Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 13
Radiographic and Esthetic Evaluation Following Immediate Implant Placement with or without Socket Shield and Delayed Implant Placement Following Socket Preservation in the Maxillary Esthetic Region – A Randomized Controlled Clinical Trial
Authors Santhanakrishnan M , Subramanian V , Ramesh N , Kamaleeshwari R
Received 24 August 2021
Accepted for publication 4 November 2021
Published 19 November 2021 Volume 2021:13 Pages 479—494
DOI https://doi.org/10.2147/CCIDE.S332687
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Christopher E. Okunseri
Muthukumar Santhanakrishnan,1 Vedavalli Subramanian,2 Nithyakalyani Ramesh,2 R Kamaleeshwari2
1Faculty of Dental Sciences, Department of Periodontology, Sri Ramachandra Institute of Higher Education and Research, Chennai, Tamil Nadu, India; 2Department of Periodontology, Sri Ramachandra Institute of Higher Education and Research, Chennai, Tamil Nadu, India
Correspondence: Muthukumar Santhanakrishnan Tel +9884118681
Email [email protected]
Objective: The purpose of this study was assessment of the changes in soft and hard tissues in the esthetic zone of maxilla following immediate implant placement (IIP) with and without the socket shield technique (SST) and placement of implants 4 months following socket preservation (DIP) in terms of alterations in crestal bone thickness (CBT) and soft tissue changes evaluated by means of pink esthetic scores (PES) following placement of implants in the esthetic zone of maxilla.
Materials and Methods: In the maxillary esthetic region, 75 dental implants were placed totally, with 25 implants each in the SST, IIP, and DIP groups. All participants were subjected to undergo CBCT for assessing the variations in thickness of crestal aspect of facial/buccal/labial alveolar bone (CBT). PES and PROMS (patient-related outcome measures) were assessed using VAS for pain threshold and esthetic satisfaction following implant placement and after 6th post-operative month.
Results: The mean reduction in CBT showed a statistically significant difference between and within the groups, in comparison to IIP and DIP groups, which demonstrated an average reduction in CBT 0.4 ± 0.1 and 0.2 ± 0.1 at 6 months following implant placement, respectively. The SST group showed a significantly lesser reduction in CBT of 0.05 ± 0.02. However, the mean difference in PES within and among the groups showed no significant difference statistically at P < 0.05. On comparison of individual scores of PES between the groups, the results showed significant difference statistically at P < 0.001.
Conclusion: The SST group demonstrated minimal reduction in CBT and a superior PES at the end of 6 months compared with the IIP and DIP groups.
Keywords: socket shield technique, immediate implant placement, socket preservation, randomized controlled trial, pink esthetic score
Introduction
Recently, there has been a growing demand for implants placed immediately following extraction (IIP) in the maxillary esthetic region since, apart from esthetic reasons, patients experience a sense of social well-being when the extracted tooth is immediately replaced.
Although the ideal protocol would be to wait for complete bone healing to occur to reduce the risk of failures following implant placement. Resorption following extraction has been shown to be inevitable, with an average bone loss of 50% occurring within 1 year of extraction in the maxillary anterior region with a 3.5 mm reduction facio-lingually and 1.24 mm apico-coronally.1 The changes in dimensions also affect esthetics. Hence, there is a need to maintain a three-dimensional socket volume immediately following extraction.2 Currently, techniques based on the use of biomaterials have been widely used, such as socket grafting following implant placement (delayed implant placement protocol – DIP) and IIP with socket grafting. Socket grafting followed by implant placement (DIP) has been advocated to reduce the magnitude of bone contraction. However, it takes a minimum of 3–6 months for complete bone maturation before implant placement following socket preservation.3
The various forms of biomaterials used for socket grafting include allografts, xenografts, alloplasts, and platelet concentrates for socket preservation. There is currently no documented evidence on the ideal material to be used for alveolar ridge preservation.4 Recent literature has shown enhanced regeneration while combining matrix scaffold material (DBBM) and biologic modifiers (platelet concentrates).5–7
The newer biologic modifiers, in the form of platelet concentrates—advanced platelet-rich fibrin (A-PRF)—prepared using slow speed and timing, help in promoting regeneration through sustained growth factor release, apart from providing a rich source of leukocytes, which help in recruitment of undifferentiated mesenchymal cells during socket healing process, which would be detrimental in bone regeneration.8
Moreover, in the esthetic region, particularly in maxillary anterior teeth and premolars, a delayed protocol and socket preservation are not acceptable to the patient, since they involve a minimum duration of 3–6 months.4
The second materialistic approach is IIP with a socket-grafting technique. This, however, has been associated with severe soft tissue loss and compromised esthetics. Immediate implant placement (IIP) is indicated ideally only when the facial bone wall is intact and >1 mm thick, although, clinically, the facial bone wall thickness has been <0.5 mm and <1 mm in 50% and 90% of cases, respectively, in the anterior maxilla.9 The evidence regarding the amount of alveolar bone remodeling following immediate implant placement is limited.10
However, both of these approaches demonstrate considerable soft-tissue changes. In this scenario, the biologic approach based on retaining the labial part of the root was introduced by Hurzeler.11 This helps in preventing the resorption of bundle bone by maintaining the marginal periodontium in the facial aspect of the implant. Since such loss can result in bone resorption leading to loss of soft tissue in the peri implant region, thereby resulting in compromised esthetics.12 They can provide better outcome with good function and improved esthetics in particular situations but not on a regular basis. Moreover, they demonstrated a better vascularity as the flapless approach helps in maintaining the blood supply via the periosteal vasculature integrated within the buccal plate of the ridge.11 It would be informative to know if optimal results could be achieved by placement of implants immediately following extraction with or without a socket shield similar to delayed implant placement following bone healing, which could reduce the treatment time by several months. Based on a systematic review13 and randomized controlled trials,2,11 there are no prospective RCTs comparing these three protocols in terms of hard and soft tissue alterations. Hence, the current study was carried out for comparative analysis of hard and soft tissue changes following these three protocols (SST, IIP, and DIP).
