Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Racial Disparities in Diabetes Technology Adoption and Their Association with HbA1c and Diabetic Ketoacidosis
Authors Conway RB, Gerard Gonzalez A , Shah VN, Geno Rasmussen C, Akturk HK, Pyle L, Forlenza G , Alonso GT, Snell-Bergeon J
Received 7 April 2023
Accepted for publication 18 July 2023
Published 2 August 2023 Volume 2023:16 Pages 2295—2310
DOI https://doi.org/10.2147/DMSO.S416192
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Rebecca Baqiyyah Conway,1 Andrea Gerard Gonzalez,2 Viral N Shah,2 Cristy Geno Rasmussen,2 Halis Kaan Akturk,2 Laura Pyle,2 Gregory Forlenza,2 Guy Todd Alonso,2 Janet Snell-Bergeon2
1Department of Epidemiology, Colorado School of Public Health, University of Colorado Anschutz Medical Campus, Aurora, CO, USA; 2School of Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Correspondence: Rebecca Baqiyyah Conway, Colorado School of Public Health, Anschutz Medical Campus, 13001 East 17th Place, Mail Stop B119, Aurora, CO, 80045, USA, Email [email protected]
Aim: Poorer glycemic control and higher diabetic ketoacidosis (DKA) rates are seen in racial/ethnic minorities with type 1 diabetes (T1D). Use of diabetes technologies such as continuous glucose monitors (CGM), continuous subcutaneous insulin infusion (CSII) and automated insulin delivery (AID) systems has been shown to improve glycemic control and reduce DKA risk. We examined race/ethnicity differences in diabetes technology use and their relationship with HbA1c and DKA.
Methods: Data from patients aged ≥ 12 years with T1D for ≥ 1 year, receiving care from a single diabetes center, were examined. Patients were classified as Non-Hispanic White (n=3945), Non-Hispanic Black (Black, n=161), Hispanic (n=719), and Multiracial/Other (n=714). General linear models and logistic regression were used.
Results: Black (OR=0.22, 0.15– 0.32) and Hispanic (OR=0.37, 0.30– 0.45) patients were less likely to use diabetes technology. This disparity was greater in the pediatric population (p-interaction=0.06). Technology use associated with lower HbA1c in each race/ethnic group. Among technology users, AID use associated with lower HbA1c compared to CGM and/or CSII (HbA1c of 8.4% vs 9.2%, respectively), with the greatest difference observed for Black adult AID users. CSII use associated with a lower odds of DKA in the past year (OR=0.73, 0.54– 0.99), a relationship that did not vary by race (p-interaction =0.69); this inverse association with DKA was not observed for CGM or AID.
Conclusion: Disparities in diabetes technology use, DKA, and glycemic control were apparent among Black and Hispanic patients with T1D. Differences in technology use ameliorated but did not fully account for disparities in HbA1c or DKA.
Keywords: continuous subcutaneous insulin infusion, continuous glucose monitoring, automated insulin delivery systems, diabetic ketoacidosis, racial disparities
Introduction
Elevated HbA1c levels are observed in US racial and ethnic minorities with type 1 diabetes (T1D).1 African American and Hispanic patients in the US also have a greater rate of diabetic ketoacidosis (DKA), a life-threatening acute complication of diabetes driven by excessive hyperglycemia and insulin insufficiency. Technologies such as continuous glucose monitors (CGM) and continuous subcutaneous insulin infusion (CSII) enhance diabetes management and improve glycemic management. These technologies reduce incidence of severe hypoglycemia and DKA. However, racial and ethnic minorities are less likely to use CGM or CSII.1–6
There have been tremendous advances in diabetes care and improvements in glycemic control over the past several decades, including insulin analogs,7,8 CGM,9 insulin pumps, i.e. CSII10,11 and automated insulin delivery systems (AID).12–14 Studies with AID have been shown to improve glycemic control and reduce hypoglycemia in children, adolescents, and adults with T1D.15–17 However, knowledge of its use in racial ethnic minorities is limited, as is its association with improved glycemic management and reduction in DKA events among these populations.
