Back to Journals » OncoTargets and Therapy » Volume 12
RASSF6 Is Downregulated In Human Bladder Cancers And Regulates Doxorubicin Sensitivity And Mitochondrial Membrane Potential Via The Hippo Signaling Pathway
Authors Tan S, Bian X, Wu B, Chen X
Received 24 May 2019
Accepted for publication 25 September 2019
Published 5 November 2019 Volume 2019:12 Pages 9189—9200
DOI https://doi.org/10.2147/OTT.S217041
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Shutao Tan,1 Xiaobo Bian,2 Bin Wu,1 Xiaonan Chen1
1Department of Urology, Shengjing Hospital of China Medical University, Shenyang 110004, People’s Republic of China; 2Department of General Surgery, Shengjing Hospital of China Medical University, Shenyang 110004, People’s Republic of China
Correspondence: Xiaonan Chen
Department of Urology, Shengjing hospital of China Medical University, Shenyang 110004, People’s Republic of China
Tel +8618940252862
Email [email protected]
Background: The present study aimed to investigate the clinicopathological significance and biological roles of RASSF6 in human bladder cancers.
Materials and methods: Immunohistochemistry and Western blots were used to examine the protein expression of RASSF6 in bladder cancer tissues. Biological roles of RASSF6 were examined using MTT, colony formation assay, Matrigel invasion assay, cell cycle analysis, AnnexinV/PI staining and JC-1 staining. Western blot analysis was used to examine the potential mechanism.
Results: We found that RASSF6 was downregulated in 73 of 138 bladder cancer specimens, which correlated with advanced stages. RASSF6 overexpression decreased the cell growth rate and inhibited invasion ability in T24 cell line. Downregulation of RASSF6 using siRNA increased the cell proliferation rate and promoted invasion in 5637 cell line. Cell cycle studies showed that RASSF6 overexpression suppressed the process of cell cycle progression. RASSF6 overexpression also increased the cellular response to doxorubicin (DOX) treatment. AnnexinV/PI staining showed that RASSF6 overexpression promoted DOX-induced apoptosis with increased cytochrome c and cleavage of caspase-3 and caspase-9. We also showed that RASSF6 overexpression downregulated the mitochondrial membrane potential, while RASSF6 depletion showed the opposite effect. Western blot analysis demonstrated that RASSF6 overexpression repressed p-Rb and Bcl-xL while upregulating p21 expression. In addition, we found that RASSF6 overexpression affected the Hippo signaling pathway by downregulating YAP. Depletion of YAP downregulated Bcl-xL expression and abolished the effect of RASSF6 on Bcl-xL. Depletion of YAP also upregulated the level of apoptosis and downregulated mitochondrial membrane potential. YAP siRNA abolished the effects of RASSF6 on DOX-induced apoptosis and loss of mitochondrial membrane potential.
Conclusion: Taken together, our results showed that RASSF6 was downregulated in bladder cancers. RASSF6 inhibited cell proliferation and invasion, as well as the progression of cancer, by regulating DOX sensitivity and mitochondrial membrane potential, possibly via the Hippo signaling pathway.
Keywords: RASSF6, bladder cancer, Hippo, YAP
Introduction
Bladder cancer is a common malignancy affecting the urinary tract, and it is one of the leading causes of death worldwide.1 Although the combination of surgery and chemotherapy has achieved a significant progress, the prognosis of advanced stage bladder cancer remains poor. The development of chemoresistance is one of the most important causes. Therefore, it is necessary to seek and identify more effective targets involved in chemo-resistance that can serve as molecular markers to predict the risk of bladder cancer.
RASSF6 belongs to the RASSF family with a Ras association domain, which has been reported to be involved in the Hippo signaling pathway. RASSFs are frequently inactivated through loss of function mutations or promoter methylation. Like other RASSF family proteins, downregulation of RASSF6 has been reported in childhood leukemias.2 Decreased RASSF6 was also reported as an independent prognostic marker in gastric cancer patients3 and RASSF6 also shows frequent DNA methylation in neuroblastoma.4 In metastatic melanoma, hypermethylation was found in the RASSF6 promoter. However, the clinical significance and biological role of RASSF6 in human bladder cancer remain unknown.
