Back to Journals » Journal of Pain Research » Volume 13
Parasternal Intercostal Block Complementation Contributes to Postoperative Pain Relief in Modified Radical Mastectomy Employing Pectoral Nerve Block I and Serratus-Intercostal Block: A Randomized Trial
Authors Song WQ , Wang W , Yang YC, Sun Q, Chen H, Zhang L, Bu XS, Zhan LY, Xia ZY
Received 5 November 2019
Accepted for publication 18 February 2020
Published 30 April 2020 Volume 2020:13 Pages 865—871
DOI https://doi.org/10.2147/JPR.S237435
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Wen-Qin Song, Wei Wang, Ying-Cong Yang, Qian Sun, Hui Chen, Lei Zhang, Xue-Shan Bu, Li-Ying Zhan, Zhong-Yuan Xia
Department of Anesthesiology, Renmin Hospital of Wuhan University, Wuhan, People’s Republic of China
Correspondence: Zhong-Yuan Xia; Liying Zhan
Department of Anesthesiology, Renmin Hospital of Wuhan University, No. 238 Jiefang Road, Wuchang District, Wuhan City, Hubei Province 430060, People’s Republic of China
Email [email protected]; [email protected]
Purpose: Pectoral nerve block I (PECS I) and serratus-intercostal plane block (SIPB) can anesthetize the majority mammary region, while parasternal intercostal block (PSI) targets the internal area during breast resection surgery. The aim of this study was to determine whether including PSI with PECS I and SIPB is more effective compared to PECS I and SIPB alone.
Patients and Methods: Sixty-two adult females undergoing unilateral modified radical mastectomy (MRM) were randomly assigned to receive either PECS I and SIPB (PS group, n=31) or a combination of PECS I, SIPB, and PSI (PSP group, n=31). The outcomes were measured with a numerical rating scale (NRS) score, and in terms of opioid consumption and anesthesia-related complications within 48 h after surgery.
Results: Although there were no differences in the NRS scores between the two groups during the inactive periods, the combination of three nerve blocks significantly reduced the NRS scores during movement. In addition, morphine equivalent consumption was lower in the PSP group compared to the PS group. Postoperative adverse events were similar in both groups in terms of regional anesthesia-related complications.
Conclusion: The combination of PECS I block, SIPB, and PSI block provides superior pain relief and postoperative recovery for patients undergoing MRM.
Keywords: pectoral nerve block, serratus-intercostal plane block, parasternal intercostal block, postoperative analgesia, modified radical mastectomy
Introduction
Modified radical mastectomy (MRM), the preferred treatment for 30–40% of patients with breast cancer,1 refers to the removal of the entire breast in addition to several lymph nodes under the arm.2 However, this procedure is associated with both acute and chronic post-surgical pain.3–6 In addition, acute postoperative pain is also a risk factor for the development of chronic pain.7 Studies in recent years have shown that adequate postoperative pain management can prevent these complications.8,9 However, a recent survey conducted by the Stony Brook University showed that postoperative pain in MRM remains poorly controlled.10 Therefore, a novel analgesic approach is required to prevent post-MRM pain and improve patient outcomes.