Minimizing hyperglycemia is critical to preventing both acute and chronic complications of T1D in racial and ethnic minorities. We examined differences, and whether insurance coverage status and type explain these differences, in the use of diabetes self-management technology by race/ethnicity among both adolescents and adults and their association with HbA1c and DKA among patients with T1D receiving regular care at a large diabetes center in the US.
Materials and Methods
Individuals included in this study consisted of patients with T1D being treated at the Barbara Davis Center for Diabetes, Colorado, between January 1st 2020 and October 31st 2021. Electronic Health Records were queried to identify patients with T1D. Demographics, information on technology use and type of technology, HbA1c, and type of medical insurance at their most recent visit, as well as a history of having DKA within the previous 12 months were extracted from the electronic health record (EHR). Persons aged 12 years and older with a diagnosis of T1D were included, resulting in a final study population of 5539 patients. This study was approved by the Colorado Multiple Institutional Review Board, with a waiver of consent since all data were already collected and this was secondary data analysis. This study is in compliance with the Declaration Helsinki.
Diabetes technology use was defined as the use of CSII, use of CGM, or an AID. All patients at our center are offered CGM and insulin pumps, and there are no requirements for them to have failed previous therapy to be eligible for these technologies. Our insulin pump training program is a structured series of three classes. Training for CGM is less structured, and usually consists of a single, 20-min class. Target HbA1c was <7% in adults aged 18 years and older and in the pediatric population.
Statistical analyses
The Student’s t-test was used to test for differences in continuous variables and the chi-square test was used to test for differences in categorical data. Multivariable logistic regression analysis was used to test the association of a) race/ethnicity with diabetes technology use; b) diabetes technology use with target glycemic control within each race/ethnic group; and c) diabetes technology use with DKA. General linear models were used to test the relationship between diabetes technology use and HbA1c in each of the race/ethnic groups. Tests for multiplicative interaction by age group (pediatric vs adult population) were conducted, and, where appropriate, stratified analyses were carried out. Analyses included terms for age, sex, diabetes duration, race/ethnicity (non-Hispanic White (White), non-Hispanic Black (Black), Hispanic, Other races/multiracial (Other)), medical insurance (private, military, Medicaid, other, none), and number of visits with the provider in the previous year. The criterion for statistical significance was a two-tailed P-value of <0.05. Statistical analysis was conducted using SAS version 9.4 (Cary, North Carolina).
Results
Patient characteristics are presented in Table 1. Mean age was 28.7 years, with slightly over a quarter of patients under age 18 years. Overall, half of the patients were female and approximately 70% of the patients were White. Hispanic patients represented the largest non-White population, representing 19% and 11%, respectively, of the pediatric and adult populations. Over 80% of both the adult and pediatric patients were using either CSII or CGM, with both CSII and CGM more common among the pediatric patients than among the adults.
 |
Table 1 Descriptive Characteristics of Barbara Davis Study Participants with Type 1 Diabetes, Mean ± Std or % (n) |
Characteristics of the study population by race are presented in Table 2 (overall) and Table 3 (stratified by age group). The mean age of the pediatric patients with type 1 diabetes was 15 years in each of the race/ethnic groups, with mean diabetes duration also similar in each group, ranging from 5.8 among Black patients to 6.2 among White patients. Private health insurance was highest among White children (72%) and lowest among Hispanic children (25%). Technology use was also highest among White children, at 93.5%, but lowest among Black children, at 55.1%. For most characteristics, those in the Other races group were either similar to Whites or between that of Whites and Black/Hispanic patients (Table 2).
 |
Table 2 Descriptive Characteristics of Barbara Davis Study Participants with Type 1 Diabetes, Stratified by Race/Ethnicity, Mean ± Std or % (n) |
Mean age of the adult population ranged from 27.5 years in Hispanic patients to 34.7 years in the White patients, with diabetes duration ranging from 13.3 years to 18.4 years, respectively. Like the pediatric population, technology use was highest among White patients; however, unlike the pediatric population technology use was similar among Black and Hispanic patients. Similarly, having private health care insurance was highest among White patients, but similar among Black and Hispanic adult patients. As with the pediatric population, characteristics of those in the Other races group were either similar to White patients or between that of White and Black/Hispanic patients (Table 3).