Materials and Methods
Study Design
This study was designed as a prospective, randomized clinical trial and conducted following the CONSORT guidelines (http://www.consortstatement.org/). All materials and procedures were approved by the institutional ethics committee and review board, Sri Ramachandra Institute of Higher Education and Research (REF: IEC/19/APR/150/20), and followed Good Clinical Practices. Registration of the trial was done at CTRI, http://www.clinicaltrials.gov/ (REF: CTRI/2019/06/019723).
Sample Size
The sample size was calculated using the G-power software, V.3.1, based on the summary statistics for facial bone thickness derived from a previous article, which resulted in 19 individuals for each group with a power of 95% and significance level of alpha set at 5%.14 The effect size was 1.1, and this value was used for sample size determination based on a two-independent-sample Mann–Whitney test (two-tailed).
Population
Individuals were selected for the trial from patients reporting to the Outpatient Department of Faculty of Dental Sciences, Sri Ramachandra Institute of Higher Education and Research, Chennai. A written informed consent was obtained from all the patients who participated in the study according to the Helsinki declaration for experimentation on human subjects, as revised in 2008.
Inclusion Criteria
Patients aged between 18 and 50 years were included if they had single missing anterior maxillary teeth and premolars with healthy teeth on either side. Patients with Type I extraction sockets (intact facial bone), facial bone thickness of ≤2 mm and without any soft tissue defects.
Exclusion Criteria
Individuals with systemic diseases, patients on medications affecting periodontal healing or anticoagulant therapy were excluded. Patients demonstrating pathological lesions and loss of facial bone plate were excluded from the study. Patients who underwent radiation therapy within 2 years preceding the study or patients currently undergoing radiation therapy were excluded. Patients with a history of drug allergy or allergy to anesthetic agents, psychiatric illness, un-co-operative patients, were also excluded from the study.
Pre-Operative Evaluation
Pre-operative evaluation of oral hygiene was evaluated by means of the Oral Hygiene Index–Simplified,15 evaluation of tissue biotype - thick or thin,16 probing pocket depth and pink esthetic score (PES)17 for evaluation of soft tissue esthetics.
CBCT Standardization
Labial cortical thickness was evaluated by means of the Ray Scan Alpha Plus (LED Medical Diagnostics Inc.) cone beam 3D imaging system, with tube voltage of 90 KVp, 10 Ma, standard exposure time of 14 sec, high resolution of 70 µm voxels, and FOV (Field of View) collimated to 5 cm by 5 cm to limit exposure to radiation. Data acquisition was done as volume in multiple planes.
Pre-operative and post-operative measurements were done using a standard reference point (r) acquired by a line drawn parallel to the inner aspect of the facial alveolar plate (r2) to intersect the floor of the maxillary sinus/nasal floor (r1).18
The sagittal views were plotted to measure the changes in dimensions of bone as follows:
The thickness of the facial cortical plate was measured 1 mm from the coronal most aspect of the facial bone crest, evaluated in cross-sections using 1 mm sections with the distance measurement tool in the pre-operative labio-palatal direction (m1) (Figure 1A and B).
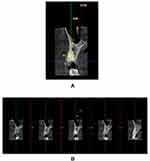 |
Figure 1 Original. Demonstration of a treated case, Radiographic measurements. (A) Pre-operative CBCT - sagittal view. (B) Thickness of labial plate assessed in section of 1 mm. |
Esthetic Evaluation
The secondary outcome variables included PES evaluation, following placement of crown and 6 months following occlusal loading. Soft tissue evaluation was conducted independently by two evaluators with the PES scoring system.14 The PES system is based on seven variables: mesial papilla, distal papilla, soft-tissue level, soft-tissue contour, alveolar process deficiency, soft-tissue color, and texture. The same weightage was given to each of the seven variables by the evaluators with a maximum possible score of 14. (Score 2 indicating the best status and 0 indicating the worst state).
Patient-Related Outcome Measures (PROMS)
Esthetics
Each patient was asked to rate his/her overall satisfaction on a scale of 0–10 on a visual analogue scale (VAS) (least satisfied to highly satisfied).19
Pain
The patients filled out a questionnaire form 1–6 h postoperatively and by the end of every day after surgery for 7 days and at the end of 6 months. This questionnaire used a VAS, labeled from 1 to 10 (absence of pain to severe pain). The patients handed in the questionnaire at the time of the first postoperative control, 7–10 days after surgery, and 6 months after occlusal loading of the implants.20
Randomization Process and Allocation Concealment
A computer‐generated list was used for randomization by a person who was not a part of the study following extraction of tooth and assessment of facial bone plate. The allocation concealment was done with the help of continuously numbered sealed envelopes (Figure 2). Immediately following tooth extraction, the surgeon disclosed the treatment assignment. Clinical and radiographic measurements were recorded by different persons not involved in the other aspects of the study.