To address the above questions, we evaluated RASSF6 protein expression in bladder cancer tissues and analyzed its clinical significance. We also examined whether RASSF6 could influence the biological behavior and investigated the possible mechanism in bladder cancer cells.
Materials And Methods
Patient And Specimen
The present study protocol was approved by the institutional reviewer board of Shengjing Hospital. Bladder cancer specimens and adjacent normal bladder tissues were obtained from patients who underwent surgical resection between 2012 and 2017. Written informed consent was provided by the patients and this was conducted in accordance with the Declaration of Helsinki. Clinical data including histopathological diagnosis and tumor grade were extracted from medical records. Ten cases of fresh specimens including both tumor tissue and corresponding normal tissue were stored at −80°C after resection for further protein extraction.
Immunohistochemistry
Tissue sections (4-μm thick) were prepared and were deparaffinized using xylene. Graded alcohol (100%, 10 mins; 90%, 2 mins; 80%, 2 mins; and 70%, 2 mins) was utilized for rehydration. Citrate buffer (pH 6.0) was used for antigen retrieval in an autoclave. A 3% solution of H2O2 solution was used for blocking the endogenous peroxidase. Sections were incubated with normal goat serum to reduce nonspecific binding. Then, sections were incubated with RASSF6 antibody (1:200; Proteintech, USA) overnight at 4°C. Immunohistochemistry was performed using the Elivision Plus kit purchased from Maixin (MaiXin, Fuzhou, People’s Republic of China). The sections were stained with DAB+ kit (Maixin) and counterstained with hematoxylin.
Two pathologists randomly examined all tumor slides. Immunostaining of RASSF6 was scored following a semiquantitative scale by evaluating in representative tumor areas for the intensities and percentages of tumor cells. Cytoplasmic localization was interpreted as positive staining. Staining intensity was scored as 0 (no/weak staining), 1 (moderate staining), 2 (strong staining). Percentage scores were classified into 1: 1–25%, 2: 26–50% 3: 51–75% and 4: 76–100%. These scores were multiplied to obtain the final score. The tumors were finally determined as RASSF6 low expression (RASSF6 downregulation) when the score was <4, and RASSF6 high expression when the tumor score was ≥4.
Cell Culture And Transfection
BIU-87, 5637 and T24 cell lines were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai, People’s Republic of China). The cells were maintained in PRMI-1640 (Invitrogen, Carlsbad, CA, USA) with 10% FBS (Invitrogen, USA).
RASSF6 plasmid was purchased from Origene (Origene, Rockville, USA) and transfected into cells using Lipofectamine 3000 (Invitrogen, USA). RASSF6 siRNA target sequence was: GACCCAGAUUCCUAUGUCU. Dharmafect1 reagent (Dharmacon, USA) was used for siRNA knockdown transfection.
Quantitative Real-Time PCR (SYBR Green Method)
RNA was extracted using RNAiso plus reagent (TaKaRa, Dalian, People’s Republic of China). Reverse transcription was performed using a TaKaRa RT kit (TaKaRa, Dalian, People’s Republic of China). Real-time PCR was performed using SYBR Green master mix (TaKaRa, Dalian, People’s Republic of China). PCR was performed using Analytik-jena qTOWER PCR System (Jena, Germany). β-actin was used as endogenous control. The change of gene amplification was calculated using the 2−ΔΔCt method.