 |
Table 3 Descriptive Characteristics of the Participants, Stratified by Race and Age Group, Mean ± Std or % (n) |
Table 4 shows the relationship between race/ethnicity and diabetes technology use. Technology use by race varied significantly between the pediatric and adult populations, with the racial disparity greater for adolescents than adults and the p-values for tests of interaction ranging from 0.04 to <0.0001. While the race disparity in usage was similar among the pediatric and adult Hispanic population compared to non-Hispanic White patients, Black adolescents were approximately one-tenth as likely to use diabetes technology compared to non-Hispanic White adolescents whereas Black adult patients were about one-fourth as likely compared to non-Hispanic White adults. Insurance status/type was also an important correlate of diabetes technology use, with those with either Medicaid or no insurance approximately a third to half as likely to be using diabetes technology compared to those with private or military insurance. This finding was similar among both the pediatric and adult populations.
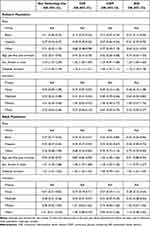 |
Table 4 Multivariable Adjusted Relationship of Race/Ethnicity with Technology Use in Type 1 Diabetes, Stratified by Age Group |
When stratified by race (Table 5 and Table 6), younger age was a significant correlate among White and Hispanic pediatric patients for both CSII and CGM use (Table 5), while longer duration of diabetes was significantly associated with some form of diabetes technology use among all race/ethnic groups except Black patients. Only among the Hispanic pediatric population was a gender difference in technology use observed, with girls more likely than boys to be using diabetes technology. Having private or military insurance was an important correlate of technology usage among all race/ethnic groups, but particularly for Black patients. Black pediatric patients with private or military insurance were twelve times more likely to be using CSII and seven times more likely to be using CGM than Black pediatric patients with no insurance or with Medicaid. Among adults (Table 6), while the relationship between insurance status/type with CGM usage was similar among the race/ethnic groups, having private or military insurance was associated with a 2- to 3-fold increased likelihood for CGM use; for CSII use this relationship was stronger in all race/ethnic groups than in non-Hispanic Whites (non-overlapping CIs). Longer duration of diabetes increased the likelihood of CSII use among all race/ethnic groups; by contrast longer duration of diabetes had no relationship with CGM use among any of the race/ethnic groups.
 |
Table 5 Race Stratified Multivariable Adjusted Factors Associated with Technology Use in the Pediatric Population |
 |
Table 6 Race/Ethnicity Stratified Multivariable Adjusted Factors Associated with Technology Use in the Adult Population |
Figure 1 shows the relationship of technology use with HbA1c in each race/ethnicity group. For both the pediatric and adult populations, regardless of technology use, mean HbA1c was highest among Black patients, intermediate among Hispanic patients, and lowest among non-Hispanic White patients and patients in the Other races category. However, technology use was associated with a lower HbA1c in every race/ethnicity group; this was stronger for CGM use among Black, Hispanic and patients of Other races category, and stronger for CSII for non-Hispanic White pediatric patients. For adults as well, technology use was associated with a lower HbA1c among patients of each race/ethnicity category.
Figure 2 shows mean HbA1c among technology users using AID compared to other technology users. Among pediatric technology users, AID was associated with lower HbA1c in every race/ethnicity group except for Black children. By contrast, among the adult population AID use was associated with lower HbA1c. The lower HbA1c associated with AID use was greatest among Black adults, with a mean HbA1c of 8.4% vs 9.2% among AID users compared to CSII and/CGM users (p<0.05). Among technology users, AID use was associated with a 1.8 to 2.9-fold greater likelihood of achieving target glycemic levels for White and Hispanic children, respectively, but showed no significant association among Black children or children of other races or among adults of any racial/ethnic group (data not depicted).