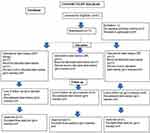 |
Figure 2 Original. CONSORT flow chart (n) representing the implant sites. |
Treatment Procedures
Immediate Implant with Socket Shield Group (SST)
Partial extraction was done by maintaining the labial segment of the root intact (socket shield). The shield was prepared according to Hurzeler’s criteria, and implants were placed immediately.12
Immediate Implant Placement Group (IIP)
Immediate implant placement included a mixture of xenograft (DBBM; Bio-Oss, Geistlich) and autologous bone particles (obtained during osteotomy) at a 1:1 ratio.
Delayed Implant Placement Group (DIP)
The socket was grafted with a mixture of xenograft (DBBM; Bio-Oss, Geistlich) and advanced platelet-rich fibrin (A-PRF) in a 1:1 ratio up to the facial bone crest, covered using a partially epithelialized connective tissue graft obtained from the hard palate. Implants were placed after 4 months of socket preservation.
Surgical Procedures
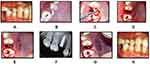
All patients were subjected to CBCT (Planmeca Promax 3D, Planmeca, Finland) analysis for assessment of facial bone thickness and bone height were analyzed for selecting appropriate size of implants. The DIO implant system (Busan, Korea) with diameters of 3.3 and 3.8 mm and lengths of 13 and 15 mm was used in the study. All patients were instructed to strictly adhere to optimum plaque control measures 1 week before surgery. Following extra-oral disinfection, the patients were instructed to rinse their mouths using Chlorhexidine HCL 1.25% mouthwash. Local infiltration anesthesia [xylocaine 2%, 1:100,000 epinephrine] was used for all procedures. Among the SST group cases, tooth de-coronation was done 1 mm above the gingival level, by means of a diamond bur and a high-speed handpiece under copious irrigation. A long shank bur was then used for sectioning the root longitudinally and mesiodistally, midway through the root with the canal as the reference, resulting in separation of the facial and oral halves from each other, from the coronal to the apical aspect (Figure 3A–C). A fine periotome was used to sever the periodontal ligament between the oral alveolar plate and root section after which the separated oral section was carefully extracted without affecting the patient’s facial segment. The facial segment was reduced coronally to the level of the alveolar crest, by a series of long shanked burs (Socket Shield kit, Darco Fonseca, Portugal) used to reduce the facial segment coronally to the level of alveolar crest after which careful thinning was done mesio-distally and apico-coronally resulting in a concave contour of the shield.
The remaining tissue debris was removed by carefully curetting the socket after which a gentle probing was done to rule out the mobility of the shield. Osteotomy drills were used at 800–1000 RPM and 40 N cm to create an osteotomy oral to the shield with a gap of roughly 2 mm (Figure 3C–E). The provisional crown was fabricated chairside in such a way that emergence profile supported the soft tissues (Figure 3F and G).
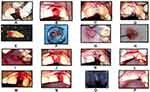
In the IIP group, extraction was done atraumatically by using periotomes so as to preserve the facial alveolar bone, after which debridement of the socket was done by using curettes and irrigating with normal saline solution (Figure 4A–D). Osteotomy was prepared to leave a space of 2 mm between the implant and facial bone plate (Figure 4E). The implant was placed subcrestally by 2–3mm (Figure 4E–G). The gap between the shield and the implant and the alveolar socket wall and the implant was grafted with a mixture of Xenograft (DBBM; Bio-Oss, Geistlich) and autogenous bone particles (obtained during osteotomy) at a 1:1 ratio to allow for soft tissue fill in the groups.
The torque registered on the drilling console was used for assessing the initial implant stability in both groups, with a 2-mm gap between the surgical site and a provisional restoration, maintained for soft tissue to fill in the groups. In both groups, all provisional crowns were kept out of occlusion with 1 mm clearance and patients were instructed to avoid occlusal overloads. An oral antibiotic (Amoxicillin 500 mg), three times a day for 5 days and analgesic (Ibuprofen 400 mg) thrice daily for 5 days were given as post-operative medications. The patients strictly adhered to optimal oral hygiene practices with regular use of Chlorhexidine mouthwash 0.2% for 2 weeks. Patients were reviewed on a day-to-day basis during the first post-operative week, followed up at 10-day intervals for the first month and 6 months postoperatively.
In the immediate group, the stability of implants was assessed 3 months following implant placement. Implant-level impressions were taken using transfer coping and individualized trays. PFM crown (Porcelain fused metal) was fabricated and cemented onto customized abutments within 14 days after impressions were taken (Figure 4H).
In the DIP group, atraumatic extraction was performed with the help of periotomes to preserve the facial alveolar bone, after which debridement of the socket was done gently by using curettes and irrigating with normal saline (Figure 5A and B). Indexed stents were used to record the morphology of the extracted sockets by direct measurements with UNC periodontal probe (Figure 5C and D). A trained phlebotomist collected 10 mL of venous blood in a sterile glass tube from the antecubital vein in the forearm of the participants and centrifuged it at 1300 rpm (200 × g) for 14 min to procure advanced platelet-rich fibrin (APRF).21
DBBM was mixed with APRF clot, which was cut into small pieces in a 1:1 ratio and filled into the socket with gentle compression up to the level of the bony crest. The socket was closed with a partially epithelialized connective tissue graft procured from the hard palate with a help of a guiding suture. The patients were followed up at regular intervals, and they were instructed to adhere to strict oral hygiene measures throughout the study period (Figure 5E–K). At 4 months following the procedure, implants were placed (Figure 5L).
