Back to Journals » Cancer Management and Research » Volume 13
Radiation Therapy Efficacy and Toxicity for Orbital and Ocular Adnexal Mucosa-Associated Lymphoid Tissue (OAMALT): A Single-Center, Retrospective Study of 32 Cases
Authors Xu L , Tang X, Jiang N, Zhang S, Cao Y, Sun X
Received 18 August 2021
Accepted for publication 15 October 2021
Published 21 October 2021 Volume 2021:13 Pages 8017—8024
DOI https://doi.org/10.2147/CMAR.S334396
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Rudolph Navari
Liping Xu,* Xinyu Tang,* Nan Jiang,* Sheng Zhang, Yuandong Cao, Xinchen Sun
Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinchen Sun; Yuandong Cao
Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, No. 300 Guangzhou Road, Nanjing, Jiangsu Province, 210029, People’s Republic of China
Email [email protected]; [email protected]
Purpose: Lymphomas of the orbit and ocular adnexa are histologically heterogeneous and their incidence rate has been increasing lately. However, because of their rarity and diversity, few cases have been analyzed. This study evaluated patients with orbital and ocular adnexal mucosa-associated lymphoid tissue (OAMALT), who received radiotherapy, and categorized their clinical characteristics, treatment outcomes, and complications.
Patients and Methods: We collected data on clinical presentation, age, sex, imaging, tumor location, treatment methods, pathological diagnosis, cataract incidence, cataract incidence periods, overall survival (OS), and disease-free survival (DFS) from 32 patients with orbital involvement and pathologically confirmed marginal zone B-cell lymphoma of MALT who were treated between 2009 and 2018. Twenty-two patients received 20 Gy/10 Fr using intensity-modulated radiation therapy (IMRT) plus 14 Gy/7 Fr using a 6– 14-MeV electron beam therapy using a lens-sparing approach. Ten patients received 32 Gy/16 Fr or 34 Gy/17 Fr using IMRT without the lens shield technique. Kaplan–Meier analysis was used to estimate DFS and OS.
Results: The median follow-up time was 83.4 ± 24.5 months. No patient had local recurrence, although three patients developed distant metastases. The 5-year and 10-year OS rates were both 100%. The 5-year and 10-year DFS rates were 96.7% and 74.2%, respectively. Overall, 11 (32.4%) of the 34 lenses developed cataracts. The estimated 5-year, 7-year, and 10-year cumulative cataract rates were 6.9%, 30.9%, and 60.8%, respectively. The median cumulative cataract incidence period was 107.0 months. Age was the only significant parameter associated with cataract formation.
Conclusion: A radiation dose of 32– 34 Gy yields excellent local control, DFS, and OS for OAMALT. Some patients may have systemic relapse, and better identification of these patients is necessary. Reducing the prescription radiation dose or using better radiation techniques to spare the ipsilateral lens could reduce cataract formation.
Keywords: orbital lymphoma, adnexal lymphoma, mucosa-associated lymphoid tissue, radiotherapy
Introduction
Lymphomas of the orbit and ocular adnexa, including the orbit, extraocular muscles, conjunctiva, eyelids, and lacrimal gland, are histologically heterogeneous and their incidence rates have been increasing lately. The most frequently observed subtypes are orbital and ocular adnexal mucosa-associated lymphoid tissue (OAMALT) lymphoma,1–5 accounting for 49.5–67.9% of lymphomas, although other common subtypes include diffuse large B-cell lymphoma (9–19.5%) and follicular lymphoma (5–20%). The clinical signs and symptoms of these cancers differ greatly depending on the tumor location, volume, and the relationship with the surrounding tissues. The most common manifestations of the disease are asymptomatic presentation, proptosis, ocular dysmotility, ptosis, periorbital swelling, blurry vision, and chemosis.2,5
Treatment options for orbital lymphoma include surgical excision, radiation therapy, chemotherapy, and immunotherapy. Pathological results, including histological and immunophenotypic information, should be obtained by surgery. A definitive diagnosis is made following surgery. Radiation therapy provides excellent local control of the disease and is the most important modality for localized primary orbital lymphoma after surgery.6 Chemotherapy should be administered to patients with progressive distant diseases. However, because of the rarity and diversity of orbital lymphoma, only a small cohort of patients over a wide time span have been analyzed. In this study, we retrospectively analyzed patients with OAMALT who were treated at our institute between October 2009 and January 2018 to review the efficacy of intensity-modulated radiation therapy (IMRT) on OAMALT and determine complications after radiation therapy (RT).
