Back to Journals » Journal of Pain Research » Volume 14
Quercetin Alleviates Neuropathic Pain in the Rat CCI Model by Mediating AMPK/MAPK Pathway
Authors Ye G, Lin C, Zhang Y, Ma Z, Chen Y, Kong L, Yuan L , Ma T
Received 24 December 2020
Accepted for publication 23 April 2021
Published 19 May 2021 Volume 2021:14 Pages 1289—1301
DOI https://doi.org/10.2147/JPR.S298727
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Guangyao Ye,1,* Chunyan Lin,1,* Yu Zhang,1 Zihan Ma,2 Yuebo Chen,1 Lingsi Kong,1 Liyong Yuan,1 Tao Ma3
1Department of Anesthesiology, Ningbo No.6 Hospital, Ningbo, 315040, People’s Republic of China; 2School of Medicine, Ningbo University, Ningbo, 315211, People’s Republic of China; 3Department of Anesthesiology and Pharmacology, Xuzhou Medical University, Xuzhou, 221000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liyong Yuan
Department of Anesthesiology, Ningbo No.6 Hospital, Ningbo, 315040, People’s Republic of China
Email [email protected]
Tao Ma
Department of Anesthesiology and Pharmacology, Xuzhou Medical University, Xuzhou, 221000, People’s Republic of China
Email [email protected]
Context: Quercetin (que) is one abundant flavonol with a variety of biological activities. Previous studies have shown quercetin can reduce neuropathic pain in rats with chronic constriction injury (CCI).
Objective: To evaluate the effects of quercetin on neuropathic pain in CCI model and explore its underlying mechanism in vivo.
Materials and Methods: CCI model was established by ligating the sciatic nerve of right leg on the SD rats. They were divided into ten groups: sham group, CCI model, sham+ que, CCI+ que group (30, 60, 120 mg/kg), CCI+ AICAR, CCI+ que+ compound C, CCI+etoricoxib, and the control group. They were administered for 28 days, and were performed the mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) during the experiment. At the end of the experiment, sciatic nerves and spinal cord segments of rats were collected, ELISA detected the expression of inflammatory factors, detected the microglia and astrocytes with fluorescence, and Western blot detected AMPK/MAPK pathway.
Results: Que could increase the MWT of CCI rats, improve the TWL of plantar, and reduce the inflammatory cells at the ligation site of the sciatic nerve. Also, que could reduce the levels of TNF-α, IL-6, and IL-1β. Western blotting results showed that p-38 MAPK, p-ERK, and p-JNK were activated in the spinal dorsal horn of CCI model group. After treatment with que and AMPK agonists, the phosphorylation levels of related proteins were inhibited. In addition, the analgesic effect of que was abolished when the AMPK inhibitor was added.
Discussion and Conclusion: Quercetin alleviated the inflammatory response of sciatic nerve and spinal dorsal horn in rats induced by CCI. Quercetin alleviates neuralgia in CCI rats by activating AMPK pathway and inhibiting MAPK pathway and its downstream targets, p-38, p-ERK, and p-JNK.
Keywords: quercetin, neuropathic pain, AMPK, MAPK, CCI
Introduction
Neuropathic pain (NPP) is a type of chronic pain which is ascribed to somatosensory nervous system damage or diseases, such as nerve compression, pathological changes, autoimmune diseases, and incisions.1 The annual incidence of NPP is about 7–8%.2 It is mainly manifested as spontaneous pain and hyperalgesia. The commonly used drugs in NPP’s clinical treatment include antiepileptic drugs, antidepressants, and opioid analgesics, but their effects are limited, and there are many adverse reactions.3 Therefore, it is of great significance to develop new NPP drugs with sound analgesic effects and few adverse reactions.