Ultrasound-based applications have enabled regional anesthetization for managing postoperative pain and is routinely recommended to prevent discomfort.11 Based on the origin, alignment, branch, and distribution of the intercostal nerve within the anterior thoracic region, postoperative analgesia in breast cancer patients can be classified into the thoracic paravertebral block (TPVB),12 intercostal nerve block (ICNB),13 pectoral nerve block I (PECS I),14 and serratus-intercostal plane block (SIPB).15 Thoracic paravertebral block is regarded as the priority option for regional anesthesia during breast cancer surgery; however, the invasive procedure requires excellent skill, experience, and deep puncturing.16
PECS I involves local anesthetic injection between pectoralis major and minor muscle, and it is a controlled and reliable technique that intercepts the innervation of the medial and lateral pectoralis nerves originating from cervical and brachial plexuses.17 SIPB, an inter-fascial approach between the serratus anterior muscle and external intercostal muscle or below serratus anterior muscle, was initially proposed as an alternative regional anesthetic technique that targets the lateral cutaneous branches of the thoracic intercostal nerves (TICN). These nerves are targeted since they penetrate the plane just below the anterior superior serratus anterior muscle to relay sensation to most of the chest wall.18 However, since drug infiltration is consistently circumscribed in the parasternal area dominated by the anterior cutaneous branches of the intercostal nerves, the latter also need to be blocked.19 Parasternal intercostal block (PSI block), wherein the anesthesia is injected into the plane deep underneath pectoralis major muscle, was developed for median sternotomy and anesthetizes the anterior cutaneous branches of TICN.20 Therefore, PSI can supplement PECS I and SIPB in breast cancer survivors.21
Considering the scope of the surgical incision in MRM, we hypothesized that the addition of PSI block to the typical PECS I and SIPB will improve peri-operative pain relief in MRM patients.
Patients and Methods
Study Participants
The prospective single-center, randomized trial was conducted in accordance with the ethical guidelines outlined in the Declaration of Helsinki, approved by the Institutional Review Board (IRB) of Renmin Hospital of Wuhan University, Wuhan, PR China, and registered at the Chinese Clinical Trial Registry (ChiCTR-1800020250).
Female patients aged 18 to 70 years with American Society of Anesthesiologists (ASA) physical classification I to II undergoing MRM were enrolled. Patients that presented contraindications to regional anesthesia or local anesthetics, chronic opioid use, morbid obesity (body mass index > 35 kg/m2), severe cardiopulmonary, renal and liver dysfunction and mental incapacity, or those who refused to participate were excluded.
Blinding and Randomization
All patients provided written informed consent and were randomly divided into the PECS I + SIPB (PS) or PSI block + PECSI + SIPB (PSP) groups. Each patient was assigned a number, and the grouping results were seen by the anesthesiologist prior to administering the nerve block. The investigator responsible for group assignment and the anesthesiologist were not involved any further, and the anesthesiologists, surgeons, PACU nurses, and investigators in the follow-up study were blinded to the group assignment.
Nerve Block Procedure
For PECS I block, the patients were instructed to lie in the supine position, and their electrocardiogram (ECG), non-invasive blood pressure (NIBP) and pulse oxygen saturation (SpO2) were monitored according to standard ASA recommendations. After disinfecting the chest skin with 1% povidone-iodine (YUNZUO, China) and locally applying 1% lidocaine, a high-frequency linear probe (6 to 13 MHz; Acclarix AX8 Compact Ultrasound System, EDAN, China) was placed horizontally at the junction of the middle and lateral thirds of the clavicle and the third rib. The transducer was then moved laterally till the thoracoacromial artery, and the pectoralis major and minor muscles were clearly observed. An atraumatic needle for peripheral nerve blocks (22G, 50 mm, B. BRAUN, Germany) was inserted in-plane into the fascia between the pectoralis major and minor muscles, and 10 mL 0.3% ropivacaine was injected in a medial to lateral direction.
SIPB was initiated with patients in the lateral position. The ultrasound transducer was placed parallel to the mid-axillary line and then moved in the sagittal plane to visualize the fifth rib and the serratus anterior muscle. A needle was inserted from the caudal to cranial direction via an in-plane technique, and the tip was placed in the fascial plane between the serratus anterior muscle and the external intercostal muscle or deep underneath the serratus muscle where 20 mL 0.3% ropivacaine was administered.
During PSI block, ultrasonography was performed longitudinally from the medial to lateral direction in the parasternal area until the pectoralis major muscle, the lower border of the third rib and upper border of the fourth rib were identified. Ten milliliters 0.3% ropivacaine was injected past the fourth rib towards the third rib between the pectoralis major muscle and the internal intercostal muscle or deep in the pectoralis major muscle.