Materials and Methods
Patients
We performed a retrospective analysis on all patients who were diagnosed with MALT lymphoma and treated at our RT department between October 2009 and January 2018 to identify patients with OAMALT.
This study was conducted in accordance with the ethical standards of the World Medical Association Declaration of Helsinki and was approved by the Ethical Board of the First Affiliated Hospital of Nanjing Medical University (Ethical No. 2019-SR-317). Due to the retrospective nature of the study and lack of interference with treatment, the requirement for written informed consent for participation was waived. In addition, our research data are confidential.
We gathered basic clinical information of the patients, including clinical presentation, age, sex, computed tomography (CT) findings, magnetic resonance imaging findings, positron emission tomography/computed tomography (PET/CT) findings, tumor location, surgical management, pathological diagnosis, flow cytometry diagnosis, bone marrow biopsy results, initial treatment and subsequent therapy, antibiotic/hormonal therapy, RT modes and doses, chemotherapy, side effects, and follow-up.
RT was administered as follows: all patients received RT to the involved orbit at a dose of 32–34 Gy in 2.0 Gy per fraction. Of the 32 patients who received local radiotherapy, 22 patients received 20 Gy/10 Fr of X-ray irradiation using the IMRT technique plus 14 Gy/7 Fr of 6–14-MeV electron beam therapy using a custom-made contact lens to shield the cornea and lens. The other 10 patients received 32 Gy/16 Fr or 34 Gy/17 Fr of 6-MV X-ray irradiation using the IMRT technique without the lens shield technique. Acute and late side effects, particularly cataract incidence, were evaluated and graded according to the Radiation Therapy Oncology Group (RTOG) toxicity score version 3.0.
Statistical Analysis
Overall survival (OS) and disease-free survival (DFS) were calculated. OS was calculated from the date of initial diagnosis to the date of death or the last follow-up. DFS was calculated from the date of RT completion to the date of any local recurrence or the occurrence of metastases (identified by CT scan and confirmed by pathological test), whichever occurred first. Patients who remained alive were censored at the date of the last follow-up visit. Kaplan–Meier curves were used to estimate survival distributions and cumulative cataract events in various subgroups. Hazard ratios were calculated using multivariate analyses. All tests were two-sided. Statistical significance was set at P < 0.05. All statistical analyses were performed using SPSS (version 20.0; SPSS, Chicago, IL, USA).
Results
Patient Characteristics
Patient characteristics are summarized in Table 1. The median age at diagnosis was 56.0 ± 11.9 years (range, 32–83 years). There were 21 men and 11 women (Table 1).
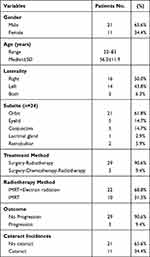 |
Table 1 Charcteristics of 32 Patients with Orbital MALT Lymphomas |
The median time between symptom onset and first hospital visit was 8.24 months (range, 0.5–36 months). Signs and symptoms included proptosis (n = 15), eyelid mass (n = 21), tearing (n = 3), chemosis (n = 3), decreased visual acuity or blurred vision (n = 5), and pain, itch, or foreign body sensation (n = 7). All patients underwent surgery by an ophthalmologist. There were 14 left orbital MALT lymphomas, 16 right orbital MALT lymphomas, and two bilateral MALT lymphomas. Overall, 21 of the 34 lesions were located in the orbit, five in the eyelid, five in the conjunctiva, one in the lacrimal gland, and two in the retrobulbar area.
Clinical Outcomes
The median follow-up time was 83.4 ± 24.5 months (range, 44.5–143.2 months). All 32 patients received local RT. None of the patients developed local recurrence, but three women developed distant metastases, without local recurrence within the treatment field, at a median of 76.5 ± 26.3 months (range, 33.8–143.2 months). One patient each developed metastases in the submandibular lymph nodes, lung, and lateral orbit. The 5-year and 10-year OS rates were both 100%, and the 5-year and 10-year DFS rates were 96.7% and 74.2%, respectively (Figure 1A). The estimated median DFS and OS for the entire cohort were not reached.
