Back to Journals » Journal of Asthma and Allergy » Volume 16
Racial and Ethnic Minorities at the Highest Risk of Uncontrolled Moderate-to-Severe Asthma: A United States Electronic Health Record Analysis
Authors George M , Camargo Jr CA , Burnette A, Chen Y, Pawar A, Molony C, Auclair M, Wells MA, Ferro TJ
Received 26 July 2022
Accepted for publication 31 March 2023
Published 12 May 2023 Volume 2023:16 Pages 567—577
DOI https://doi.org/10.2147/JAA.S383817
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Amrita Dosanjh
Maureen George,1 Carlos A Camargo Jr,2 Autumn Burnette,3 Yuning Chen,4 Ajinkya Pawar,4 Cliona Molony,4 Melissa Auclair,4 Michael A Wells,4 Thomas J Ferro4
1Office of Research and Scholarship, Columbia University School of Nursing, New York, NY, USA; 2Department of Emergency Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; 3Division of Allergy and Immunology, Howard University Hospital, Howard University College of Medicine, Washington, DC, USA; 4Sanofi, Cambridge, MA, USA
Correspondence: Maureen George, Office of Research and Scholarship, Columbia University School of Nursing, New York, NY, 10032, USA, Tel +1 212-305-1175, Email [email protected]
Purpose: The identification of risk factors associated with uncontrolled moderate-to-severe asthma is important to improve asthma outcomes. Aim of this study was to identify risk factors for uncontrolled asthma in United States cohort using electronic health record (EHR)-derived data.
Patients and Methods: In this retrospective real-world study, de-identified data of adolescent and adult patients (≥ 12 years old) with moderate-to-severe asthma, based on asthma medications within 12 months prior to asthma-related visit (index date), were extracted from the Optum® Humedica EHR. The baseline period was 12 months prior to the index date. Uncontrolled asthma was defined as ≥ 2 outpatient oral corticosteroid bursts for asthma or ≥ 2 emergency department visits or ≥ 1 inpatient visit for asthma. A Cox proportional hazard model was applied.
Results: There were 402,403 patients in the EHR between January 1, 2012, and December 31, 2018, who met the inclusion criteria and were analyzed. African American (AA) race (hazard ratio [HR]: 2.08), Medicaid insurance (HR: 1.71), Hispanic ethnicity (HR: 1.34), age of 12 to < 18 years (HR 1.20), body mass index of ≥ 35 kg/m2 (HR: 1.20), and female sex (HR 1.19) were identified as risk factors associated with uncontrolled asthma (P < 0.001). Comorbidities characterized by type 2 inflammation, including a blood eosinophil count of ≥ 300 cells/μL (as compared with eosinophil < 150 cells/μL; HR: 1.40, P < 0.001) and food allergy (HR: 1.31), were associated with a significantly higher risk of uncontrolled asthma; pneumonia was also a comorbidity associated with an increased risk (HR: 1.35) of uncontrolled asthma. Conversely, allergic rhinitis (HR: 0.84) was associated with a significantly lower risk of uncontrolled asthma.
Conclusion: This large study demonstrates multiple risk factors for uncontrolled asthma. Of note, AA and Hispanic individuals with Medicaid insurance are at a significantly higher risk of uncontrolled asthma versus their White, non-Hispanic counterparts with commercial insurance.