Intraoperative Management
After conventional intravenous anesthetization with propofol (1.5–2mg/kg) and sufentanil (0.3–0.5mcg/kg), orotracheal intubation was performed using a direct laryngoscope, followed by volume-controlled mechanical ventilation. Based on the desired Narcotrend index range between 20 and 46, or Narcotrend staging between E1 to D2, the depth of anesthesia was monitored to ensure consistent anesthetic maintenance. Propofol was continuously infused, along with discontinuous application of muscle relaxants and remifentanil. An IV dose of 5 mg dexamethasone and 3–5 mg metoclopramide was administered to prevent postoperative nausea and vomiting (PONV). Once the patients were able to follow verbal commands after the surgery, the trachea was extubated and the patient was transferred to the post-anesthesia care unit (PACU).
Postoperative Management
All patients were admitted to the surgical ward when their Steward score reached 4 or above. If the patient complained of moderate or severe incision pain, corresponding to 4–6 points and 7–10 points (NRS) respectively, they were treated with sufentanil 2–5 mcg IV. PONV was treated with tropisetron 5 mg IV.
Outcomes
All baseline information and postoperative measurements were assessed by an investigator. Postoperative pain score was assessed using a numerical rating scale (NRS) (0 = no pain to 10 = worst, severe pain). NRS pain scores during inactive and active (upper limb abduction or coughing) periods were measured 1, 3, 6, 12, 24, and 48 h after surgery. Complications associated with the nerve blocks and general anesthesia, such as pneumothorax, nausea, vomiting, and itching, were treated and recorded.
Statistical Analysis
Preliminary data in 16 subjects suggested that 24 h morphine equivalent consumption, calculated by the equivalent conversion of sufentanil, agonist/antagonist of opioid receptors and non-steroidal anti-inflammatory drugs (NSAIDs), were 51.88±10.64 and 39.25±6.86 mg in the PS and PSP groups, respectively. To achieve a type 1 error of 0.05 and 90% power to assume a 10 mg difference in opioid consumption, the required sample size was estimated to 17 patients per group. SPSS 16.0 software (IBM, USA) was used for statistical analysis, and the data were presented as mean ± standard deviation for numerical variables, and as frequencies and proportions for categorical variables. Numerical variables were analyzed using the Student independent two-sample t-test or nonparametric Mann–Whitney U-tests, depending on whether the data distribution was normal or not as per Shapiro–Wilk test. The categorical data were analyzed using the chi-square test. P < 0.05 was considered significantly different.
Results
Seventy potential patients were assessed for eligibility from December 2018 to May 2019, of which 8 were excluded. The remaining 62 subjects consented to participate in this study and completed all the assessments (Figure 1). The PS and PSP groups were similar in terms of demographic characteristics such as age, height, weight, ASA physical status, surgical side, duration of surgery, anesthesia, etc. (Table 1).
 |
Table 1 Demographic Characteristics and Baseline Data |
 |
Figure 1 Consort flow diagram. PECS I, pectoral nerve block I; SIPB, serratus-intercostal plane block. |
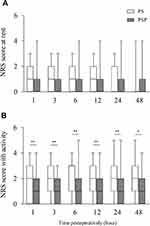
While the NRS scores were similar between both groups during the rest periods at all time points (Figure 2A), the addition of PSI to PECS I and SIPB attenuated pain severity during active periods compared to the PS group (Figure 2B).
In addition, PSI block supplementation significantly reduced morphine requirement in the first 24 h after MRM when compared to the PS group (40.58 ± 6.49 mg vs 52.34 ± 11.14 mg; difference of 11.76 mg; P < 0.001) (Table 2). Furthermore, the duration of PACU stay was significantly shorter for the PSP compared to the PS group (46.03±12.11 min vs 53.19±13.56 min; P=0.032) (Table 2).
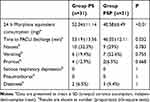 |
Table 2 Opioid Consumption and Postoperative Adverse Events of Patients |
The postoperative adverse events were not significantly different between the two groups (Table 2). Taken together, compound regional anesthesia can relieve pain and minimize opioid-related complications.