We performed further subgroup analyses. Three of the eleven female patients had disease progression, whereas no male patients developed local recurrence or distant metastasis. There was a significant difference in DFS between the male and female patients (P = 0.006) (Figure 1B). No statistically significant difference in DFS was observed based on the treatment protocol (surgery + radiotherapy vs surgery + chemotherapy + radiotherapy) (P = 0.619) (Figure 1C). Three of twenty-one patients younger than 60 years old had disease progression, whereas no patients older than 60 years had disease progression. However, there was no statistically significant difference between the two groups (P = 0.189) (Figure 1D). Similarly, no statistically significant difference was found in DFS according to tumor location (P = 0.373) (Figure 1E) or radiation mode (P = 0.458) (Figure 1F).
Complications
Acute RT reactions were minimal in all patients. A mild degree of periorbital soft tissue swelling, eye drying, tearing in the wind, or acute conjunctivitis that subsided gradually in a few months was noted in most patients. All symptoms were successfully relieved using artificial tears. No radiation retinopathy, glaucoma, optic neuropathy, or retinopathy was observed in these patients.
Overall, 11 (32.4%) of the 34 lenses developed cataracts. Seven of the eleven cataracts underwent cataract surgery, and the visual ability partially recovered after surgery. Figure 2A shows the cumulative cataract incidence over time after radiation. The estimated 5-year, 7-year, and 10-year cumulative cataract rates were 6.9%, 30.9%, and 60.8%, respectively. The median cumulative cataract incidence period was 107.0 months (95% confidence interval [CI], 81.9–157.3).
For female and male patients, the estimated median cataract incidence periods after RT were 97.7 ± 7.9 months (95% CI: 71.4–135.7) and 112.0 ± 8.7 months (95% CI: 65.3–173.9), respectively. There was no significant difference in the cumulative cataract incidence periods between male and female patients (P = 0.832) (Figure 2B). Cataracts were observed in 10 (34.5%) of the 29 patients who did not receive chemotherapy. One (33.3%) of the three patients who received chemotherapy developed cataracts. However, no significant difference in the cumulative cataract incidence period was observed between patients treated with surgery + radiotherapy (110.0 ± 7.7 months, 95% CI: 94.9–125.2) and those treated with surgery + chemotherapy + radiotherapy (103.5 ± 0 months) (P = 0.695) (Figure 2C).
Four (19.0%) of the twenty-one patients who were ≤60 years old developed cataracts, whereas seven (63.6%) of the eleven patients >60 years old developed cataracts. Patients >60 years (89.9 ± 7.9 months, 95% CI: 74.3–105.6) were likely to develop cataracts sooner after radiotherapy than those aged ≤60 years (121.9 ± 9.4 months, 95% CI: 103.4–140.3) after orbital RT (P = 0.025) (Figure 2D).
Through the end of the study period, three (30%) of the ten patients who received IMRT with 32–34 Gy/17 Fr X-ray beam radiation developed cataracts, and eight (36.4%) of the twenty-two patients receiving 20 Gy/10 Fr plus 14 Gy/7 Fr electron beam radiation developed cataracts. The estimated median cataract incidence period was 114.0 ± 8.3 months (95% CI: 97.7–130.2) for patients who received IMRT plus X-ray beam radiation, whereas it was only 76.8 ± 9.0 months (95% CI: 59.1–94.4) for those who received only X-ray beam radiation. However, no statistical difference was observed between the two groups (P = 0.347) (Figure 2E).
Cox regression analysis revealed that age (P = 0.019) was an independent factor related to cataract incidence in patients with OAMALT who received radiotherapy. Compared to patients aged <60 years, patients aged ≥60 years had a 4.43-times higher risk of cataract incidence (95% CI: 1.28–15.4), as shown in Figure 2F.
Discussion
Orbital lesions can be primary or metastatic and may be vascular, neural, congenital, inflammatory, autoimmune, infectious, neoplastic, or malignant in nature. Each category contains multiple sub-etiologies. Treatment options for these lesions include surgery, hormonal therapy, chemotherapy, and radiotherapy. Our study focused only on patients with OAMALT and had a relatively long follow-up of 83.4 months. We believe that the implications of RT treatment and late complication analysis conducted in this study is convincing.