Keywords: asthma, underserved, uncontrolled, Medicaid
Introduction
Asthma is one of the most common chronic diseases, affecting >300 million people worldwide.1 Among the 26 million Americans with asthma, approximately 5% to 10% of patients have severe asthma. In about half of these patients, severe asthma remains uncontrolled.2 In the United States (US), the total annual economic burden of asthma (2008–2013) was estimated at $81.9 billion, of which 61% was associated with medical costs and the remaining was attributable to either absenteeism or mortality.3 The total economic burden of uncontrolled asthma in the US during the next 20 years is estimated to be $963.5 billion when indirect costs are included, and adults and adolescents are expected to lose >15 million quality-adjusted life years (QALYs) during the next two decades because of uncontrolled asthma.4
Type 2 inflammation, found in at least 50% of people with severe asthma, is an important disease mechanism,5 characterized by immunoglobulin E (IgE)-mediated allergy, eosinophilia (lung, sputum, and/or blood), and/or increased fractional exhaled nitric oxide (FeNO) levels.6 Type 2 asthma is associated with co-morbidities, such as chronic rhinosinusitis with nasal polyps, atopic dermatitis, and eosinophilic esophagitis, which are also driven by Type 2 inflammation.6
Socioeconomic and insurance status, asthma severity, and prior healthcare utilization have been shown to predict the risk of hospitalization,3,5–8 in the US. Thus, identifying patients who are at the higher risk for uncontrolled asthma and proactively applying targeted interventions to high-risk asthma patients could lead to reduced asthma morbidity and mortality.8–12 There are few large, longitudinal studies that have examined factors contributing to asthma exacerbations and these studies have shown patient characteristics such as race and ethnicity to be predictors of uncontrolled asthma.13–15 Electronic health record (EHR)-derived data offer convenient and low-cost access to information for the study of asthma exacerbations and comorbidity patterns among asthma patients over longer time periods than is possible in clinical trials.13–15
The objective of this study was to identify risk factors for uncontrolled asthma in adolescent and adult patients with moderate-to-severe asthma using the Optum® Humedica EHR-derived data.
Materials and Methods
Study Design and Patients
In this retrospective real-world study, de-identified EHR-derived data of adolescent and adult patients (≥12 years old) with moderate-to-severe asthma were used. Patients were identified based on asthma medications prescribed within the 12 months prior to an asthma-related visit (index date) as follows: (1) medium- to high-dose inhaled corticosteroid (ICS) plus ≥1 additional controller medication, or (2) oral corticosteroids (OCS) for ≥90 days (OCS-dependent asthma), or (3) ≥3 OCS prescriptions for asthma within the 12 months prior to an asthma-related visit (OCS-dependent asthma). Patients were required to have ≥12 months of EHR data coverage before and after the index date. Uncontrolled asthma was defined as asthma with any of the following: (1) ≥2 outpatient OCS bursts for asthma within any 12-month period (defined as <14 days of OCS in an outpatient setting with asthma diagnosis within 7 days of the corticosteroid prescription date); (2) ≥2 emergency department (ED) visits; or (3) ≥1 hospitalization/inpatient visit for asthma. Patients were excluded if they had a diagnosis of chronic obstructive pulmonary disease.
The baseline period was the 12 months prior to the index date. The follow-up period was the ≥12-month period after the index date. As this study met the preapproved criteria for real-world evidence under an umbrella institutional review board (IRB) of Sanofi (protocol number: AA_00029), this study was exempt from obtaining IRB approval and informed patient consent because it constitutes research of anonymized data. Further, preapproval criteria’ suggests the exemption of IRB for real-world studies such as this utilizing secondary data ie claims/EHR data from Optum (instead of primary data of patients collected for research purposes).
Data Sources and Collection
Data were extracted from the Optum® Humedica EHR database of US patients with Medicaid or commercial insurance from January 1, 2012, to December 31, 2018. Optum® Humedica is a US EHR database with >95 million patient lives. The Humedica longitudinal clinical repository combines data from >50 US integrated delivery networks (IDNs) and includes >700 hospitals and 7000 clinics, covering patients from all US census regions and with all types of insurances. The following data were collected at baseline: (1) asthma medication use, including additional medication-related information, such as counts of prescriptions, fills and cumulative days’ supply; (2) days’ supply, prescription counts, and fills for short-acting beta agonists (SABA) and ICS; (3) sociodemographic characteristics; (4) medical history; and (5) clinical and disease characteristics, including asthma exacerbation-related information and comorbidities (coded in ICD-9 or ICD-10 post-October 2015).