Western Blotting
Protein samples were extracted from cells using lysis buffer and were separated by SDS-PAGE. Then, proteins were then transferred to a polyvinylidene fluoride membranes and incubated with primary antibodies against RASSF6 (1:800; Porteintech), p21, MMP2, Bcl-xL, cytochrome c, caspase-3, cleaved caspase-3, caspase-9, cleaved caspase-9 and YAP (1:1000, Cell Signaling Technology, USA) and GAPDH (1:2000; Cell Signaling Technology, USA). After incubation with horseradish-peroxidase (HRP)-coupled secondary antibody (1:2000, Santa Cruz, USA). The bands were visualized with HRP substrate (Pierce) and recorded using a DNR Bio-Imaging System (DNR, Jerusalem, Israel).
Colony Formation Assay And The MTT Assay
Cells (1000) were seeded in 6-well plates and then cultured for about approximately 2 weeks. The plates were washed with PBS, fixed with paraformaldehyde, and stained with Giemsa. The number of colonies with more than 50 cells was counted.
For MTT assays, 24 hrs after transfection, the cells were seeded in a 96-well plates at a concentration of approximately 2000 cells per well. To evaluate cell viability, 5 mg/mL MTT solution (20 μL) was added to each well for 4 hrs in a CO2 incubator. Culture medium was then removed and 150 μL of DMSO was added to the well and mixed gently. The plate was measured at a wavelength of 490 nm using a plate reader.
Cell Cycle Analysis
Cell cycle analysis was conducted 48 hrs after transfection. The transfected cells were fixed with paraformaldehyde at 4°C overnight and then washed with PBS buffer. The cells were stained with propidium iodide (PI, 5 mg/mL, 30 mins) at room temperature. Flow cytometry was performed using the ACEA flow cytometer (ACEA Biosciences, San Diego, CA, USA).
Annexin V/PI Apoptosis Assay
The percentage of apoptotic cells was determined using the Annexin V/PI staining assay. Early and late apoptotic cells were determined via flow cytometry using the ACEA flow cytometer.
Matrigel Invasion Assay
The cell invasion assay was performed using a Transwell chamber coated with 18 µL Matrigel from BD Bioscience (dilution, 1:4). The cells were suspended and 100 µL of serum-free medium and transferred to the upper chamber and incubated for 20 hrs. The noninvading cells in the upper chambers were removed. The cells passing through the filter were fixed with paraformaldehyde (room temperature, 10 mins), stained with hematoxylin (room temperature, 5 mins) and counted under a light microscope.
Mitochondrial Membrane Potential
The JC-1 staining method was used for examination of the mitochondrial membrane potential (Δψm). Briefly, cells were washed with PBS buffer and then incubated with JC-1 fluorescent dyes (5 μM) for 30 mins in an incubator. The cells were washed with PBS buffer and applied to ACEA flow cytometer (ACEA, USA) for analysis. Novoexpress software (ACEA, USA) was used for data analysis.
Statistical Analysis
We used SPSS statistical software for Windows, version 16 software (SPSS, Chicago, IL, USA) for statistical analyses. The correlations between RASSF6 levels and clinicopathological factors were analyzed according to the χ2 test. Differences between transfection/control groups were analyzed using Student’s t-test. A value of p<0.05 was considered as statistically significant.
Results
The Clinical Significance Of RASSF6 In Bladder Cancer Tissues
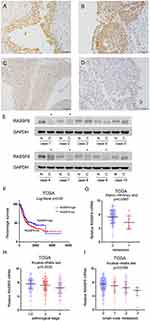
Strong cytoplasmic RASSF6 expression was found in normal urothelial transitional epithelial tissue (Figure 1A). According to the immunohistochemical (IHC) scoring system, we stratified all cases into 2 groups: low RASSF6 expression (scores 0–4) and high RASSF6 expression (scores 4–8). Of the 138 cases examined, 65 (47.1%) showed high RASSF6 expression and 73 (52.9%) showed low RASSF6 expression (Figure 1B–D). As shown in Table 1, statistical significance was found between RASSF6 downregulation and local invasion status (Ta-T1 vs T2-T4, p=0.0014). The percentage of RASSF6 protein downregulation (65.3%) in muscle invasion bladder cancers was higher than that (38.1%) in non-muscle invasion bladder cancers, suggesting RASSF6 downregulation was an indicator of malignant progression. The associations of RASSF6 downregulation with age, sex and tumor grade were not significant. To validate the IHC results, we also examined RASSF6 protein status in ten bladder cancer tissues and paired adjacent normal tissues using Western blot analyses. The results showed significant RASSF6 downregulation in six of ten of paired cases (Figure 1E).