Discussion
Supplementing PECS I and SIPB with PSI block increases peri-operative pain relief during and after breast cancer surgery, compared to PECS I and SIPB alone. Several reports in recent years support the combined application of PECS I and II blocks for superior postoperative analgesia in patients receiving breast cancer surgery.14,22,23 In addition, ultrasound-guided PECS I and SIPB is a suitable alternative to conventional regional anesthesia.24,25 However, we observed in a previous study that some breast cancer patients subjected to PECS I block and SIPB complained of mild to moderate pain in the internal mammary area, which is in accordance with other studies.26,27
Transversus thoracic muscle plane (TTP) block has been successively applied to median sternotomy, pericardial drainage, and modified radical mastectomy.28–30 Ueshima et al found that the combination of PECS and TTP blocks decreased NRS pain scores during both rest and movement after MRM, as did pentazocine addition, compared to PECS block only.30 A study on 299 patients receiving TTP block did not detect complications like hematomas and pneumothorax.31 However, inducing a TTP block is technically challenging since it is difficult to detect the transversus thoracic muscle by ultrasound and the injection site is close to pleura.21 Since anterior branches of the intercostal nerve penetrate through the pectoral major muscle, and the external intercostal muscle innervates the internal mammary area, PSI block tends to produce a uniform sensory deficit with TTP block, and is technically simple. Therefore, a PSI block is a better alternative if the relatively thin transversus thoracic muscle is not clearly confirmed.
Thoracic paravertebral block has been considered an optimal choice for analgesic modality in patients undergoing breast cancer surgery.32 The analgesic benefits of TPVB have been thoroughly studied, including relieved acute and chronic pain following modified radical mastectomy, reduced opioid consumption, lower incidence of adverse events, and improved survival quality.33,34 However, evidence derived from an efficacy and safety trial indicates that superb skill and rich practical experience are required in the procedure since the failure rate of ultrasound-guided TPVB is 5.3%.35 Furthermore, TPVB is technically difficult to learn and perform compared with pectoral nerve block.36 Hussain N and colleagues undertook a systematic review and meta-analysis to provide evidence that pectoral nerve blocks could be a promising alternative to paravertebral block.37
To the best of our knowledge, this study is the first to evaluate the efficacy and safety of co-administering PECS I, SIPB, and PSI block in patients undergoing MRM. In contrast to another prospective clinical trial,30 our findings indicate that combining all three nerve blocks reduced NRS scores and morphine requirement compared to PECS I block and SIPB alone. The modest decrease in pain score and opioid requirements may be attributed to the preservation of the internal mammary nodes in a few patients.
There were several limitations in our study, such as the relatively small sample size, absence of sensory spread analysis due to a lack of presurgical pinprick test data, and not measuring the duration of regional anesthesia, hemodynamic changes, and the plasma levels of stress-associated proteins. Subsequent studies should focus on these parameters. In conclusion, PSI block can be an effective and safe adjuvant to PECS to provide superior pain relief. Further studies should assess the ideal analgesic pattern to prevent chronic post-surgical pain.
Conclusion
Supplementary parasternal intercostal block during pectoral nerve block I and serratus-intercostal block reduced early postoperative pain in adult female patients undergoing MRM.
Data Sharing Statement
The complete data is available in Supplemental file 1. No further data will be shared.