Table 7 shows the relationship of technology use with DKA in the past year. Compared to non-Hispanic White patients, Black and Hispanic patients were more than twice as likely to have experienced a DKA event in the past year. Further controlling for health insurance status/type attenuated but did not eliminate this excess risk, particularly for Hispanic patients. Patients with private or military insurance were less than half as likely to have experienced a DKA event in the past year. Technology use had no effect on the relationship of race with DKA in the past year. Results were similar for both the pediatric and adult populations (p-value for interaction >0.80 for each: any technology use, CSII use and CGM use).
 |
Table 7 Multivariable Adjusted Association of Technology Use with Diabetic Ketoacidosis in the Past Year |
Discussion
In this study of approximately 5000 pediatric and adult patients with type 1 diabetes, we examined the relationship of diabetes technology use by race/ethnicity and its relationship with HbA1c and DKA. The major findings of this study are that 1) technology use was lower in Black and Hispanic patients, a relationship that was attenuated, but not eliminated, by health insurance status and type; 2) technology use was associated with a lower HbA1c in every race/ethnic group; 3) AID use among technology users was associated with the greatest difference in HbA1c among Black adults compared to other races, but had no beneficial relationship on HbA1c among Black children; and 4) CSII reduced the likelihood of DKA with no difference in effect by race. We also observed that racial disparities in both diabetes technology use and HbA1c were greater in the pediatric population, with the greatest disparities observed for Black compared to White adolescents. Finally, medical insurance, but not diabetes technology use, was associated with having experienced a DKA event in the past year, with this relationship being strongest among Black patients.
Consistent with other reports, use of technologies such as CSII, CGM and AID delivery systems for glucose management was much lower among our Black and Hispanic patients than among non-Hispanic Whites in our population. This was observed among both the pediatric and the adult patient populations. Similarly, the Pediatric Diabetes Consortium T1D-New Onset (NeOn) Study observed a greater transition to CSII use among non-Hispanic Whites than among minorities (36% vs 11%) during the first year since diabetes diagnosis even after accounting for socioeconomic factors such as high income and having private health insurance.6 In the SEARCH for Diabetes in Youth study as well, racial and ethnic minorities were less likely to use CSII, a lower usage which was also associated with a higher HbA1c.5,18 One contributing factor may be the lack of representation of minorities among clinical trials of diabetes.19 Due to their lack of representation in clinical trials, these devices may not be user friendly to minority populations and may thus contribute to the low uptake. Other factors may be socioeconomic factors such as income, education, and health insurance, language-related numeracy and ability to count carbohydrates, as well as provider bias in prescribing practices.
Among all race and ethnic groups in our population, having health insurance and the type of health insurance were significant factors in whether patients were using diabetes technology for glucose management. This was true for both children and adults, and appeared most striking for Black pediatric patients, despite diabetes technology being covered by Medicaid for pediatric patients in Colorado. Thus, factors related to the patient having insurance and the type of insurance may account for some of the racial disparities in technology use. Indeed, there is some evidence that even when socioeconomic factors are similar, technology use, and prescribing of technology use, for diabetes management is still lower for minority patients.3–5 In Colorado, people younger than age 22 who are insured by Colorado’s Medicaid and Medicaid expansion program (CHP+) have had access to CGM technology since at least 2016, yet pediatric patients who had Medicaid as their source of insurance were approximately 70% less likely to be using any diabetes technology for management of diabetes compared to those with private insurance. Disparities in physician prescribing of treatment regimen have been shown to account for some of the disparities in diabetes technology adoption.3–5
There is considerable evidence that the use of diabetes technologies, including continuous glucose monitoring (CGM) systems,12,13,20–22 insulin pumps and automated insulin delivery systems (AID) systems, improve glycemic control and quality of life.12,13,23 Lower A1c reduces acute and chronic diabetes complications, including DKA24,25 microvascular diseases,26 and cardiovascular disease,27,28 the leading cause of mortality in people with T1D. We showed that diabetes technology use for the management of glycemic control was associated with reductions in HbA1c across all race/ethnic groups and among both pediatric and adult patients, with the exception of CSII use among Black adolescents. While CGM use was associated with a significantly lower HbA1c among the Black pediatric patients, no difference was observed for CSII use in this sub-patient population in contrast to approximately 1.5% lower HbA1c associated with CSII use among Black adults. Black adults showed the greatest difference in HbA1c associated with CSII use of all the race/ethnic groups. Reasons for this race-specific disparity between the pediatric and adult population in the difference in HbA1c associated with CSII use are not clear since patients at our center are offered CGM and insulin pumps, and there are no requirements for them to have failed previous therapy to be eligible for these technologies. Our insulin pump training program is a structured series of three classes, offered to all patients regardless of race.21 Nevertheless, it is possible that outside of these three structured classes the non-Black pediatric patients are provided more help in using CSII. Indeed, the pediatric clinic has a robust Latino program implemented by one of our authors (AGG) while the adult clinic does not and thus we may have been observing the impact of this program among the pediatric Hispanic patients rather than a specific lack among the Black pediatric patients. Though the sample size was small and thus power to detect a significant difference was limited, the automated insulin delivery system appeared more effective in lowering HbA1c in Black children, with the same 5% point reduction in HbA1c compared with non-users as was observed for Hispanic children.