Because of the rarity and diversity of orbital lymphoma, only a small cohort of patients with wide treatment spans has been analyzed. Primary radiotherapy, usually 25–35 Gy, is the standard treatment for stage IE orbital lymphoma.8–12 Son et al reported on a series of 46 patients who were treated with radiotherapy and defined a dose of 30.6 Gy applied in fractions of 1.8–2.0 Gy as a reasonable treatment in terms of tolerability and efficacy.13 A previous study reported that doses of 24 Gy for indolent lymphoma and 30 Gy for aggressive non-Hodgkin lymphoma have comparable efficacy to doses of 40–45 Gy.14–16 The standard schedule for indolent lymphoma is 24 Gy in 12 Fr. However, 32 or 34 Gy was chosen for OAMALT in our study (higher than the present standard dose of 24–30.6 Gy but lower than the previous standard dose of 40–45 Gy) because 32 or 34 Gy dose prescription is relatively effective and safe.
In our study, 32 patients with MALT orbital lymphoma had favorable outcomes after radiation treatment with a total dose of 32–34 Gy. With a median follow-up period of 83.4 months, we observed no local recurrences within the treatment field. However, three female patients experienced a relapse after a median 76.5 months. Our results are similar to those reported by Hashimoto et al,17 where local control rates were excellent, as no patient had local recurrence, and 10 of 78 patients with OAMALT (13%) experienced relapse at a distant site after radiotherapy. This suggests that OAMALT has excellent local control rate after RT and relapse may still occur in a distant part of the body after some time.
In this study, the 5-year and 10-year OS rates were both 100%, and the 5-year and 10-year DFS rates were 96.7% and 74.2%, respectively. The estimated median DFS and OS for the entire cohort were not reached. Therefore, given the low incidence of locoregional and distant failure in this retrospective study, the role of primary radiotherapy is clear and important. Our results are similar to previously published data for MALT orbital lymphoma showing excellent local control rates with doses ranging from 24 to 36 Gy.18–23
Our study revealed that the treatment method, age (≤60 y vs >60 y), tumor location, and radiation mode (IMRT+E vs IMRT) were not correlated with DFS among these patients. Sex was the only risk factor for DFS. In this retrospective study, three female patients developed distant metastases without local recurrence within the treatment field. Therefore, women were more likely to develop disease progression after RT than men. However, we are not certain whether sex is a real risk factor in this retrospective study because of the relatively small number of patients.
Local control with RT for orbital lymphomas is excellent. Local control rates range from 89% to 100%, with a distant metastasis rate of 0–25%.6,24–27 In this study, 9.4% of patients developed distant metastases, which is consistent with the results of previous studies. Therefore, radiotherapy provides excellent disease outcomes and should be used as first-line treatment for primary orbital lymphomas.6
In a Japanese study of 78 patients, 23 (30%) patients subsequently developed grade III cataracts.17 In our study, 11 (32.4%) of the 34 lenses developed cataracts. Seven of the eleven cataracts underwent cataract surgery, and visual ability partially recovered after surgery. The estimated 5-year, 7-year, and 10-year cumulative cataract rates were 6.9%, 30.9%, and 60.8%, respectively, and the estimated median cumulative cataract incidence period was 107.0 months. In the Rare Cancer Network study,28 the incidence of cataract formation was 30%, irrespective of the use of lens shielding, with a median tumor dose of 34.2 Gy. The incidence of cataracts increases with time after RT. To reduce the incidence of cataracts without reducing the local control rate, we think that the best treatment choices include reducing the dose of RT and taking effective measures to protect the eye lens.
Univariate analysis revealed that sex, treatment method, and radiation mode were not significantly associated with cataract formation. Age was the only statistically significant parameter associated with cataract formation. Patients older than 60 years had a higher risk of cataract formation than those aged 60 years or younger, according to the univariate analysis, and Cox regression analysis further confirmed this conclusion. Patients aged ≥60 years had a 4.43 times higher risk of cataract development than those aged <60 years. It is worth noting that IMRT+E was associated with a lower incidence of cataract and a longer cataract incidence than IMRT. However, no significant correlation was found between cataractogenesis and IMRT+E in our study, which is similar to the results of previous studies.28 Therefore, we recommend the lens shielding technique (IMRT+E) whenever feasible.