Uncontrolled Asthma
The following variables were extracted during the 12-month baseline period to assess risk factors associated with uncontrolled asthma: (1) sociodemographic variables, such as age, sex, race (African-American [AA], Asian, White, other/unknown), ethnicity (Hispanic, non-Hispanic, and unknown), insurance type (Medicaid and commercial), body mass index) BMI (<25, 25 to <30, 30 to <35, and ≥35 kg/m2), and smoking status (current smoker, former smoker, non-smoker, and unknown); (2) asthma-related healthcare resource utilization (HCRU), such as outpatient visits, ED visits, and inpatient visits (hospitalizations); (3) blood eosinophil count:<150 cells/μL, 150 to <300 cells/μL, and ≥300 cells/μL; (4) filled prescription for medications/treatments, such as SABA use, OCS bursts, flu vaccine, and ICS use; (5) comorbidities including chronic rhinosinusitis, eosinophilic esophagitis, allergic conjunctivitis, hives, depression, anxiety, obesity, food allergy, atopic dermatitis, nasal polyps, allergic rhinitis (AR), pneumonia, and acute sinusitis; (6) Charlson comorbidity index (CCI) score (0, 1, 2, ≥3). CCI is the most used index in health outcomes studies, which assigns a weight ranging from 1 to 6 according to disease severity for 19 conditions.16
Statistical Analysis
A Cox proportional hazard model was applied to discover factors associated with uncontrolled asthma, adjusting for baseline characteristics, including age, sex, race, ethnicity, insurance, asthma clinical factors (eg, OCS dependence), and other clinical factors (eg, smoking, BMI, depression, and anxiety). The current analysis is a multivariable analysis, ie all risk factors are included in the Cox proportion hazard model and assessed at the same time. P < 0.001 was considered the threshold for statistical significance. Primary analysis was performed on patients with uncontrolled asthma defined as OCS≥3, ED≥2, and Hospitalization (Hosp) ≥1. Sensitivity analyses were performed to analyze the impact of the differing definitions of uncontrolled asthma in our findings: (1) OCS ≥2, ED ≥1, Hosp ≥1; (2) OCS≥3, ED≥2, and Hosp ≥1; (3) OCS ≥3 and ED ≥1, and Hosp ≥1. Data were analyzed using Python 3.6 and module lifeline.
Results
There were 402,403 patients in the EHR between January 1, 2012, and December 31, 2018, who met the inclusion criteria and were included for analysis. Most patients were female (68%), non-Hispanic (90%), White (79%), and non-smokers (41%). The most common asthma medications were ICS (93%), long-acting bronchodilator inhaler (LABA) (80%), and SABA (73%). Demographic and baseline characteristics are shown in Table 1. The results are based on longitudinal analyses.
 |
Table 1 Patient Characteristics During 12-Month Baseline Period |
Sociodemographic Factors
The following sociodemographic variables were associated with a significantly increased risk of uncontrolled asthma: AA race, smoking, Medicaid insurance, adolescent age, Hispanic ethnicity, female sex, and obesity. AA and Medicaid insurance showed the greatest association with a 2.08 (95% CI 2.00–2.16) and 1.71 (95% CI 1.65–1.78) -times increased risk of uncontrolled asthma, respectively (Table 2). The results of sensitivity analyses were consistent with the primary analysis for sociodemographic-related risk factors (eFigure S1).
 |
Table 2 Summary of Sociodemographic Related Risk Factors |
Healthcare Utilization
All asthma related HCRU, such as outpatient visits, ED visits, and inpatient visits were associated with a significantly increased risk of uncontrolled asthma (Table 3). Inpatient and ED visits showed a 2.01 (95% CI 1.92–2.11) - and 1.41 (95% CI 1.40–1.43) -times increased risk of uncontrolled asthma, respectively, whereas outpatient visit showed a smaller relative risk of 1.03 of uncontrolled asthma. The results of sensitivity analyses were consistent with the primary analysis for HCRU-related risk factors (eFigure S2).
 |
Table 3 Summary of Risk Factors Related to Healthcare Resource Utilization |
Eosinophils Count
Elevated blood eosinophil count (150 to <300 cells/μL and ≥300 cells/μL) was associated with a significantly increased risk of uncontrolled asthma (Table 4). This indicates that the patients with blood eosinophil count of 150 to <300/μL and ≥300/μL were significantly more likely to be diagnosed with uncontrolled asthma than patients with blood eosinophil count of <150 cells/μL. Blood eosinophil count of ≥300 cells/μL showed the greatest association with a 1.40 (95% CI 1.34–1.47)-times increased risk of uncontrolled asthma. The results of sensitivity analyses were consistent with that of the primary analysis for blood eosinophil count-related risk factors (eFigure S3).