Automated insulin delivery systems reduce HbA1c, increase time in range (70–180 mg/dL), and reduce time above and below range.14,29–32 We have previously shown that AID use improved HbA1c levels, glycemic management and time spent in the appropriate glycemic range in our adult population and was associated with lower A1c in our pediatric population.21,29 In the current study as well, among diabetes technology users, AID use was associated with further reductions in HbA1c in adults but only among non-Hispanic Black and non-Hispanic White patients. In our pediatric adolescent population, AID use was associated with lower HbA1c in all race/ethnic groups except non-Hispanic Black patients. In contrast to the 1% point difference in glycemic control among Black adults using an AID system, only a 0.5% difference in HbA1c levels was observed in Black adolescent AID users and non-users. This lack of a significant difference in HbA1c among Black adolescents using an AID vs not using AID may be related to the small numbers of Black patients in our population since the same 0.5% point difference in HbA1c associated with AID use was also observed in Hispanic patients.
Finally, CSII use and having health insurance other than Medicaid were associated with a reduced likelihood of DKA in our population with no difference by race. Racial and ethnic differences in DKA in the US have been well documented by the T1DX registry and others,2,33–37 with higher rates generally observed among Black patients, particularly children, with T1D than in other racial/ethnic groups.2,34,35 In our population, we also observed an increased risk of DKA among racial/ethnic minorities, with the greatest excess risk among Hispanic patients compared to non-Hispanic White patients (it should also be noted that some of our patients were part of the T1DX registry). However, despite the higher DKA among Hispanic patients, CSII reduced DKA similarly among Hispanic patients. CSII was associated with a significantly 27% lower risk of DKA in our population, an association that did not differ by race or ethnicity. While insulin pump infusion site failure can contribute to DKA genesis, CSII users in our population had lower DKA risk. This may not be an indication of a protective effect of CSII per se, but rather that patients using CSII were more facile diabetes technology users. Similarly, AID may help the less engaged users get more exogenous insulin and thus reduce DKA risk, though we did not observe differences in DKA by AID use. The strong 60% reduction in DKA associated with having private, military or other type of health insurance besides Medicaid and its attenuation of the association of race with DKA underscores how SES factors other than access to health care and having health insurance affect critical health outcomes in diabetes.
Strengths and Limitations
Strengths of this study included its large sample size, with over 5000 patients who were being treated at a single diabetes center. The diversity of the population being treated at this single center is another strength, as nearly 30% of the patents were Hispanic, Black, or of other non-White race/ethnic groups, allowing for racial comparisons in diabetes technology use and this effect on glycemic control among patients being treated at the same health care center and thus theoretically minimizing differences in healthcare center practices. We also had a large population of both pediatric and adult patients and were able compare racial differences by age group. While we had information on the patients’ insurance status and type, a limitation is that other socioeconomic data such as education and income were not available. Thus, while in theory health insurance in Colorado, including Medicaid, is supposed to cover diabetes technology for all Colorado children with T1D, in practice other costs associated with these technologies, such as co-payments, may limit their adoption by youth belonging to lower income families. The lack of information on education and household income also limited our ability to assess whether disparities in racial technology use might be due to differential prescribing practices among physicians to patients in the same income and education strata. Thus, while provider bias may have played an important role in the racial differences in diabetes technology use among our population, provider bias in prescribing technology is not easy to capture. Another limitation was our reliance on electronic health record data. As such, misclassification of type 1 diabetes is possible; however, this is unlikely to have changed our results of racial differences in diabetes technology use and their associations with HbA1c. We also used EHR data to capture which patients were using diabetes technology and the type of technology they were using; however, we did not have information about adherence to these devices among the users. High adherence to the use of CGM and AID has been shown to be critical to HbA1c improvement.38 Finally, data presented in this report are from a single diabetes center serving a very large geographical area and patients may go to an outside emergency department or hospital outside of our network to be treated for DKA, thus our results on the relationship between diabetes technology use and DKA may be underestimated if these visits to outside clinics happened more often among those not using diabetes technology. Additionally, being a single diabetes center, our results may not be generalizable to sites nationwide.