In recent years, research on NPP’s mechanism has revealed that activation of adenosine monophosphate-activated protein kinase (AMPK) could inhibit two signaling pathways which are closely related to central sensitization and changes in neuronal synaptic plasticity after nerve injury. They are the mammalian target of rapamycin (mTOR) and mitogen-activated protein kinases (MAPK), which could inhibit the occurrence and development of chronic pain.4 MAPK is a protein serine/threonine kinase. This vital signal transduction protein receives signals from membrane receptors and carries them into the nucleus. It regulates the transcription and translation through protein phosphorylation.5,6 When the MAPK family is activated, it will increase the excitability of spinal cord dorsal horn (SCDH) microglia and astrocytes. It also increases the synthesis and releases of inflammatory factors, enhances excitatory synaptic transmission, and increases neurons’ excitability, central sensitization, including pain allergies and hyperalgesia allodynia, which can produce an aggravated pain response to stimuli outside the area of injury.7,8 Therefore, some scholars have proposed that AMPK can be used as a new target of analgesics for research and development.9,10 Studies have shown that the plant monomer quercetin (que) has anti-inflammatory, neuroprotective, and analgesic effects,11 and has relatively mild adverse reactions.12 Furthermore, quercetin’s pharmacological effect is achieved by activating AMPK,13,14 and it has a significant analgesic effect on a variety of chronic pain.13–15 However, whether quercetin mediates MAPK-related analgesia through AMPK and which MAPK signaling pathways are more closely related to these problems still need further research.
In this study, we proposed the following hypothesis: quercetin may stimulate the AMPK signal transduction pathway, causing changes of the key proteins in MAPK signal transduction pathway, including p38, p-JNK, and p-ERK, thereby inhibiting NPP. We will establish chronic constriction injury of sciatic (CCI) rats, evaluate the pain behavior, clarify the analgesic effect of quercetin on NPP rats, and combine molecular biology techniques to explore its mechanism.
Materials and Methods
Experimental Reagents
Acadesine (AICAR, A838530), quercetin (Q817161), etoricoxib (E831542), compound C (D864421) were all purchased from Shanghai MACKLIN; TNF-α (Cat no. SEKR-0009), IL-1β (Cat no. SEKR-0002), IL-6 (Cat no. SEKR-0005) content was detected by ELISA kit (Solarbio, Beijing, China). Antibodies: Iba1 Rabbit mAb (Cat no. 17198), AMPKα Antibody (Cat no. 2532), phospho-AMPKα (Thr172) (40H9) Rabbit mAb (Cat no. 2535), JNK (Cat no. 9252), p-JNK (Thr183/Tyr185) Cat no. 9251, ERK, p-ERK (Thr202/Tyr204) (Cat no. 4377), p38 MAPK Antibody (Cat no. 9212) and p-p38 (Thr180/Tyr182) (Cat no. 9212), and HRP-labeled secondary antibody were all purchased from Cell Signaling Technology.
Animals
SPF Sprague-Dawley (SD) male rats (120 rats), body weight 250–300 g, were provided by Shanghai JieSiJie Laboratory Animal Co., Ltd., and the production license number was SCXK (Shanghai) 2018–0004. The rats were reared in an independent environment with 12 h day/night, kept at room temperature (24 ± 1°C), left freely to drink and eat, and adapted to the environment for 1 week in advance. All experimental operations follow the Ethics Committee of the International Pain Research Association regulations. The experiment was approved by the Animal Care and Use Committee of the Medical School of Ningbo University.
CCI Model Construction
With reference to Bennett’s16 method, the following steps were followed: using 350 mg/kg chloral hydrate to anesthetize the rat, we fixed it on the operating table, prepared and sterilized the skin of the right thigh, and cut the middle and back of the right thigh skin, bluntly separated the muscle, exposed the upper and middle section of the sciatic nerve, freed the nerve from the bifurcation of the peroneal nerve, surrounded the nerve with 4-0 sutures, made 4 light ligation loops with a spacing of 1 mm, and tied the knot with the strength, by which it would not affect the epineurium’s blood supply, and the thigh muscle twitches or kick reflex would be retained. After spraying the penicillin powder locally for disinfection, the muscle, skin, and incision were sutured with silk thread. The rats in the Sham group did not ligate the sciatic nerve, and the remaining steps were the same as those in the model group. A series of self-protection behaviors, such as licking, biting, or throwing the foot, occurred in animals with obvious spontaneous pain (spontaneously lifting the injured limbs, sometimes licking the feet, biting the feet, or kick) and the threshold of withdrawal threshold (MWT) and thermal withdrawal latency (TWL) are significantly decreased compared with the control group, indicating the model was successful.