 |
Table 4 Summary of Risk Factors Related to Eosinophil Count |
Comorbidities
Among comorbidity-related risk factors, pneumonia, food allergy, and anxiety/depression were associated with a significantly increased risk of uncontrolled asthma by 1.35 (95% CI 1.27–1.44), 1.31 (95% CI 1.16–1.49) and 1.10 (95% CI 1.06–1.14) -times, respectively (Table 5). AR was associated with a significantly lower risk of uncontrolled asthma, with a hazard ratio of 0.84 (95% CI 1.06–1.14). The results of sensitivity analyses were consistent with that of the primary analysis for comorbidities-related risk factors (eFigure S4).
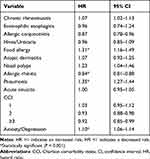 |
Table 5 Summary of Risk Factors Related to Comorbidities |
Baseline Medication
The use of SABA and OCS at baseline were associated with a significantly increased risk of uncontrolled asthma (Table 6). The use of SABA and OCS bursts showed an increased risk of uncontrolled asthma by 1.04 (95% CI 1.04–1.04)- and 1.45 (95% CI 1.41–1.50)-times, respectively. Use of ICS and administration of the flu vaccine were associated with a significantly lower risk of uncontrolled asthma, with HR of 0.91 (95% CI 0.89–0.93) and 0.90 (95% CI 0.86–0.93), respectively (Table 6). The results of sensitivity analyses were consistent with that of the primary analysis for baseline medication-related risk factors (eFigure S5).
 |
Table 6 Summary of Risk Factors Related to Baseline Medication |
Discussion
Using the Optum® Humedica EHR with >400,000 patients with asthma, we found a higher risk of uncontrolled asthma among patients (1) of AA race, (2) with Medicaid insurance, (3) of adolescent age, (4) of Hispanic ethnicity, (5) of female sex, (6) who are current or previous smokers, (7) with obesity (BMI ≥ 35 kg/m2), (8) with type 2 comorbidities (food allergy, nasal polyposis, and chronic rhinosinusitis) or biomarkers (elevated eosinophil count), and (9) with HCRU. To our knowledge, this is the largest recent study with large mix of Medicaid and commercial insurance highlighting the leading risk factors associated with uncontrolled asthma using an EHR database.
Our results are generally consistent with earlier findings of demographic risk for uncontrolled asthma. Previous studies have shown an increased risk of acute exacerbation of asthma in AA and Hispanic patients.17,18 Further, our results are consistent with the findings showing that AA race was significantly and positively associated with severe asthma exacerbation (4.3-fold increased risk).19 Our findings in Medicaid patients are consistent with a previously published study20 that showed a significant association of Medicaid insurance with poorly controlled asthma. Our study also showed that the risk of uncontrolled asthma was higher in the adolescent age range (12 to <18 years) than in the elderly. This finding may be attributable to lower adherence among adolescent patients, a lower likelihood of receiving inhaled steroids, lower self-monitoring, and/or lack of connection with an asthma care provider.21–23 This issue warrants further study, but a detailed analysis of uncontrolled asthma in minority adolescents is beyond the scope of the current study. In the present study, female sex was associated with an increased risk of uncontrolled asthma, consistent with previously published studies24,25 reporting that adolescent females were more likely to report severe and uncontrolled asthma, with frequent visits to healthcare professionals. The obesity-related asthma phenotype in female patients is associated with severe symptoms and preserved lung function; molecular mechanisms include oxidative stress, airway neutrophil activation, and increased innate immune activity.26 Current or previous smokers were also identified to be at higher risk of uncontrolled asthma in this study. Although the potential mechanisms underlying smoking-associated asthma are unclear, T2-low neutrophilic and steroid-resistant phenotypes may be considered.27
We found that certain co-morbidities are associated with an increased risk of uncontrolled asthma. Anxiety/depression was associated with a significantly increased risk of uncontrolled asthma, consistent with a previous real-life study indicating that these are common and relevant comorbidities in individuals with asthma and are associated with uncontrolled asthma.28 Furthermore, the present study shows that the risk of uncontrolled asthma is 1.40 times greater in patients who had blood eosinophil count of ≥300 cells/μL than in patients with blood eosinophil count of <150 cells/µL. This finding is consistent with previous work,29 which reported that a high blood eosinophil count was an independent risk factor for two or more asthma exacerbations, or any asthma-related ED visit