 |
Table 1 Distribution Of RASSF6 In Bladder Cancer According To Clinicopathological Characteristics |
The Cancer Genome Atlas (TCGA) bladder cancer cohort revealed that the overall survival of patients with low RASSF6 expression was worse than those with high RASSF6 expression (log-rank test, p=0.05, Figure 1F). TCGA dataset also showed that expression of RASSF6 was significantly lower in bladder cancers with metastasis (Mann–Whitney test, p=0.0093, Figure 1G), higher pathological stage (Kruskal–Wallis test, p=0.0032, Figure 1H) and more lymph node metastasis (Kruskal–Wallis test, p=0.0109, Figure 1I). Collectively, these data indicated that RASSF6 was downregulated in human bladder cancers and correlated with malignant features.
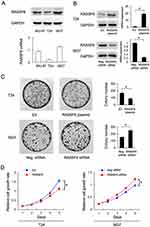
RASSF6 Inhibits Cell Proliferation And Invasion
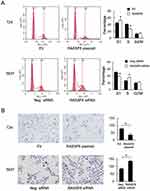
We analyzed RASSF6 protein and mRNA levels in a panel of bladder cancer cell lines (including BIU-87, 5637 and T24). We found relative weak expression of RASSF6 in the T24 cell line compared with the 5637 cell line (Figure 2A). We used T24 cell line for RASSF6 plasmid transfection and 5637 cell line for siRNA transfection. The efficiency of RASSF6 transfection and knockdown were confirmed by PCR and Western blotting, respectively (Figure 2B). Colony formation assay and MTT assays were used to investigate the influence of RASSF6 on proliferation. Colony formation assay showed that the ectopic expression of RASSF6 reduced colony numbers while siRNA treatment enhanced colony numbers (Figure 2C). MTT results showed that RASSF6 transfection inhibited proliferation rate, while RASSF6 siRNA treatment increased proliferation (Figure 2D). In addition, the role of RASSF6 on cell invasion was investigated using the Matrigel invasion assay. RASSF6 transfection increased the invasion ability of bladder cancer cells while siRNA decreased this ability (Figure 3B).
RASSF6 Inhibits Cell Cycle Progression And Enhances Doxorubicin Sensitivity
Cell cycle analysis showed that the S phase percentage was decreased after RASSF6 overexpression. The S phase percentage was increased after RASSF6 knockdown (Figure 3A) indicating that RASSF6 inhibited cell proliferation possibly through cell cycle control.
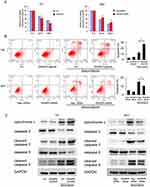
We then investigated the effect of RASSF6 on chemosensitivity. A solution of 1μM doxorubicin (DOX) was used to treat bladder cancer cells and MTT assay was used to determine the inhibition rate of DOX on cell viability. As shown in Figure 4A, RASSF6 overexpression increased the inhibition percentage after treatment with 1μM DOX, while RASSF6 siRNA induced DOX resistance by downregulating the inhibition percentage (Figure 4A). Annexin V/PI was utilized for examination of apoptosis level. RASSF6 transfection significantly increased apoptosis rate in bladder cancer cells after DOX (1μM) treatment for 24 hrs, while siRNA depletion downregulated apoptosis rate (Figure 4B). Together, these results showed that RASSF6 inhibited DOX resistance in bladder cancer.