Animal Grouping and Treatment
After the establishment of the CCI model and sham group, all experimental rats were divided into 10 groups, 10 rats in each group (100 of the 120 rats were selected for the follow-up experiment, excluding death due to surgery or other causes). 1) The normal control group (control), without any treatment; 2) Sham operation group (sham), the sciatic nerve is not ligated, other steps are the same as the CCI model; 3) Sham operation + 120 mg/kg que treatment group (sham+ que120). In addition to not ligating the sciatic nerve, gavage 120 mg/kg que; 4) CCI model group (CCI model), ligating the sciatic nerve; 5) CCI+30 mg/kg que treatment group (CCI+que 30); 6) CCI+60 mg/kg que treatment group (CCI+que60), 7) CCI+120 mg/kg que treatment group (CCI+que120); 8) CCI+AMPK agonist (CCI+AICAR) group, 15 μg/rat AICAR was injected, 9) CCI+AMPK inhibitor (CCI+que+ Compound C) group, 120 mg/kg que orally, 20 μg/rat compound C was injected by syringe injection; 10) in the CCI+5.5 mg/kg etoricoxib treatment group, CCI+5.5 mg/kg etoricoxib was administered intragastrically. Seven days after establishing the CCI model and the successful model being confirmed by MWT and TWL test, the drug treatment was started and continued until 28 days after the operation. For administration of compound C and AICAR, the needle is inserted into the skin between the greater trochanter and the nerve ligation site of the rat, and the needle went forward and medially, then the compound was injected along the fan-shaped area of the sciatic nerve.
Behavior Tests
Bio-signal acquisition system (MINITR, Nanjing Calvin Biotechnology Co., Ltd.) and Von-Frey pain test kit (CA91367, San Diego Instruments. Inc) were used on the 1st day before the operation and on the 7th, 14th, and 28th day after the operation. Von-Frey and the reaction time of hot and cold plate measured the mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) of each rat group. Experiments are carried out in Am 8:00~12:00. The laboratory was kept quiet and the room temperature was maintained at 24 ± 1°C. Five days before the start of the experiment (before operation), the rats were placed in the observation box for 30 min every day to adapt the rats to the observation box’s environment.
MWT determination: Place a transparent plexiglass box with a length of 30 cm in length, width, and height on a 30 cm high shelf, and the top of the shelf is a wire mesh with small holes. Place the rat to be tested in the box. After the rat adapts to being quiet, the experimenter holds the Von-Frey and vertically stimulates the middle of the sole of the rat’s hind limbs through the wire mesh to make it slightly s-shaped for 4–6 s. The rat’s rapid foot-lifting response during the stimulation time or when the Von-Frey is recorded as a positive reaction. The foot-lifting response caused by the animal’s limb movement is not included in the positive category. It is measured once every 30 s for 5 consecutive times. The average value of the pressure value corresponding to Von-Frey is used as the foot lift threshold. Use 15 g Von-Frey as the maximum folding force. When the measured value is more than 15 g, record 15 g.
TWL measurement: Put the rat in the radiant heat pain meter’s glass box to ensure that the rat can move freely throughout the body. After the rat is allowed to stand for 10 min, focus on the left and right heels of the rat with the same intensity of intense light, and record the time from the start of the light to the time the rat withdraws the foot. The measurement is performed every 5 min, and the measurement is three times in total. The average value of the results, to evaluate the thermal hyperalgesia of rats. In order to prevent heat radiation burns, the upper limit of TWL is set to 25 s.
HE Staining
After the experiment, the L4-L5 spinal cord segments and sciatic nerves of each group of rats were fixed with neutral formaldehyde, and then dehydrated and paraffin-embedded to make 5 μm tissue sections. Dewax xylene, rehydrated with a gradient concentration of alcohol, stained with hematoxylin and eosin, dehydrated with conventional alcohol, after xylene is transparent, sealed with neutral gum, observed and took pictures under an optical microscope.