Implants were placed in the DIP group similar to the procedures displayed previously for the IIP group. Local anesthesia was administered following which flap elevation was done and the implants sites were prepared without debriding the preserved sockets, following which implant placement was done and immediately loaded as previously described (Figure 5L–P). Provisionalisation was done within 24 h with acrylic crowns, which were kept out of contact with opposing teeth. Patients in the DIP group were assessed for stability of implants 3 months following implant placement. Following which permanent restorations were placed similar to other groups.
Post-Operative Analysis by CBCT
All patients were subjected to CBCT scans, 6 months following placement of implants for assessment of horizontal bone loss (reduction in crestal bone thickness), which was the primary outcome of this clinical trial. Thickness of the facial cortical plate was studied using sections of 1 mm in cross-sectional view with thickness measured postoperatively (m2) in the labio-palatal direction with the help of a distance measurement tool (Figure 6). The difference between baseline and 6 months following implant placement was calibrated as reduction in facial plate thickness (m1-m2).
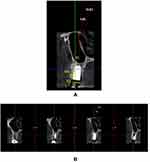 |
Figure 6 Original. Post-operative CBCT of a treated case. (A) Sagittal view. (B) Labial cortical thickness assessed in sections of 1-mm thickness. |
Esthetic Evaluation
The secondary outcome variables included PES evaluation recorded immediately, postoperatively, and 6 months following restoration of implant according to PES.19
Patient-Related Outcome Measures (PROMS)
Pain
Patients were asked to complete a visual analogue scale (VAS), with markings ranging from 1 to 10 as described before, 1–6 h postoperatively and every day following surgery for 7 days. All patients handed in the questionnaire at the time of the first postoperative control, 7–10 days after surgery, and 6 months following occlusal loading.20
Esthetics
Additionally, each patient was asked to rate his/her esthetic satisfaction following implant restoration based on a VAS labelled from 0 to 10, as described earlier.19
Statistical Analysis
A SPSS software package (version 20.0; SPSS Inc.) was used for statistical analysis. Kolmogorov–Smirnov test and the Shapiro–Wilk test was used to check the normality of the numerical data. Changes in CBT showed a parametric distribution and PES showed a non-parametric distribution, respectively.
Mean ± SD was used to express parametric data, and median (IQR) was used to express non-parametric data, respectively. One-way ANOVA was used for the analysis of parametric data for comparison within and between the groups. Within group differences were analyzed using repeated-measures ANOVA.
The non-parametric data for comparison between and within groups were done by Mann–Whitney “U” test and the Wilcoxon signed-rank test, respectively. The inter-rater variability was assessed using the intraclass correlation coefficient test. For analysis of the visual analogue scale for esthetics and pain, Fischer’s exact test was used.
Results
General Information
The clinical trial was conducted between May 2019 and July 2019 in which 75 subjects participated, 25 of whom were allocated to each of the three groups, the SST, IIP, and DIP groups. No significant difference was found statistically at baseline for the parameters age, gender, and CBT, within and among the groups. However, for PES, there was a statistically significant difference present between and within the groups (Table 1).
 |
Table 1 Baseline Characteristics of the Study Participants (n = 75) |
A single implant was placed in every patient in esthetic zone contributing to one site for the SST, IIP and DIP groups. No complications were recorded during the course of the study.
Clinical Outcomes
Radiographic Outcomes
Radiographic analysis was performed 1 mm apically on the coronal most bone crest to evaluate alterations in the buccal cortical thickness.
The alterations in the CBT were assessed from baseline and at 6 months after placement. Nevertheless, patients in the SST group demonstrated significantly greater values of CBT at 6 months in comparison with those in the IIP and DIP groups (P<0.001) (Table 2, Figure 7).
 |
Table 2 Comparison of Labial Bone Thicknesses in the Studied Groups at Different Time Intervals (n = 75) |
The PES assessment showed no significant differences statistically within and between the groups at 6 months from baseline following placement of implants. The patients in the SST, IIP and DIP groups showed preoperative PES values of 12.1±1.6, 12.2±1.9, and 10.9±1.5, respectively, which were not statistically significant (P=0.07). The SST group demonstrated slightly greater PES scores compared to IIP and DIP groups at 6 months, with no significant difference statistically (11.7±1.8 vs 11.2 ± 2.1 in the IIP group and 10.2±1.4 in the DIP group). However, when the patients were grouped into three categories based on individual values of PES (score<10, score>11<12, and scores>13 and <14) and were analyzed for changes within and between the groups, there was a significant difference statistically between and within the groups, with a greater number of patients with PES values of (scores >13 and <14) in the SST group, which was highly significant statistically at P<0.001. The patients in the IIP and DIP groups demonstrated lower PES values (scores >13 and <14) when compared to SST group, 6 months post-operatively, which showed a statistical significance (P<0.001), denoting a better outcome esthetically in the SST group (Table 3, Figure 8).
 |
Table 3 Changes in PES Scores of the Study Participants (n = 75) |
Inter-evaluator consistency was tested by means of the intraclass correlation coefficient test, which showed excellent agreement between the evaluators (Table 4).
 |
Table 4 Test and Re-Test Reliability Scores of PES of the Study Participants (n = 75) |
Patient-Centered Outcome
VAS scores for esthetics and pain between and within the groups showed no statistically significant difference. However, patients in the SST group showed better trend with regard to esthetic satisfaction (84% of scores 9 and 10) when compared to IIP group (64%) and the DIP group (52%), respectively (Tables 5 and 6).