Taken together, the present retrospective case series of 32 patients reflects the excellent local control rate of OAMALT after RT. We also analyzed cataract formation with time after RT. However, it has to be noted that due to the retrospective nature of our study with a small number of patients from a single institution, the generalizability of the findings is limited. In view of current data, we strongly suggest that clinicians make great efforts to protect the eye lens without reducing curative effect. Further, prospective studies and randomized trials are warranted in this regard in the future.
Conclusion
Our study demonstrated excellent local control, DFS, and OS with a radiation dose of 32–34 Gy for OAMALT. A small proportion of patients had systemic relapse, and better identification of this subset of patients is necessary. To reduce the incidence of cataract formation and other radiation toxicities, reducing the radiation dose to the planning target volume or using better radiation techniques to spare the ipsilateral lens and other critical organs around the target structures should be explored.
Abbreviations
OAMALT, ocular adnexal mucosa-associated lymphoid tissue; IMRT, Intensity-Modulated Radiation Therapy; CT, Computed Tomography; PET, Positron Emission Tomography; RT, Radiation Therapy; RTOG, Radiation Therapy Oncology Group; OS, overall survival; DFS, disease-free survival; CI, confidence interval.
Acknowledgments
We would like to thank Editage for English language editing.
Disclosure
The authors report no conflict of interest in this work.
References
1. Gerbino G, Boffano P, Benech R, et al. Orbital lymphomas: clinical and radiological features. J Craniomaxillofac Surg. 2014;42(5):508–512. doi:10.1016/j.jcms.2013.07.017
2. Ferry JA, Fung CY, Zuckerberg L, et al. Lymphoma of the ocular adnexa: a study of 353 cases. Am J Surg Pathol. 2007;31:170–184. doi:10.1097/01.pas.0000213350.49767.46
3. Jenkins C, Rose GE, Bunce C, et al. Histological features of ocular adnexal lymphoma (REAL classification) and their association with patient morbidity and survival. Br J Ophthalmol. 2000;84:907–913. doi:10.1136/bjo.84.8.907
4. Couland SE, Krause L, Delecluse HJ, et al. Lymphoproliferative lesions of the ocular adnexa. Analysis of 112 cases. Ophthalmology. 1998;105:1430–1441. doi:10.1016/S0161-6420(98)98024-1
5. Hsu CR, Chen YY, Yao M, Wei YH, Hsieh YT, Liao SL. Orbital and ocular adnexal lymphoma: a review of epidemiology and prognostic factors in Taiwan. Eye. 2020. PMID: 32994547. doi:10.1038/s41433-020-01198-y
6. Kharod SM, Herman MP, Morris CG, et al. Radiotherapy in the management of orbital lymphoma: a single institution’s experience over 4 decades. Am J Clin Oncol. 2018;41(1):100–106.
7. Eckardt AM, Lemound J, Rana M, Gellrich NC. Orbital lymphoma: diagnostic approach and treatment outcome. World J Surg Onc. 2013;11:1–7.
8. Gveroviæ-Antunica A, Markoviæ I, Bohaè M, Coroviæ-Arneri E, Ivaniin-Baraè A, Karaman K. Primary orbital non-Hodgkin's lymphoma. case report. Acta Clin Croat. 2007;46:113–116.
9. Cicco LD, Cella L, Liuzzi R, et al. Radiation therapy in primary orbital lymphoma: a single institution retrospective analysis. Radiat Oncol. 2009;4:1–6.