or hospitalization. Type 2 inflammation is the key characteristic feature of uncontrolled, persistent asthma and several other inflammatory diseases, including chronic rhinosinusitis and AR, which can manifest as distinct or comorbid diseases with asthma.6 In the present study chronic rhinosinusitis was found to be associated with higher risk of uncontrolled asthma. However, in contrast to the previous findings where chronic rhinosinusitis was significantly (OR: 1.45, 95% CI 1.06–1.99, P=0.02) associated with increased risk of uncontrolled asthma,30 this association was not significant in the present study. Among other comorbidities-related risk factors, risk of uncontrolled asthma was significantly lower in patients who had AR. This is not consistent with the previous reports, in which the patients with moderate-to-severe rhinitis had a 12.7-fold increase (95% confidence interval [CI], 1.73–92.85) in the odds of having uncontrolled asthma compared with those without rhinitis.31 The contradictory findings could possibly be due to greater exposure of patients, included in our study, to the healthcare system and, thus, more regular touchpoints with the health system for disease management including asthma. The nature of this finding is beyond the scope of this study and should be investigated further. Also, the use of ICD coded data to identify comorbidities may have limited accuracy which might have led to overlap in reporting of these comorbidities, including chronic rhinosinusitis and AR. The present study showed that asthma-related healthcare utilization, such as inpatient visits or ED visits, was also highly associated with uncontrolled asthma risk. These findings are consistent with the TENOR study showing that the occurrence of a recent severe exacerbation requiring an ED/hospital admission increased the odds of a future exacerbation nearly three-fold, compared with patients with exacerbations not requiring admission.32
Limitation
The definitive interpretation of our study data may be limited by certain methodologic considerations, including the definition of uncontrolled asthma, the inherent variability of uncontrolled asthma, and the use of an EHR. Our definition of uncontrolled asthma is consistent with international guidelines33,34 as we have captured the components of the uncontrolled asthma definition which are considered outcomes (eg, OCS use, ED visits, and hospitalization); therefore, our calculations may be an underestimate of uncontrolled disease in this patient population as non-outcome-related measures, eg, daily utilization of SABA, were not feasible. We conducted sensitivity analyses to mitigate the inherent variability in the definition of uncontrolled asthma because there is no gold standard definition for uncontrolled asthma. The results from the sensitivity analyses were generally consistent with those from the primary analysis.
By using the Optum® Humedica EHR data, we were able to identify a large sample of patients with moderate-to-severe asthma. Long-term follow-up, breadth of coverage, and representativeness of the US population allowed for considerable statistical power while drawing robust conclusions about the risk factors associated with uncontrolled asthma. In the present study, ZIP code-level data were not available in the Optum® Humedica EHR database, so we could not analyze data geographically. The role of these risk factors in uncontrolled asthma in various patient populations (eg, urban vs rural) merits further investigation.
Conclusion
This large study demonstrates multiple risk factors for uncontrolled asthma. Of note, AA and Hispanic individuals with Medicaid insurance are at a significantly higher risk of uncontrolled asthma versus their White, non-Hispanic counterparts with commercial insurance. These findings may assist healthcare providers in identifying patients who are at the highest risk for uncontrolled asthma, such as AA adolescent females with Medicaid insurance, and may need closer and more frequent follow up to ensure their asthma is adequately controlled. Additionally, these data may assist providers in identifying patients who may warrant additional on adequate management of their asthma.
Abbreviations
AA, African-American; AR, allergic rhinitis; BMI, Body mass index; CCI, Charlson comorbidity index; ED, emergency department; EHR, electronic health record; FeNO, fractional exhaled nitric oxide; HR, hazard ratio; HCRU, healthcare resource utilization; IgE, immunoglobulin E; ICS, inhaled corticosteroid; IDNs, integrated delivery networks; IRB, institutional review board; LABA, long-acting bronchodilator inhaler; OCS, oral corticosteroids; QALYs, quality-adjusted life years; SABA, short-acting beta agonists; US, United States.