Acknowledgments
There was no external funding in the preparation of this manuscript.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62. doi:10.3322/caac.21203
2. PDQ Adult Treatment Editorial Board. Breast cancer treatment (PDQ®): patient version. PDQ Cancer Inf Sum. 2018.
3. Andersen KG, Duriaud HM, Jensen HE, et al. Predictive factors for the development of persistent pain after breast cancer surgery. Pain. 2015;156(12):2413–2422. doi:10.1097/j.pain.0000000000000298
4. Carpenter JS, Andrykowski MA, Sloan P, et al. Postmastectomy/postlumpectomy pain in breast cancer survivors. J Clin Epidemiol. 1998;51(12):1285–1292. doi:10.1016/S0895-4356(98)00121-8
5. Peuckmann V, Ekholm O, Rasmussen NK, et al. Chronic pain and other sequelae in long-term breast cancer survivors: nationwide survey in Denmark. European J Pain. 2009;13:478–485. doi:10.1016/j.ejpain.2008.05.015
6. Hayes SC, Johansson K, Stout NL, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118:2237–2249. doi:10.1002/cncr.27467
7. Meretoja TJ, Andersen KG, Bruce J, et al. Clinical prediction model and tool for assessing risk of persistent pain after breast cancer surgery. J Clin Oncol. 2017;35:1660–1667. doi:10.1200/JCO.2016.70.3413
8. Kehlet H1, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. doi:10.1016/S0140-6736(06)68700-X
9. Sinatra R. Causes and consequences of inadequate management of acute pain. Pain Med. 2010;11(12):1859–1871. doi:10.1111/j.1526-4637.2010.00983.x
10. Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017;10:2287–2298. doi:10.2147/JPR.S144066
11. Chou R, Gordon DB, de Leon-casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of regional anesthesia and pain medicine, and the American Society of Anesthesiologists’ Committee on regional anesthesia, executive committee, and administrative council. J Pain. 2016;17:131–157. doi:10.1016/j.jpain.2015.12.008
12. Hussain N, Shastri U, McCartney CJL, et al. Should thoracic paravertebral blocks be used to prevent chronic postsurgical pain after breast cancer surgery? A systematic analysis of evidence in light of IMMPACT recommendations. Pain. 2018;159(10):1955–1971. doi:10.1097/j.pain.0000000000001292
13. Wijayasinghe N, Duriaud HM, Kehlet H, et al. Ultrasound guided intercostobrachial nerve blockade in patients with persistent pain after breast cancer surgery: a pilot study. Pain Physician. 2016;19(2):E309–18.
14. Kamiya Y, Hasegawa M, Yoshida T, et al. Impact of pectoral nerve block on postoperative pain and quality of recovery in patients undergoing breast cancer surgery: A randomised controlled trial. Eur J Anaesthesiol. 2018;35(3):215–223. doi:10.1097/EJA.0000000000000762
15. Ohgoshi Y, Yokozuka M, Terajima K. Serratus-intercostal plane block for breast surgery. Masui. 2015;64(6):610–614.
16. Hussain N, Brull R, McCartney CJL, et al. Pectoralis-II myofascial block and analgesia in breast cancer surgery: a systematic review and meta-analysis. Anesthesiology. 2019;131(3):630–648.
17. Blanco R. The ‘pecs block’: a novel technique for providing analgesia after breast surgery. Anesthesia. 2011;66:847–848. doi:10.1111/j.1365-2044.2011.06838.x
18. Blanco R, Parras T, McDonnell JG, Prats-Galino A. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia. 2013;68:1107–1113. doi:10.1111/anae.12344
19. Blanco R, Fajardo M, Parras Maldonado T. Ultrasound description of pecs ii (modified pecs I): a novel approach to breast surgery. Rev Esp Anestesiol Reanim. 2012;59:470–475. doi:10.1016/j.redar.2012.07.003
20. Song WQ, Wang W, Zhan LY. Perioperative analgesia during thymectomy via median sternotomy: ultrasound-guided bilateral parasternal block. Anaesthesist. 2019;68(12):848–851. doi:10.1007/s00101-019-00700-w
21. Ohgoshi Y, Ino K, Matsukawa M. Ultrasound-guided parasternal intercostal nerve block. J Anesth. 2016;30(5):916. doi:10.1007/s00540-016-2202-5
22. Bashandy GM, Abbas DN. Pectoral nerves I and II blocks in multimodal analgesia for breast cancer surgery: a randomized clinical trial. Reg Anesth Pain Med. 2015;40(1):68–74. doi:10.1097/AAP.0000000000000163
23. Karaca O, Pınar HU, Arpacı E, et al. The efficacy of ultrasound-guided type-I and type-II pectoral nerve blocks for postoperative analgesia after breast augmentation: A prospective, randomised study. Anaesth Crit Care Pain Med. 2018;S2352–5568(17):2–30358.