10. Kiesewetter B, Lukas J, Kuchar A, et al. Clinical features, treatment and outcome of mucosa-associated lymphoid tissue (MALT) lymphoma of the ocular adnexa: single center experience of 60 patients. PLoS One. 2014;9:e104004. doi:10.1371/journal.pone.0104004
11. Rasmussen PK, Ralfkiaer E, Prause JU, et al. Follicular lymphoma of the ocular adnexal region: a nation-based study. Acta Ophthalmol. 2015;93:184–191. doi:10.1111/aos.12525
12. Cohen V. Treatment options for ocular adnexal lymphoma (OAL). Clin Ophthalmol. 2009;3:689–692. doi:10.2147/OPTH.S5828
13. Son SH, Choi BO, Kim GW, et al. Primary radiation therapy in patients with localized orbital marginal-zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT Lymphoma). Int J Radiat Oncol Biol Phys. 2010;77:86–91. doi:10.1016/j.ijrobp.2009.04.018
14. Lowry L, Smith P, Qian W, et al. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised Phase III trial. Radiother Oncol. 2011;100(1):86–92. PMID: 21664710. doi:10.1016/j.radonc.2011.05.013
15. Hoskin PJ, Kirkwood AA, Popova B, et al. 4 Gy versus 24 Gy radiotherapy for patients with indolent lymphoma (FORT): a randomised Phase 3 non-inferiority trial. Lancet Oncol. 2014;15(4):457–463. PMID: 24572077. doi:10.1016/S1470-2045(14)70036-1
16. Hoskin P, Popova B, Schofield O, et al. 4 Gy versus 24 Gy radiotherapy for follicular and marginal zone lymphoma (FoRT): long-term follow-up of a multicentre, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2021;22(3):332–340. PMID: 33539729. doi:10.1016/S1470-2045(20)30686-0
17. Hashimoto N, Sasaki R, Nishimura H, et al. Long-term outcome and patterns of failure in primary ocular adnexal mucosa-associated lymphoid tissue lymphoma treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:1509–1514. doi:10.1016/j.ijrobp.2011.04.052
18. Bhatia S, Paulino AC, Buatti JM, et al. Curative radiotherapy for primary orbital lymphoma. Int J Radiat Oncol Biol Phys. 2002;54:818–823. doi:10.1016/S0360-3016(02)02966-8
19. Le QT, Eulau SM, George TI, et al. Primary radiotherapy for localized orbital MALT lymphoma. Int J Radiat Oncol Biol Phys. 2002;52:657–663. doi:10.1016/S0360-3016(01)02729-8
20. Uno T, Isobe K, Shikama N, et al. Radiotherapy for extranodal, marginal zone, B-cell lymphoma of mucosa-associated lymphoid tissue originating in the ocular adnexa: a multiinstitutional, retrospective review of 50 patients. Cancer. 2003;98:865–871. doi:10.1002/cncr.11539
21. Fung CY, Tarbell NJ, Lucarelli MJ, et al. Ocular adnexal lymphoma: clinical behavior of distinct World Health Organization classification subtypes. Int J Radiat Oncol Biol Phys. 2003;57:1382–1391. doi:10.1016/S0360-3016(03)00767-3
22. Kennerdell JS, Flores NE, Hartsock RJ. Low-dose radiotherapy for lymphoid lesions of the orbit and ocular adnexa. Ophthal Plast Reconstr Surg. 1999;15:129–133. doi:10.1097/00002341-199903000-00012
23. Zhou P, Ng AK, Silver B, et al. Radiation therapy for orbital lymphoma. Int J Radiat Oncol Biol Phys. 2005;63:866–871. doi:10.1016/j.ijrobp.2005.03.005
24. Fitzpatrick PJ, Macko S. Lymphoreticular tumors of the orbit. Int J Radiat Oncol Biol Phys. 1984;10(3):333–340. PMID: 6368493. doi:10.1016/0360-3016(84)90051-8
25. Bolek TW, Moyses HM, Marcus RB, et al. Radiotherapy in the management of orbital lymphoma. Int J Radiat Oncol Biol Phys. 1999;44(1):31–36. PMID: 10219791. doi:10.1016/s0360-3016(98)00535-5
26. Stafford SL, Kozelsky TF, Garrity JA, et al. Orbital lymphoma: radiotherapy outcome and complications. Radiother Oncol. 2001;59(2):139–144. PMID: 11325441. doi:10.1016/s0167-8140(00)00328-5
27. Parikh RR, Moskowitz BK, Maher E, et al. Long-term outcomes and patterns of failure in orbital lymphoma treated with primary radiotherapy. Leuk Lymphoma. 2015;56(5):1266–1270. PMID: 25356924. doi:10.3109/10428194.2014.979415
28. Martinet S, Ozsahin M, Belkacémi Y, et al. Outcome and prognostic factors in orbital lymphoma: a Rare Cancer Network study on 90 consecutive patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2003;55(4):892–898. PMID: 12605966. doi:10.1016/s0360-3016(02)04159-7
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