 |
Table 5 VAS Scores for Esthetics Among Group I, Group II, and Group III of the Study Participants (n = 75) |
 |
Table 6 VAS Scores for Pain Among Group I, Group II, and Group III of the Study Participants (n = 75) |
Discussion
The timing of implant placement in the maxillary esthetic zone poses a great challenge for clinicians, since, apart from functional rehabilitation, esthetics is an integral component in determining the success of the procedure. The IIP protocol has been widely followed due to its ability to limit bone resorption and reduce treatment time. However, it is usually associated with compromised esthetic outcomes caused by buccal soft tissue recession.22 Thus, it would be ideal to wait for bone to heal completely before implant placement, which could take 6 months or more (DIP). ARP techniques have been shown to modify bone modelling events and partially prevent bone resorption. However, this procedure also takes a minimum of 3 to 6 months for bone to mature completely before placement of an implant following socket preservation, apart from requiring a second surgical intervention, which is not attractive to patients.3
Immediate placement reduces treatment time and limits bone resorption compared with conventional implant placement, but frequently results in compromised esthetic outcomes,23–25 which could be attributed to remodeling of the alveolar ridge after extraction of the tooth,26 since this is increased in this region as the bone crest is primarily composed of vulnerable bundle bone. IIP following tooth extraction has been associated with significant hard tissue changes which is related to labial cortical plate thickness, which is about 0.8 mm in this region, thereby leading to soft tissue changes and resorption.27–30
The socket shield technique (SST) where the buccal root fragment is intentionally retained at the time of extraction prevents the buccal wall resorption by preserving the vascularity and PDL resulting in fewer changes in the soft tissues and alveolar bone structure thereby facilitating immediate placement of implants.12
Currently, only very few randomized clinical trials2,11 have compared these implant placement protocols; moreover, to the best of our knowledge, no studies have been done prospectively to compare immediate implant placement (with and without a socket shield) and delayed implant placement following socket preservation for assessing hard and soft tissue changes.
Hence, the present study was conducted to compare these three protocols to evaluate the changes in bone (CBT) and soft tissues evaluated using PES in the maxillary esthetic zone involving single dental implants. The study included sites involving maxillary anterior teeth and premolars, since most patients exhibit premolars during their “dynamic smile”,31 and would demonstrate no significant difference in the resorption of buccal bone plate and bone healing in anterior teeth compared with premolars, since the average width of the buccal bony wall was 1 mm for anterior teeth and 1.1 mm in the premolars.32 The anterior maxilla is made up of predominantly D2 and D3 bones. Hence, there would be no difference in bone healing between different sites in the anterior maxilla.33 Moreover, flapless tooth extraction involving single extraction sites, with healthy neighboring teeth demonstrated changes, which occur mainly in the facial aspect compared to the proximal aspects as the vascularity is maintained intact in the proximal areas due to retention of healthy periodontal ligament in the adjacent teeth.34
The alterations in Crestal Bone Thickness (CBT) were considered as the primary outcome variable as it is detrimental in maintaining the integrity of soft and hard tissues around the implant. The evaluation of CBT was done at 6 months following implant placement, as most of the changes occur during this time point following extraction of the tooth.34 There was a highly significant difference statistically in changes in CBT among and within the groups, with the SST group demonstrating minimal changes (0.05±0.02) compared with the IIP group (0.2± 0.02) and the DIP group (0.4± 0.1). The minimal changes in the SST group could be attributed to the presence of a criss-cross arrangement of the periodontal ligament, resulting in better root socket preservation, thereby preventing buccal wall collapse by preserving the vascularity and periodontal ligament.
Although a direct comparison with other studies comparing these three protocols was not possible, the results of the present study can be compared with studies comparing SST and IIP groups and IIP and DIP groups.
The results of our study for the SST group concurred with those of other studies that demonstrated changes in CBT of 0.09 mm,35 0.17 mm,14 0.22 mm,36 and 0.26 mm,37 respectively.
The results of our study differed from those of studies that demonstrated changes of 0.06 mm,11 0.6 mm,2 0.8 mm,38 and 1 mm39 in the CBT, which could be attributed to different surgical protocols, the inclusion of mandibular teeth, and the use of digital intraoral periapical radiographs in those studies.
The IIP group showed minimal changes in CBT (0.2 ± 0.02) compared with the DIP group (0.4± 0.1). This difference could be due to the fact that a composite graft (combination of autogenous bone and DBBM) was used for grafting the “jumping” distance in the test group, which could have reduced the number of remodeling changes in the IIP group and the delay of 4 months in the DIP group following socket preservation that could have attributed to greater changes in CBT in the DIP group.
The results of the present study for the IIP group concurred with those of a study40 that showed a difference of 0.99±0.21 mm in buccal bone changes following IIP when compared with the DIP procedure, and with those of other studies that demonstrated reductions of 0.1 mm,11 1.1 mm,41 and 1.1 mm2 in CBT, respectively.
The results of the present study for the DIP group concurred with those of other studies that reported differences in CBT of 0.24 mm,42 0.27 mm,43 0.46 mm,44 and 0.66 mm,45 respectively.
Alterations in soft tissue assessed by PES17 between the groups were considered as a secondary outcome variable in the present study, since esthetic assessment is integral in evaluating the successful implant therapy in the maxillary esthetic zone. However, there was a significant difference when the patients were grouped into three categories based on individual values of PES (score<10, score>11<12, and scores>13 and <14) which could be attributed to the re-modelling changes that could have occurred by 4 months following socket preservation prior to implant placement in the DIP group.42 However, 6 months post-operatively there was no statistically significant difference among the groups with regard to average PES but there was a significant difference when the numbers of patients with individual PES values were grouped into three categories (score<10, score>11<12, and scores>13 and <14).