To further validate the effect of RASSF6 on DOX-induced apoptosis, we examined the expressions of apoptosis proteins. RASSF6 overexpression upregulated the levels of cytochrome c, cleaved caspase-3 and cleaved caspase-9. In contrast, RASSF6 knockdown decreased cytochrome c, cleaved caspase-3 and cleaved caspase-9 in 5637 cell line treated with DOX (Figure 4C). We also examined the change of RASSF6 protein after DOX treatment. As shown in Supplementary Figure 1, DOX treatment slightly upregulated RASSF6 protein in T24 cell line. There was no change of RASSF6 protein in 5637 cell line treated with DOX (data not shown). These data supported the fact that RASSF6 could enhance DOX sensitivity.
RASSF6 Regulates Mitochondrial Membrane Potential
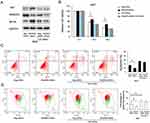
Mitochondrial function is often closely associated with drug resistance. The maintenance of normal mitochondrial membrane potential is crucial for cell survival. We therefore determined whether RASSF6 could regulate mitochondrial membrane potential (Δψm).
In normal cells with high Δψm, JC-1 is known to exhibit intense red fluorescence. However, in cells with low Δψm, JC-1 remains in a monomeric form, and exhibits green fluorescence. As shown in Figure 5, in T24 and 5637 cells treated with DOX, RASSF6 overexpression increased the percentage of green fluorescence, suggesting that RASSF6 downregulated Δψm in DOX-treated cells. RASSF6 siRNA treatment showed the opposite effect in both cell lines.
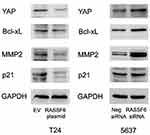
RASSF6 Regulates Hippo Pathway
To identify the potential mechanism, we measured the levels of several related proteins. Our results revealed that RASSF6 downregulated the expression of Bcl-xL, and MMP2 while upregulating p21 (Figure 6). It has been reported that the RASSF family proteins could regulate the activity of Hippo signaling. We therefore examined YAP, an important Hippo effector, to determine whether it was altered in RASSF6 overexpressed and depleted cells. RASSF6 overexpression downregulated the protein level of YAP in T24 cell line, while RASSF6 depletion upregulated YAP expression in 5637 cell lines. These results demonstrated that RASSF6 overexpression downregulated the expression of YAP in bladder cancer cells.
RASSF6 Regulates Apoptosis Through Hippo Signaling
Next, we determined if Hippo signaling was responsible for the change of chemosensitivity. We knocked down endogenous YAP in 5637 cells and tested the effect of RASSF6 depletion. Western blotting confirmed that YAP siRNA efficiently decreased YAP protein levels. As shown in Figure 7A, YAP depletion significantly downregulated Bcl-xL expression. There was amelioration of RASSF6 siRNA-induced Bcl-xL upregulation, implying that the effect of RASSF6 upon Bcl-xL was dependent upon YAP, at least in part (Figure 7A). MTT assay showed that in YAP depleted 5637 cells, the effect of RASSF6 knockdown on viability was significantly reduced (Figure 7B). Annexin V/PI staining showed that YAP siRNA upregulated the level of DOX-induced apoptosis. In YAP depleted cells, the effects of RASSF6 siRNA on apoptosis were not significant (Figure 7C). In addition, YAP siRNA decreased Δψm and abolished the protecting effect of RASSF6 knockdown (Figure 7D). Together, these data suggested that YAP played a central role during the RASSF6-induced increase of chemosensitivity in bladder cancer cells.
Discussion
Identification of novel biomarkers, especially those can control chemosensitivity, is an urgent task for the development of novel therapies for bladder cancer patients. The RASSF family proteins have received much attention due to the tumor suppressing effects. RASSFs are frequently inactivated in various human cancers. There have been several studies reporting decreased RASSF6 expression in childhood leukemia,2 gastric cancer3 and neuroblastoma.4 However, there are no reports regarding its expression pattern and biological roles in bladder cancer. Although some of the RASSF proteins display redundant activities, they seem to perform different tasks inside cells. Thus, it is important to identify the uniqueness of the RASSF6 with bladder carcinogenesis. Here, we showed that RASSF6 was downregulated in bladder cancer tissues, which correlated with the local invasion. In addition, our results were supported by RNA-seq data from TCGA. Collectively, our results emphasized that RASSF6 loss is a potential biomarker for bladder cancer.