Data Sharing Statement
The qualified researchers may request access to patient-level data and related documents [such as clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications]. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://vivli.org/.
Ethics Approval and Informed Consent
Since this study met the preapproved criteria for real-world evidence under an umbrella institutional review board (IRB) protocol, this study was exempt from obtaining IRB approval and informed patient consent because it constitutes research of anonymized data.
Acknowledgments
The authors thank Fen Ye, PhD, for initial study planning, and Rakesh Ojha, PhD, of Sanofi for medical writing support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was sponsored by Sanofi.
Disclosure
George M: AstraZeneca: Speaker and consultant; Teva, Genentech, and Sanofi: Consultant. Camargo CA: AstraZeneca and Sanofi: Scientific advisory board member; GSK: Consultant. Burnette A: Sanofi, Regeneron, Amgen, and AstraZeneca: Member of scientific advisory board member and Speaker; GSK, Sanofi, Regeneron, and Amgen: Consultant.
Chen Y: Former employee of Sanofi at the time of the study conduct and may hold stock and/or stock options in the company. Chen Y is currently employee of Meta Platforms, Inc., Hacker Way, Menlo Park, CA, USA.
Pawar A: Current employee of Sanofi and may hold stock and/or stock options in the company.
Molony C: Former employee of Sanofi at the time of the study conduct and may hold stock and/or stock options in the company. Molony C is currently employee of IDEXX, Westbrook, ME, USA.
Auclair M: Former employee of Sanofi at the time of study conduct and may hold stock and/or stock options in the company; current employee for Novartis Pharmaceuticals.
Wells MA: Former employee of Sanofi at the time of study conduct and may hold stock and/or stock options in the company; current employee/Shareholder for Novartis Pharmaceuticals.
Ferro TJ: Former employee of Sanofi at the time of the study conduct and may hold stock and/or stock options in the company. Ferro TJ is currently employee of Sun Pharma, Princeton, NJ, USA.
The authors report no other conflicts of interest in this work.
References
1. The Global Asthma Report 2018. Auckland, New Zealand: Global Asthma Network; 2018. Available from: http://globalasthmareport.org/resources/Global_Asthma_Report_2018.pdf.
2. Hankin CS, Bronstone A, Wang Z, Small MB, Buck P. Estimated prevalence and economic burden of severe, uncontrolled asthma in the United States. J Allergy Clin Immunol. 2013;131(Suppl 2):AB126. doi:10.1016/j.jaci.2012.12.1118
3. Nurmagambetov T, Kuwahara R, Garbe P. The Economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348–356. doi:10.1513/AnnalsATS.201703-259OC
4. Yaghoubi M, Adibi A, Safari A, FitzGerald JM. The projected economic and health burden of uncontrolled asthma in the United States. Am J Respir Crit Care Med. 2019;200(9):1102–1112.
5. Fahy JV. Type 2 inflammation in asthma — present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65.
6. Busse WW. Understanding the key issues in the treatment of uncontrolled persistent asthma with type 2 inflammation. Eur Respir J. 2021;58(2):2003393.
7. Papaioannou AI, Kostikas K, Zervas E, Kolilekas L, Papiris S, Gaga M. Control of asthma in real life: still a valuable goal? Eur Respir Rev. 2015;24(136):361–369.
8. National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138.
9. Jackson JE, Doescher MP, Hart LG. A National Study of Lifetime Asthma Prevalence and Trends in Metro and Non-Metro Counties, 2000-2003; 2007. Working paper posted on the WWAMI Rural Health Research Center website. Available from: https://familymedicine.uw.edu/rhrc/wp-content/uploads/sites/4/2021/10/RHRC_WP108_Jackson.pdf.
10. Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim. Care Respir J. 2007;16(1):22–27.
11. Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol. 2011;127(1):145–152.
12. Denlinger LC, Phillips BR, Ramratnam S, et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017;195(3):302–313.
13. Himes BE, Dai Y, Kohane IS, Weiss ST, Ramoni MF. Prediction of chronic obstructive pulmonary disease (COPD) in asthma patients using electronic medical records. J Am Med Inform Assoc. 2009;16(3):371–379.