Conclusion
Use of diabetes technologies for the management of glycemia in T1D appears to be associated with lower HbA1c in all race/ethnic groups, though the association is minimal in Black youth and most pronounced among Black adults. Factors associated with medical insurance or medical insurance type appear to be barriers to technology use among all race/ethnic groups, most pronounced among Black youth, despite diabetes technology being free of charge for Medicaid users in Colorado, suggesting that provider bias may play a role. Additionally, despite having a robust, culturally relevant pediatric Latino program, we still struggle to get diabetes technology usage in this population approaching the rates in non-Hispanic Whites. Thus, more needs to be done to decrease provider bias and increase technology acceptability among all racial/ethnic groups and insured groups. Given the apparent reduced risk of DKA with CSII use regardless of race/ethnicity and the reduction in HbA1c with technology use, efforts to increase utilization of these technologies among minority populations should be strongly encouraged.
Acknowledgments
We thank Bing Wang for database support.
Funding
Supported by the University of Colorado Diabetes Research Center Clinical Resources Core NIH, NIDDK grant P30-DK116073.
Disclosure
VNS’ employer, University of Colorado, has received research supports from NovoNordisk, Insulet, Tandem Diabetes Care, Dexcom, JDRF and NIH and received honoraria for advisory works for NovoNordisk, Medscape, Lifescan, and DKSH Singapore and from Dexcom, Embecta and Insulet for speaking.
HKA’s employer, University of Colorado, received research support from Dexcom, Eli Lilly, Senseonics, Medtronic, Tandem, IM Therapeutics, REMD Biotherapeutics, received honoraria for advisory board attendance for Mannkind and Senseonics. GF reports grant and/or personal fees from Medtronic, Dexcom, Tandem, Insulet, Abbott, Lilly, and Beta Bionics, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Agarwal S, Kanapka LG, Raymond JK, et al. Racial-ethnic inequity in young adults with type 1 diabetes. J Clin Endocrinol Metab. 2020;105(8):e2960–9. PubMed PMID: 32382736; PMCID: PMC7457963. doi:10.1210/clinem/dgaa236
2. Willi SM, Miller KM, DiMeglio LA, et al. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135(3):424–434. PubMed PMID: 25687140; PMCID: PMC4533245. doi:10.1542/peds.2014-1774
3. Agarwal S, Schechter C, Gonzalez J, Long JA. Racial-ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diabetes Technol Ther. 2021;23(4):306–313. PubMed PMID: 33155826; PMCID: PMC7994432. doi:10.1089/dia.2020.0338
4. Valenzuela JM, La Greca AM, Hsin O, Taylor C, Delamater AM. Prescribed regimen intensity in diverse youth with type 1 diabetes: role of family and provider perceptions. Pediatr Diabetes. 2011;12(8):696–703. PubMed PMID: 21457425. doi:10.1111/j.1399-5448.2011.00766.x
5. Paris CA, Imperatore G, Klingensmith G, et al. Predictors of insulin regimens and impact on outcomes in youth with type 1 diabetes: the SEARCH for Diabetes in Youth study. J Pediatr. 2009;155(2):183–9.e1. PubMed PMID: 19394043. doi:10.1016/j.jpeds.2009.01.063
6. Lin MH, Connor CG, Ruedy KJ, et al. Race, socioeconomic status, and treatment center are associated with insulin pump therapy in youth in the first year following diagnosis of type 1 diabetes. Diabetes Technol Ther. 2013;15(11):929–934. PubMed PMID: 23869706; PMCID: PMC3817890. doi:10.1089/dia.2013.0132
7. Shah VN, Akturk HK, Joseph H, Schneider N, Snell-Bergeon JK. A randomized controlled trial of transition from insulin pump to multiple daily injections using insulin degludec. Diabetes Obes Metab. 2021;23(8):1936–1941. PubMed PMID: 34180122. doi:10.1111/dom.14423