Based on the MTT and colony formation assay results we observed that RASSF6 overexpression suppressed cell proliferation, while its depletion showed the opposite effects. Cell cycle analysis demonstrated that RASSF6 overexpression decreased the percentage of S phase cells. The decrease in S phase after RASSF6 overexpression is likely due to the observed increase in G1 (so an G1/S cell cycle arrest), which correlates with the observed upregulation of p21, whereas the opposite seems to be true after RASSF6 knockdown. Because p21 is an inhibitor of cell cycle progression, its loss indicated uncontrolled growth.5,6 It has been reported that RASSF6 promoted p21 dependent cell cycle arrest in renal cell carcinoma.7
These results indicated that RASSF6 inhibited bladder cancer growth by blocking cell cycle progression. In additional, Transwell assay showed that RASSF6 reduced invading cell numbers while its depletion promoted cell invasion. Thus, RASSF6 is an inhibitor of both proliferation and invasion.
The effect of RASSF6 upon chemosensitivity has not yet been investigated in bladder cancer. Our current findings revealed the role of RASSF6 as a positive regulator of chemosensitivity. It has been reported that mitochondria are involved in apoptosis, and chemotherapeutic drugs induce apoptosis through mitochondria-dependent pathways.8–10 The JC-1 staining assay was first performed to examine Δψm to characterize the role of RASSF6 on mitochondrial function. We found that RASSF6 overexpression reduced Δψm while RASSF6 depletion maintained Δψm when cells were treated with DOX. Loss of Δψm resulted in increased membrane permeability, which caused the release of cytochrome c and triggered mitochondria-dependent apoptosis pathway.11 Our finding therefore indicated that RASSF6 facilitated DOX-induced apoptosis through downregulation of Δψm.
To determine the potential molecular mechanism, we screened several proteins and found that RASSF6 negatively regulated Bcl-xL expression. Bcl-xL is a member of the pro-survival Bcl-2 family of proteins, which is more functional than Bcl-2 when induced by chemotherapy drugs such as DOX.12,13 Bcl-xL specifically binds to cytochrome c and prevent apoptosis. It was reported that Bcl-xL could prevent decreases in Δψm and ROS production.14 Thus, RASSF6 may inhibit Δψm through downregulation of Bcl-xL. A recent report showed that Bcl-xL could bind and antagonize RASSF6 function.15 This indicated a positive feedback loop between RASSF6 and Bcl-xL function. Our results provide mechanistic insight into interplay between RASSF6 and anti-apoptotic proteins.
The Hippo signaling pathway plays an important role in maintaining tissue growth balance, mainly through inhibiting cell proliferation and inducing apoptosis. Dysregulation of Hippo signaling during the development of human cancers has been reported. The RASSF family proteins are involved in Hippo regulation. We found that RASSF6 overexpression downregulated YAP protein while it promoted phosphorylation of LATS1/2. YAP is a transcriptional co-activator which is upregulated in a range of human cancers including bladder cancer.16 YAP has been reported to regulate proliferation, invasion, migration and chemoresistance in cancer cells.17,18 The RASSF family protein has been reported as a positive regulator of LATS phosphorylation,19 which could in turn downregulate YAP protein. Furthermore, Bcl-xL has been reported as a YAP target.20 We further validated the correlation by checking the effect of RASSF6 knockdown in YAP-depleted cells. The results confirmed that YAP was required in the regulation of RASSF6 on Bcl-xL and apoptosis. Together, these results showed that RASSF6 regulated DOX resistance through YAP/Bcl-xL in bladder cancer cells.