14. Himes BE, Kohane IS, Ramoni MF, Weiss ST. Characterization of patients who suffer asthma exacerbations using data extracted from electronic medical records. AMIA Annu Symp Proc. 2008;1:308–312.
15. Xie S, Greenblatt R, Levy MZ, Himes BE. Enhancing electronic health record data with geospatial information. AMIA Jt Summits Transl Sci Proc. 2017;2017:123–132.
16. Glasheen WP, Cordier T, Gumpina R, Haugh G, Davis J, Renda A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am Health Drug Benefits. 2019;12(4):188–197.
17. Grossman NL, Doros GD, Fandino N, et al. Susceptibility to exacerbations in Black adults with asthma. J Asthma. 2019;56(7):704–710.
18. Lee DS, Gross E, Hotz A, Rastogi D. Comparison of severity of asthma hospitalization between African American and Hispanic children in the Bronx. J Asthma. 2020;57(7):736–742.
19. Rumpel JA, Ahmedani BK, Peterson EL, et al. Genetic ancestry and its association with asthma exacerbations among African American subjects with asthma. J Allergy Clin Immunol. 2012;130(6):1302–1306.
20. Camargo CA Jr, Ramachandran S, Ryskina KL, Lewis BE, Legorreta AP. Association between common asthma therapies and recurrent asthma exacerbations in children enrolled in a state Medicaid plan. Am J Health Syst Pharm. 2007;64(10):1054–1061.
21. DiMango E, Rogers L, Reibman J, et al. Risk factors for asthma exacerbation and treatment failure in adults and adolescents with well-controlled asthma during continuation and step-down therapy. Ann Am Thorac Soc. 2018;15(8):955–961.
22. Bruzzese J-M, Stepney C, Fiorino EK, et al. Asthma self-management is sub-optimal in urban Hispanic and African American/black early adolescents with uncontrolled persistent asthma. J Asthma. 2012;49(1):90–97.
23. Lintzenich A, Teufel RJ 2nd, Basco WT. Younger asthmatics are less likely to receive inhaled corticosteroids and asthma education after admission for exacerbation. Clin Pediatr (Phila). 2010;49(12):1111–1116.
24. Borges RC, Alith MB, Nascimento OA, Jardim JR. Gender differences in the perception of asthma respiratory symptoms in five Latin American countries. J Asthma. 2022;59(5):1030–1040.
25. Cydulka RK, Emerman CL, Rowe BH, et al. Differences between men and women in reporting of symptoms during an asthma exacerbation. Ann Emerg Med. 2001;38(2):123–128.
26. Kuruvilla ME, Lee FE, Lee GB. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin Rev Allergy Immunol. 2019;56(2):219–233.
27. Takahashi K, Pavlidis S. Ng Kee Kwong F, et al. Sputum proteomics and airway cell transcripts of current and ex-smokers with severe asthma in U-BIOPRED: an exploratory analysis. Eur Respir J. 2018;51(5):463.
28. Ciprandi G, Schiavetti I, Rindone E, Ricciardolo FLM. The impact of anxiety and depression on outpatients with asthma. Ann Allergy Asthma Immunol. 2015;115(5):408–414.
29. Zeiger RS, Schatz M, Dalal AA, et al. Blood eosinophil count and outcomes in severe uncontrolled asthma: a prospective study. J Allergy Clin Immunol Pract. 2017;5(1):144–153.e8.
30. Peters SP, Jones CA, Haselkorn T, et al. Real-world Evaluation of Asthma Control and Treatment (REACT): findings from a national Web-based survey. J Allergy Clin Immunol. 2007;119(6):1454–1461.
31. Ponte EV, Franco R, Nascimento HF, et al. Lack of control of severe asthma is associated with co-existence of moderate-to-severe rhinitis. Allergy. 2008;63(5):564–569.
32. Miller MK, Lee JH, Miller DP, Wenzel SE. Recent asthma exacerbations: a key predictor of future exacerbations. Respir Med. 2007;101:481–489.
33. GINA report 2019. Available from: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf.
34. Chun KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