The SST group demonstrated a highly significant difference (P<0.01) with greater PES values (scores>13 and 14) compared to IIP and DIP groups. The trend showed that 48% of patients in the SST group demonstrated PES (>13<14) whereas the IIP group and DIP group demonstrated 40% and 4%, respectively. The results could be attributed to greater reductions in crestal bone thickness in the IIP group and DIP groups compared with that in the SST group, resulting in reduced labial contour and soft-tissue changes in those groups, and IIP demonstrated better PES than the DIP groups. The reason for the better PES in the IIP and SST groups compared with the DIP groups could be due to the provisionalisation that was done within 24 h in the IIP and SST groups, since it provided a barrier for grafting material as well as facilitating the formation of junctional epithelium46,47 and helped in the maintenance of proximal contacts, which preserved the papillary height and positions.48
Further, the results of PES for the SST group with a median IQR value of 12 (2.0) concurred with the PES scores from other RCTs,2,37 where the authors reported an average value of 12 (11–14) and 12.15 ± 0.76, after 6 months, respectively, and with that of another observational study,38 which reported a mean PES of 12. The results of this study differed from those of another study36 that reported a PES score of 13.5, which could be due to the smaller sample size in that study.
The IIP group demonstrated better PES values compared with the DIP group. Based on individual PES values between the groups, a highly significant difference statistically was observed in the IIP group, with a higher number of patients (40%) demonstrating PES values (scores>13 and 14) in comparison with the DIP group, which demonstrated significantly lower values (4%) of PES scores (scores>13 and <14) in the IIP group when compared to DIP group that could be due to gingival recession, which occurs in the delayed group following socket preservation facially44 and is attributed to remodeling changes that occur after 3–6 months following extraction in the DIP group before implant placement.3
The IIP group demonstrated PES value of 11.2±2.1, 6 months following occlusal loading, which was comparable with the results of other studies that showed PES scores of 10.3 mm,2 12.42 mm,42 and 12.38 mm,43 respectively.
In results of our study, there was a better PES in the IIP than in the DIP group, in contrast to another study49 where the authors used mPES for esthetic evaluation with excellent and acceptable results in the DIP group with regard to mPES, compared with the early implant placement protocol. However, they demonstrated fewer acceptable cases of delayed implant placement compared to immediate implant placement, due to facio-lingual and apico-coronal bone loss that occurred during the healing process in the delayed protocol.
At present, Patient-Reported Outcomes (PROs) are considered a basic measure for evaluation of therapeutic success and are widely used in clinical trials as a primary outcome measure.50 The current literature, however, demonstrates only a limited number of studies evaluating PROMS in the maxillary esthetic zone apart from objective evaluations of implant restoration. Hence, VAS was used for evaluation of pain and esthetic satisfaction of the patients.
Although there was no statistically significant difference for pain and esthetic satisfaction within and between the groups, the trend showed clearly a better esthetic satisfaction in the SST group (84%) of the patients having scores of 9 and 10 compared to IIP group and DIP groups, which showed 64% and 52%, respectively, which could be due to lesser reductions in soft-tissue recession and buccal plate loss facilitated by the maintenance of periodontal ligament, resulting in the maintenance of keratinized tissue width facilitated by preservation of inserting dentogingival fibers.34,37
Conclusions
The SST group demonstrated minimal reduction in CBT and a superior PES at the end of 6 months compared to IIP and DIP groups. However, the present study had the following limitations: a reduced follow-up period of 6 months and the use of CBCT for evaluation of thin bone plates (<1 mm). However, to arrive at a definitive indication for the timing of implant placement in the esthetic maxillary region, future multi-centric studies with an extended follow-up period and evaluation of PROMS with more complete and validated questionnaires such as the OHIP–14 should be conducted.
Clinical Significance
The results of the present study clearly demonstrated the superiority of the socket shield technique compared to IIP and DIP as evidenced by reduced dimensional alterations in the hard and soft tissues in the SST group. This is clinically relevant information that could be employed in clinical scenarios where implants are to be placed in the esthetic maxillary zone.
Consent for Publication
We hereby transfer, assign, or otherwise convey all copyright ownership, including any and all rights incidental thereto, exclusively to this journal, in the event that such work is published by the journal.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hämmerle CH, Araújo MG, Simion M; Osteology Consensus Group 2011. Evidence-based knowledge on the biology and treatment of extraction sockets. Clin Oral Implants Res. 2012;23(Suppl 5):80–82. doi:10.1111/j.1600-0501.2011.02370.x
2. Bramanti E, Norcia A, Cicciu M, et al. Postextraction dental Implant in the aesthetic zone, socket shield technique versus conventional protocol. J Craniofac Surg. 2018;29(4):1037–1041. doi:10.1097/SCS.0000000000004419
3. De Risi V, Clementini M, Vittorini G, Mannocci A, De Sanctis M. Alveolar ridge preservation techniques: a systematic review and meta-analysis of histological and histomorphometrical data. Clin Oral Implants Res. 2015;26(1):50–68. doi:10.1111/clr.12288
4. Annunziata M, Guida L, Nastri L, et al. The role of autologous platelet concentrates in alveolar socket preservation: a systematic review. J Transfus Med Hemother. 2018;45:195–203. doi:10.1159/000488061
5. Buser D, Chappuis V, Belser URSC, Chen S. Implant placement post extraction in esthetic single tooth sites: when immediate, when early, when late? Periodontology. 2017;73:84–102. doi:10.1111/prd.12170
6. Miron RJ, Zhang Y. Textbook of Next generation biomaterials for bone and periodontal regeneration. In: Chapter 6 – Synthetic Bone Substitute Materials.92.