RASSF family proteins contain 10 members (namely RASSF1 to 10), all of which contain Ras association domain. RASSF1-6 also contains a SARAH (Sav/RASSF/Hpo) domain, which could mediate protein interactions crucial in the growth pathways, including Hippo pathway. RASSF1-6 has been reported as tumor suppressor in various cancers.21 The roles of RASSF7-10 in cancers were still controversial. Thus, these RASSF family proteins seem to perform different tasks inside cancer cells. Some of them display redundant activities. In bladder cancers, only RASSF1A has been reported as a tumor suppressor and tumor biomarker.22–24 The mechanisms of RASSF1A and RASSF6 seem different in bladder carcinogenesis. RASSF1A was reported to upregulate E-cadherin and beta-catenin, which inhibited bladder cancer invasion.22 To date, RASSF6 was the only RASSF family protein which could regulate Hippo signaling in bladder cancer cells. Further research is needed to characterize the roles of other RASSF members in bladder cancer.
Collectively, our current data revealed that RASSF6 was downregulated in bladder cancers. RASSF6 inhibited proliferation and invasion, and facilitated DOX-induced apoptosis, potentially via downregulation of YAP/Bcl-xL signaling.
Acknowledgement
The study was supported by the Natural Science Foundation of Liaoning Province of China (grant no. 20170540988) and Shenyang Science and Technology Program (grant no. 17-231-1-57).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Hesson LB, Dunwell TL, Cooper WN, et al. The novel RASSF6 and RASSF10 candidate tumour suppressor genes are frequently epigenetically inactivated in childhood leukaemias. Mol Cancer. 2009;8:42. doi:10.1186/1476-4598-8-42
3. Wen Y, Wang Q, Zhou C, et al. Decreased expression of RASSF6 is a novel independent prognostic marker of a worse outcome in gastric cancer patients after curative surgery. Ann Surg Oncol. 2011;18(13):3858–3867. doi:10.1245/s10434-011-1668-5
4. Djos A, Martinsson T, Kogner P, Caren H. The RASSF gene family members RASSF5, RASSF6 and RASSF7 show frequent DNA methylation in neuroblastoma. Mol Cancer. 2012;11:40. doi:10.1186/1476-4598-11-40
5. Skirnisdottir I, Seidal T. Association of p21, p21 p27 and p21 p53 status to histological subtypes and prognosis in low-stage epithelial ovarian cancer. Cancer Genomics Proteomics. 2013;10(1):27–34.
6. Lee JH, Lee SY, Lee JH, Lee SH. p21 WAF1 is involved in interferon-beta-induced attenuation of telomerase activity and human telomerase reverse transcriptase (hTERT) expression in ovarian cancer. Mol Cells. 2010;30(4):327–333. doi:10.1007/s10059-010-0131-y
7. Liang YY, Zheng LS, Wu YZ, et al. RASSF6 promotes p21(Cip1/Waf1)-dependent cell cycle arrest and apoptosis through activation of the JNK/SAPK pathway in clear cell renal cell carcinoma. Cell Cycle. 2014;13(9):1440–1449. doi:10.4161/cc.28416
8. Buondonno I, Gazzano E, Jean SR, et al. Mitochondria-targeted doxorubicin: a new therapeutic strategy against doxorubicin-resistant osteosarcoma. Mol Cancer Ther. 2016;15(11):2640–2652. doi:10.1158/1535-7163.MCT-16-0048
9. Hajrezaie M, Paydar M, Looi CY, et al. Apoptotic effect of novel Schiff based CdCl(2)(C(1)(4)H(2)(1)N(3)O(2)) complex is mediated via activation of the mitochondrial pathway in colon cancer cells. Sci Rep. 2015;5:9097. doi:10.1038/srep09097