24. Bouzinac A, Brenier G, Dao M, et al. Bilateral association of pecs I block and serratus plane block for postoperative analgesia after double modified radical mastectomy. Minerva Anestesiol. 2015;81(5):589–590.
25. Fusco P, Scimia P, Marinangeli F, et al. The association between the ultrasound-guided serratus plane block and PECS I block can represent a valid alternative to conventional anesthesia in breast surgery in a seriously ill patient. Minerva Anestesiol. 2016;82(2):241–242.
26. Wang W, Song WQ, Yang YC, et al. Ultrasound-guided pectoral nerve block I and serratus-intercostal plane block alleviate postoperative pain in patients undergoing modified radical mastectomy. Pain Physician. 2019;22(4):E315–E323.
27. Ueshima H, Kitamura A. blocking of multiple anterior branches of intercostal nerves (TH2-6) using a transversus thoracic muscle plane block. Reg Anesth Pain Med. 2015;40(4):388. doi:10.1097/AAP.0000000000000245
28. Ueshima H, Hara E, Marui T, et al. The ultrasound-guided transversus thoracic muscle plane block is effective for the median sternotomy. J Clin Anesth. 2016;29:83. doi:10.1016/j.jclinane.2015.10.014
29. Ueshima H, Otake H. The lateral transversus thoracic muscle plane block is effective for the pericardial drainage. J Clin Anesth. 2017;42:12. doi:10.1016/j.jclinane.2017.07.011
30. Ueshima H, Otake H. Addition of transversus thoracic muscle plane block to pectoral nerves block provides more effective perioperative pain relief than pectoral nerves block alone for breast cancer surgery. Br J Anaesth. 2017;118(3):439–443. doi:10.1093/bja/aew449
31. Ueshima H, Otake H. Ultrasound-guided transversus thoracic muscle plane block: complication in 299 consecutive cases. J Clin Anesth. 2017;41:60. doi:10.1016/j.jclinane.2017.03.056
32. Tighe SQ, Karmakar MK. Serratus plane block: do we need to learn another technique for thoracic wall blockade? Anaesthesia. 2013;68:1103–1106. doi:10.1111/anae.12423
33. Abdallah FW, Morgan PJ, Cil T, et al. Ultrasound-guided multilevel paravertebral blocks and total intravenous anesthesia improve the quality of recovery after ambulatory breast tumor resection. Anesthesiology. 2014;120:703–713. doi:10.1097/ALN.0000436117.52143.bc
34. Karmakar MK, Samy W, Li JW, et al. Thoracic paravertebral block and its effects on chronic pain and health-related quality of life after modified radical mastectomy. Reg Anesth Pain Med. 2014;39(4):289–298. doi:10.1097/AAP.0000000000000113
35. Terkawi AS, Tsang S, Sessler DI, et al. Improving analgesic efficacy and safety of thoracic paravertebral block for breast surgery: A mixed-effects meta-analysis. Pain Physician. 2015;18:E757–80.
36. Kulhari S, Bharti N, Bala I, Arora S, Singh G. Efficacy of pectoral nerve block versus thoracic paravertebral block for postoperative analgesia after radical mastectomy: A randomized controlled trial. Br J Anaesth. 2016;117:382–386. doi:10.1093/bja/aew223
37. Hussain N, Brull R, McCartney CJL, et al. Pectoralis-II myofascial block and analgesia in breast cancer surgery: a systematic review and meta-analysis. Anesthesiology. 2019;131(3):630–648. doi:10.1097/ALN.0000000000002822
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.