7. Indovina A
8. Clark M, Rajendran Y, Paydar S, et al. Advanced platelet-rich fibrin and freeze-dried bone allograft for ridge preservation: a randomized controlled clinical trial. J Periodontol. 2018;89(4):379–387. doi:10.1002/JPER.17-0466
9. Braut V, Bornstein MM, Belser U, Buser D. Thickness of the anterior maxillary facial bone wall – a retrospective radiographic study using cone beam computed tomography. Int J Periodont Rest Dent. 2011;31:125–131.
10. Araujo MG, Sukekava F, Wennstrom JL, Lindhe J. Ridge alterations following implant placement in fresh extraction sockets: an experimental study in the dog. J Clin Periodontol. 2005;32:645–652. doi:10.1111/j.1600-051X.2005.00726.x
11. Tiwari S, Bedi RS, Wadhwani P, Aurora JK, Chauhan H. Comparison of immediate implant placement following extraction with and without socket-shield technique in esthetic region. J Maxillofac Oral Surg. 2020;19(4):552–560. doi:10.1007/s12663-019-01272-3
12. Hürzeler MB, Zuhr O, Schüpbach P, et al. The socket shield technique: a proof-of-principle report. J Clin Periodontol. 2010;37:855–862. doi:10.1111/j.1600-051X.2010.01595.x
13. Mourya A, Mishra SK, Gaddale R, Chowdhary R. Socket‐shield technique for implant placement to stabilize the facial gingival and osseous architecture: a systematic review. J Invest Clin Dent. 2019;10(4):124–149. doi:10.1111/jicd.12449
14. Sun C, Zhao J, Liu Z, et al. Comparing conventional flap-less immediate implantation and socket-shield technique for esthetic and clinical outcomes: a randomized clinical study. Clin Oral Implant Res. 2020;31:181–191. doi:10.1111/clr.13554
15. Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7–13. doi:10.14219/jada.archive.1964.0034
16. Muller HP, Kononen E. Variance components of gingival thickness. J Periodontal Res. 2005;40(3):239–244. doi:10.1111/j.1600-0765.2005.00798.x
17. Furhauser R, Mailath-Pokorny G, Haas R, et al. Immediate restoration of immediate implants in the esthetic zone of the maxilla via the copy-abutment technique: 5-year follow-up of pink esthetic scores. Clin Implant Dent Rel Res. 2017;19:28–37. doi:10.1111/cid.12423
18. Zhang W, Skrypczak A, Weltman R. Anterior maxilla alveolar ridge dimension and morphology measurement by cone beam computerized tomography (CBCT) for immediate implant treatment planning. BMC Oral Health. 2015;15:65. doi:10.1186/s12903-015-0055-1
19. Altay MA, Sindel A, Tezerişener HA, et al. Esthetic evaluation of implant-supported single crowns: a comparison of objective and patient-reported outcomes. Int J Implant Dent. 2019;5(1):2. doi:10.1186/s40729-018-0153-3
20. Atalay B, Ramaanoglu M, Tozan EN. Ozyuvaci. Pain intensity and its objective determinants following implant surgery and sinus lifting: a 1-year prospective study. Niger J Clin Pract. 2017;20:1139. doi:10.4103/1119-3077.217253
21. Fujioka-Kobayashi M, Miron J, Hernandez M, et al. Optimised platelet-rich fibrin with the low speed concept: growth factor release, biocompatibility and cellular response. J Periodontol. 2017;88:112–121. doi:10.1902/jop.2016.160443
22. Esposito M, Barausse C, Pistilli R, et al. Immediate loading of post-extractive versus delayed placed single implants in the anterior maxilla: 1-year after loading outcome of a pragmatic multicenter randomised controlled trial. Eur J Oral Implantol. 2015;8(4):361–372.
23. Chen ST, Buser D. Esthetic outcomes following immediate and early implant placement in the anterior maxilla–a systematic review. Int J Oral Maxillofac Implants. 2014;29(Suppl):186–215. doi:10.11607/jomi.2014suppl.g3.3
24. Evans CD, Chen ST. Esthetic outcomes of immediate implant placements. Clin Oral Implants Res. 2008;19:73–80.
25. Saeidi Pour R, Zuhr O, Hürzeler M, et al. Clinical benefits of the immediate implant socket shield technique. J Esthet Rest Dent. 2017;29:93–101. doi:10.1111/jerd.12291
26. Bodic F, Hamel L, Lerouxel E, Basle MF, Chappard D. Bone loss and teeth. Joint Bone Spine. 2005;72:215–221. doi:10.1016/j.jbspin.2004.03.007
27. De Rouck T, Collys K, Cosyn J. Immediate single-tooth implants in the anterior maxilla: a 1-year case cohort study on hard and soft tissue response. J Clin Periodontol. 2008;35:649–657. doi:10.1111/j.1600-051X.2008.01235.x
28. Malchiodi L, Cucchi G, Nocini PF, et al. Evaluation of the esthetic results of 64 nonfunctional immediately loaded post extraction implants in the maxilla: correlation between interproximal alveolar crest and soft tissues at 3 years of follow-up. Clin Implant Dent Rel Res. 2013;15:130–142. doi:10.1111/j.1708-8208.2011.00424.x
29. Raes F, Cosyn J, Crommelinck E, Coessens P, De Bruyn H. Immediate and conventional single implant treatment in the anterior maxilla: 1-year results of a case series on hard and soft tissue response and aesthetics. J Clin Periodontol. 2011;38:385–394. doi:10.1111/j.1600-051X.2010.01687.x
30. Roe P, Kan JY, Rungcharassaeng K, Caruso JM, Zimmerman G. Horizontal and vertical dimensional changes of peri-implant facial bone following immediate placement and provisionalisation of maxillary anterior single implants: a 1-year cone beam computed tomography study. Int J Oral Maxillofac Implants. 2012;27:393–400.