8. Dubé MC, Lavoie C, Weisnagel SJ. Glucose or intermittent high-intensity exercise in glargine/glulisine users with T1DM. Med Sci Sports Exerc. 2013;45(1):3–7. PubMed PMID: 22895370. doi:10.1249/MSS.0b013e31826c6ad3
9. Fonseca VA, Grunberger G, Anhalt H, et al. Continuous glucose monitoring: a consensus conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract. 2016;22(8):1008–1021. PubMed PMID: 27214060. doi:10.4158/ep161392.Cs
10. Maahs DM, Chase HP, Westfall E, et al. The effects of lowering nighttime and breakfast glucose levels with sensor-augmented pump therapy on hemoglobin A1c levels in type 1 diabetes. Diabetes Technol Ther. 2014;16(5):284–291. PubMed PMID: 24450776. doi:10.1089/dia.2013.0227
11. Yardley JE, Iscoe KE, Sigal RJ, Kenny GP, Perkins BA, Riddell MC. Insulin pump therapy is associated with less post-exercise hyperglycemia than multiple daily injections: an observational study of physically active type 1 diabetes patients. Diabetes Technol Ther. 2013;15(1):84–88. PubMed PMID: 23216304. doi:10.1089/dia.2012.0168
12. Berget C, Akturk HK, Messer LH, et al. Real-world performance of hybrid closed loop in youth, young adults, adults and older adults with type 1 diabetes: identifying a clinical target for hybrid closed-loop use. Diabetes Obes Metab. 2021;23(9):2048–2057. PubMed PMID: 34010499. doi:10.1111/dom.14441
13. Akturk HK, Giordano D, Champakanath A, Brackett S, Garg S, Snell-Bergeon J. Long-term real-life glycaemic outcomes with a hybrid closed-loop system compared with sensor-augmented pump therapy in patients with type 1 diabetes. Diabetes Obes Metab. 2020;22(4):583–589. PubMed PMID: 31789447. doi:10.1111/dom.13933
14. Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(3):155–163. PubMed PMID: 28134564; PMCID: PMC5359676. doi:10.1089/dia.2016.0421
15. Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018;392(10155):1321–1329. PubMed PMID: 30292578; PMCID: PMC6182127. doi:10.1016/s0140-6736(18)31947-0
16. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316(13):1407–1408. PubMed PMID: 27629148. doi:10.1001/jama.2016.11708
17. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707–1717. PubMed PMID: 31618560; PMCID: PMC7076915. doi:10.1056/NEJMoa1907863
18. Snyder LL, Stafford JM, Dabelea D, et al. Socio-economic, demographic, and clinical correlates of poor glycaemic control within insulin regimens among children with Type 1 diabetes: the SEARCH for diabetes in youth Study. Diabet Med. 2019;36(8):1028–1036. PubMed PMID: 31050009; PMCID: PMC6635011. doi:10.1111/dme.13983
19. Akturk HK, Agarwal S, Hoffecker L, Shah VN. Inequity in racial-ethnic representation in randomized controlled trials of diabetes technologies in type 1 diabetes: critical need for new standards. Diabetes Care. 2021;44(6):e121–e3. PubMed PMID: 34016613; PMCID: PMC8247501. doi:10.2337/dc20-3063
20. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311–320. PubMed PMID: 20587585. doi:10.1056/NEJMoa1002853
21. Sawyer A, Sobczak M, Forlenza GP, Alonso GT. Glycemic control in relation to technology use in a single-center cohort of children with type 1 diabetes. Diabetes Technol Ther. 2022;24(6):409–415. PubMed PMID: 35099306; PMCID: PMC9208858. doi:10.1089/dia.2021.0471
22. Forlenza GP, Vigers T, Berget C, et al. Predicting success with a first-generation hybrid closed-loop artificial pancreas system among children, adolescents, and young adults with type 1 diabetes: a model development and validation study. Diabetes Technol Ther. 2022;24(3):157–166. PubMed PMID: 34780306; PMCID: PMC8971998. doi:10.1089/dia.2021.0326
