https://apps.who.int/iris/bitstream/handle/10665/67438/WHO_EDM_2002.3.pdf.
10. Sema FD, Asres ED, Wubeshet BD. Evaluation of rational use of medicine using WHO/INRUD core drug use indicators at Teda and Azezo Health Centers, Gondar Town, Northwest Ethiopia. Integr Pharm Res Pract. 2021;10:51–63. doi:10.2147/IPRP.S316399
11. Angamo MT, Wabe NT, Raju NJ. Assessment of Patterns of drug use by using World Health Organization ’ s prescribing, patient care and health facility indicators in selected health facilities in Southwest Ethiopia. J Appl Pharm Sci. 2011;01(07):62–66.
12. Ahmedteha A, Girma M. ASsessment of labeling and patient knowledge of dispensed drugs at shenen gibe district hospital outpatient pharmacy, Jimma Lekovima u Shenen Gibe district. Med Rev. 2016;8(63):179–184.
13. Desta Z, Abula T, Beyene L, Fantahun M, Yohannes AG, Ayalew S. Assessment of rational drug use and prescribing in primary health care facilities in north west Ethiopia. East Afr Med J. 1997;74(12):758–763.
14. Ejeta F, Feyisa D, Kebede O, et al. Medication counseling practices in medicine retail outlets found in bench Sheko Zone, Southern Nations, Nationalities, and Peoples’ Region, South West Ethiopia. Pragmatic Obs Res. 2021;12:105–117. doi:10.2147/POR.S322407
15. FMHACA. Manual for medicines good dispensing practice; 2012.
16. DACA. Guideline for the regulation of promotion and advertisement of drugs; 2008.
17. World Health Organization. How to investigate drug use in health facilities: selected drug use indicators. Geneva: World Health Organization; 1993: 92. Available from: https://apps.who.int/iris/bitstream/handle/10665/60519/WHO_DAP_93.1.pdf?sequence=1&isAllowed=y.
18. FDA. International Conference on Harmonisation; guidance on E11 clinical investigation of medicinal products in the pediatric population; availability. Notice. Fed Regist. 2000;65(242):78493–78494.
19. Hanada K. Characteristics of clinical data packages of pediatric medical products approved in the US and comparison of the approved dosages between pediatric and adult populations; 2015.
20. World Health Organization. General regulations made in terms of the medicines and related substances act, 1965(ACT NO. 101 OF 1965), as amended; 1965.
21. Ejigu E, Gebre-Mariam T, Gedif T. Assessment of drug labeling in two selected hospitals of Addis Ababa Ethiopia. J Ethiop Pharma Assoc. 1998;14:123–212.
22. Wogayehu B, Adinew A, Asfaw M. Knowledge on dispensed medications and its determinants among patients attending outpatient pharmacy at chencha primary level hospital, Southwest Ethiopia. Integr Pharm Res Pract. 2020;9:161–173. doi:10.2147/IPRP.S274406
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