31. Kapagiannidis D, Kontonasaki E, Bikos P, Koidis P. Teeth and gingival display in the premolar area during smiling in relation to gender and age. J Oral Rehabil. 2005;32(11):830–837. doi:10.1111/j.1365-2842.2005.01517.x
32. Huynh-Ba G, Pjetursson BE, Sanz M, et al. Analysis of the socket bone wall dimensions in the upper maxilla in relation to immediate implant placement. Clin Oral Implants Res. 2010;21(1):37–42. doi:10.1111/j.1600-0501.2009.01870.x
33. Lekholm U, Zarb GA, Albrektsson T. Patient selection and preparation. In: Tissue Integrated Prostheses. Chicago: Quintessence Publishing Co. Inc; 1985:199–209.
34. Chappuis V, Araujo MG, Buser D. Clinical relevance of dimensional bone and soft tissue alterations post-extraction in esthetic sites. Periodontol. 2017;73:73–83. doi:10.1111/prd.12167
35. Barakat DA, Hassan RS, Eldibany RM. Evaluation of the socket shield technique for immediate implantation. Alexandria Dent J. 2017;42:155–161. doi:10.21608/adjalexu.2017.57919
36. Zhu YB, Qiu LX, Chen L, et al. Clinical evaluation of socket shield technique in maxillary anterior region. Zhonghua Kou Qiang Yi Xue Za Zhi. 2018;53:665–668.
37. Abd-Elrahman A, Shaheen M, Askar N, Atef M. Socket shield technique vs conventional immediate implant placement with immediate temporization. Randomized clinical trial. Clin Implant Dent Rel Res. 2020;5:1–10.
38. Abadzhiev M, Nenkov P, Velcheva P. Conventional immediate implant placement and immediate placement with socket-shield technique– which is better. Int J Clin Med Res. 2014;1(5):176–180.
39. Baumer D, Zuhr O, Rebele S, et al. The socket-shield technique: first histological, clinical, and volumetrical observations after separation of the buccal tooth segment—a pilot study. Clin Implant Dent Rel Res. 2015;17:71–82. doi:10.1111/cid.12076
40. Clementini M, Agostinelli A, Castelluzzo W, et al. The effect of immediate implant placement on alveolar ridge preservation compared to spontaneous healing after tooth extraction: radiographic results of a randomized controlled clinical trial. J Clin Periodontol. 2019;46:776–786.
41. Sanz M, Lindhe J, Alcaraz J, Sanz‐Sanchez I, Cecchinato D. The effect of placing a bone replacement graft in the gap at immediately placed implants: a randomized clinical trial. Clin Oral Implants Res. 2017;28(8):902–910. doi:10.1111/clr.12896
42. Felice P, Zucchelli G, Cannizzaro G, et al. Immediate, immediate-delayed (6 weeks) and delayed (4 months) post-extractive single implants: 4 months post-loading data from a randomised controlled trial. Eur J Oral Implantol. 2016;9(3):233–247.
43. Esposito M, Zucchelli G, Cannizzaro G, et al. Immediate, immediate-delayed (6 weeks) and delayed (4 months) post-extractive single implants: 1-year post-loading data from a randomised controlled trial. Eur J Oral Implantol. 2017;10(1):11–26.
44. Block MS, Mercante DE, Lirette D, et al. Prospective evaluation of immediate and delayed provisional single tooth restorations. J Oral Maxillofac Surg. 2009;67(11 Suppl):89–107. doi:10.1016/j.joms.2009.07.009
45. Pellicer-Chover H, Penarrocha-Oltra D, Bagan L, et al. Single-blind randomized clinical trial to evaluate clinical and radiological outcomes after one year of immediate versus delayed implant placement supporting full-arch prostheses. Med Oral Patol Oral Cir Bucal. 2014;19:295–301. doi:10.4317/medoral.19536
46. Salama M, Ishikawa T, Salama H, et al. Advantages of the root submergence technique for Pontic site development in esthetic implant therapy. Int J Periodont Rest Dent. 2007;27:521–527.
47. Gluckman H, Salama M, Du Toit J. A retrospective evaluation of 128 socket-shield cases in the esthetic zone and posterior sites: partial extraction therapy with up to 4 years follow-up. Clin Implant Dent Rel Res. 2017;20(2):122–129. doi:10.1111/cid.12554
48. Tarnow DP, Chu SJ, Salama MA, et al. Flapless post extraction socket implant placement in the esthetic zone: part 1. The effect of bone grafting and/or provisional restoration on facial-palatal ridge dimensional change—a retrospective cohort study. Int J Periodont Rest Dent. 2014;34(3):323–331.
49. Ghahroudi AAR, Rokn AR, Shamshiri AR, Samiei N. Does timing of implant placement affect esthetic results in single-tooth implants? A cohort evaluation based on mPES. J Esthet Restor Dent. 2020;7:1–11.
50. Baiju RM, Elbe P, Varghese NO, Anju P. Patient reported outcome assessment of periodontal therapy: a systematic review. J Clin Diagn Res. 2017;11(8):14–19.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.