23. Cobry EC, Hamburger E, Jaser SS. Impact of the hybrid closed-loop system on sleep and quality of life in youth with type 1 diabetes and their parents. Diabetes Technol Ther. 2020;22(11):794–800. PubMed PMID: 32212971; PMCID: PMC7698988. doi:10.1089/dia.2020.0057
24. Fazeli Farsani S, Brodovicz K, Soleymanlou N, Marquard J, Wissinger E, Maiese BA. Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): a systematic literature review. BMJ Open. 2017;7(7):e016587. PubMed PMID: 28765134; PMCID: PMC5642652. doi:10.1136/bmjopen-2017-016587
25. Lyerla R, Johnson-Rabbett B, Shakally A, Magar R, Alameddine H, Fish L. Recurrent DKA results in high societal costs - a retrospective study identifying social predictors of recurrence for potential future intervention. Clin Diabetes Endocrinol. 2021;7(1):13. PubMed PMID: 34332631; PMCID: PMC8325863. doi:10.1186/s40842-021-00127-6
26. Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. PubMed PMID: 8366922. doi:10.1056/nejm199309303291401
27. Lachin JM, Orchard TJ, Nathan DM. Update on cardiovascular outcomes at 30 years of the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):39–43. PubMed PMID: 24356596; PMCID: PMC3868002. doi:10.2337/dc13-2116
28. Cleary PA, Orchard TJ, Genuth S, et al. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes. 2006;55(12):3556–3565. PubMed PMID: 17130504; PMCID: PMC2701297. doi:10.2337/db06-0653
29. Kaur H, Schneider N, Pyle L, Campbell K, Akturk HK, Shah VN. Efficacy of hybrid closed-loop system in adults with type 1 diabetes and gastroparesis. Diabetes Technol Ther. 2019;21(12):736–739. PubMed PMID: 31347928. doi:10.1089/dia.2019.0254
30. Choudhary P, Kolassa R, Keuthage W, et al. Advanced hybrid closed loop therapy versus conventional treatment in adults with type 1 diabetes (ADAPT): a randomised controlled study. Lancet Diabetes Endocrinol. 2022;10(10):720–731. PubMed PMID: 36058207. doi:10.1016/s2213-8587(22)00212-1
31. Ware J, Boughton CK, Allen JM, et al. Cambridge hybrid closed-loop algorithm in children and adolescents with type 1 diabetes: a multicentre 6-month randomised controlled trial. Lancet Digit Health. 2022;4(4):e245–e255. PubMed PMID: 35272971. doi:10.1016/s2589-7500(22)00020-6
32. Messer LH, Forlenza GP, Sherr JL, et al. Optimizing hybrid closed-loop therapy in adolescents and emerging adults using the miniMed 670G system. Diabetes Care. 2018;41(4):789–796. PubMed PMID: 29444895; PMCID: PMC6463622. doi:10.2337/dc17-1682
33. Everett E, Mathioudakis N. Association of area deprivation and diabetic ketoacidosis readmissions: comparative risk analysis of adults vs children with type 1 diabetes. J Clin Endocrinol Metab. 2019;104(8):3473–3480. PubMed PMID: 31220288; PMCID: PMC6599429. doi:10.1210/jc.2018-02232
34. Bergmann KR, Nickel A, Hall M, et al. Association of neighborhood resources and race and ethnicity with readmissions for diabetic ketoacidosis at US Children’s hospitals. JAMA Netw Open. 2022;5(5):e2210456. doi:10.1001/jamanetworkopen.2022.10456
35. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682–690. PubMed PMID: 21325184; PMCID: PMC3118568. doi:10.1001/jama.2011.122
36. Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID-19 related hospitalizations in children with type 1 diabetes. J Diabetes. 2021;13(8):681–687. PubMed PMID: 33855813; PMCID: PMC8251108. doi:10.1111/1753-0407.13184
37. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755–e1762. PubMed PMID: 33410917; PMCID: PMC7928931. doi:10.1210/clinem/dgaa920
38. Langendam M, Luijf YM, Hooft L, Devries JH, Mudde AH, Scholten RJ. Continuous glucose monitoring systems for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2012;1(1):Cd008101. doi:10.1002/14651858.CD008101.pub2
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