10. Shi M, Zhang J, Li X, et al. Mitochondria-targeted delivery of doxorubicin to enhance antitumor activity with HER-2 peptide-mediated multifunctional pH-sensitive DQAsomes. Int J Nanomedicine. 2018;13:4209–4226. doi:10.2147/IJN.S163858
11. Yang M, Wang B, Gao J, Zhang Y, Xu W, Tao L. Spinosad induces programmed cell death involves mitochondrial dysfunction and cytochrome C release in Spodoptera frugiperda Sf9 cells. Chemosphere. 2017;169:155–161. doi:10.1016/j.chemosphere.2016.11.065
12. Fiebig AA, Zhu W, Hollerbach C, Leber B, Andrews DW. Bcl-XL is qualitatively different from and ten times more effective than Bcl-2 when expressed in a breast cancer cell line. BMC Cancer. 2006;6:213. doi:10.1186/1471-2407-6-213
13. Bertini I, Chevance S, Del Conte R, Lalli D, Turano P. The anti-apoptotic Bcl-x(L) protein, a new piece in the puzzle of cytochrome c interactome. PLoS One. 2011;6(4):e18329. doi:10.1371/journal.pone.0018329
14. Saez-Atienzar S, Bonet-Ponce L, Da Casa C, et al. Bcl-xL-mediated antioxidant function abrogates the disruption of mitochondrial dynamics induced by LRRK2 inhibition. Biochim Biophys Acta. 2016;1862(1):20–31. doi:10.1016/j.bbadis.2015.09.021
15. Xu X, Iwasa H, Hossain S, et al. BCL-XL binds and antagonizes RASSF6 tumor suppressor to suppress p53 expression. Genes Cells. 2017;22(12):993–1003. doi:10.1111/gtc.12541
16. Dong L, Lin F, Wu W, Liu Y, Huang W. Verteporfin inhibits YAP-induced bladder cancer cell growth and invasion via Hippo signaling pathway. Int J Med Sci. 2018;15(6):645–652. doi:10.7150/ijms.23460
17. Corvaisier M, Bauzone M, Corfiotti F, et al. Regulation of cellular quiescence by YAP/TAZ and Cyclin E1 in colon cancer cells: implication in chemoresistance and cancer relapse. Oncotarget. 2016;7(35):56699–56712. doi:10.18632/oncotarget.11057
18. Dai XY, Zhuang LH, Wang DD, et al. Nuclear translocation and activation of YAP by hypoxia contributes to the chemoresistance of SN38 in hepatocellular carcinoma cells. Oncotarget. 2016;7(6):6933–6947. doi:10.18632/oncotarget.6903
19. Richter AM, Pfeifer GP, Dammann RH. The RASSF proteins in cancer; from epigenetic silencing to functional characterization. Biochim Biophys Acta. 2009;1796(2):114–128. doi:10.1016/j.bbcan.2009.03.004
20. Rosenbluh J, Nijhawan D, Cox AG, et al. Beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151(7):1457–1473. doi:10.1016/j.cell.2012.11.026
21. van der Weyden L, Adams DJ. The Ras-association domain family (RASSF) members and their role in human tumourigenesis. Biochim Biophys Acta. 2007;1776(1):58–85. doi:10.1016/j.bbcan.2007.06.003
22. Bao Y, Liu X, Liu Y, Wang S, Wu B. Ras-association domain family 1 (RASSF1A) gene regulates progression, migration and invasion of bladder cancer. Surg Oncol. 2019;30:63–71. doi:10.1016/j.suronc.2019.05.009
23. Gao T, Wang S, He B, et al. The association of RAS association domain family Protein1A (RASSF1A) methylation states and bladder cancer risk: a systematic review and meta-analysis. PLoS One. 2012;7(11):e48300. doi:10.1371/journal.pone.0048300
24. Meng W, Huebner A, Shabsigh A, Chakravarti A, Lautenschlaeger T. Combined RASSF1A and RASSF2A promoter methylation analysis as diagnostic biomarker for bladder cancer. Mol Biol Int. 2012;2012:701814. doi:10.1155/2012/701814
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.