Immunofluorescence
On the 28th day after CCI modeling, rats were anesthetized by intraperitoneal injection of chloral hydrate (350 mg/kg) after the behavioral test. The tissue was removed, rinsed with PBS (pH 7.40, 0.02 M), and then 4% paraformaldehyde phosphate buffer was added to fix the tissue. The L4-L5 spinal cord segment was taken from the sciatic nerve’s ligation site, fixed in the fixative mentioned above solution for 6–8 h, and dehydrated with sucrose PBS buffer (0.01 M, 30%) at 4°C. Then, it was embedded and fixed, sectioned with a cryostat (5 μm), washed with PBS for 5 min × 3; citrate buffer (pH 6.0) for antigen retrieval; incubated with autofluorescence quencher for 15 min at room temperature, and blocked with 3% BSA for 30 min. Using goat anti-Iba-1 antibody, p-JNK (1:100) antibody was incubated, washed with PBS for 15 min ×3, and the sections were incubated with appropriate secondary antibodies for 50 min at room temperature. The sections were washed with PBS, mounted with a fluorescent sealant, observed with a fluorescent microscope, and photographed with OPLENIC software. IPP 6.0 image software calculates and analyzes the fluorescence intensity. The expressions of rat SCDH microglia (Iba-1), Iba1 and p-JNK were detected.
Western Blot
The L4-L5 spinal cord segment was taken from the sciatic nerve’s ligation site, the tissue was homogenized, and dissolved in lysis buffer (containing 1 mM phenylmethylsulfonyl fluoride and protease inhibitor). After centrifugation at 12,000 rpm 4°C for 10 min, the supernatant was collected and stored at −80°C. Coomassie brilliant blue method was used to determine protein concentration. The same number of protein samples was taken from each group, separated by SDS-PAGE, and transferred to the PVDF membrane. Five percent skim milk was sealed at room temperature for 2 h. Rabbit anti-p-AMPK (1:1000), anti-AMPK (1:1000), rabbit anti-p-p38 (1:1000), anti-p38 (1:1000), rabbit anti-p-ERK (1:1000), anti-ERK (1:1000), anti-JNK (1:1000), and rabbit anti-p-JNK (1:1000) were, respectively, incubated overnight at 4°C. It was then incubated with horseradish peroxidase-labeled goat anti-rabbit secondary antibody (1:2000) for 1 h at room temperature. The protein bands with chemiluminescence reagents were observed, and the protein content was analyzed with Image J. The ratio of the gray value of the protein band to the gray value of the GAPDH band reflects p-AMPK, p-ERK, p-JNK, and p-38 content, and co-expression with microglia or astrocytes, using β-actin as an internal control.
Enzyme-Linked Immunosorbent Assay (ELISA)
The L4-L5 spinal cord segment was taken from the sciatic nerve ligation side and stored in liquid nitrogen for inspection. The sampled tissues were homogenized in cold phosphate buffer, centrifuged at 10,000 rpm for 15 min, and the supernatant was taken to detect the contents of TNF-α, IL-6, and IL-1β by ELISA. The operation steps strictly followed the requirements of the ELISA kit.
Statistical Analysis
All data were presented as the mean ± or + standard error of the mean. Differences between multiple groups were analyzed by one-way analysis of variance followed by Duncan’s multiple range test. Differences between the two groups were measured by Student’s t-test, using SPSS 22.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of Quercetin on MWT and TWL Values in Rats
From the morphology of the sole of the rat, the model group showed obvious contraction of the foot (Figure S1). Compared with control, the model’s threshold decreased significantly (P<0.01), indicating that the CCI model was successfully established and can be used in subsequent experiments. It can be seen that quercetin had no significant effect on MWT and TWL in the Sham group (Figure 1A and B). Furthermore, from the 7th day after the operation, the MWT and TWL of the quercetin treatment group were significantly higher than that of the model (P<0.05). However, there was no significant difference in MWT between the 60 mg and 120 mg quercetin treatment + CCI groups on the 14th and 28th days (P>0.05). There was a significant difference between +AICAR and CCI+Compound C (P<0.05), indicating that AMPK pathway affects MWT. Similarly, there was no significant difference in TWL between the 60 mg and 120 mg quercetin treatment + CCI groups on the 14th and 28th days (P>0.05), but it was lower than control and CCI+etoricoxib; the difference was statistically significant (P<0.05). The results showed that quercetin could improve mechanical pain threshold (MWT) and thermal pain tolerance (TWL) of plantar in CCI rats.
Quercetin Alleviates Neuropathological Injury Induced by CCI
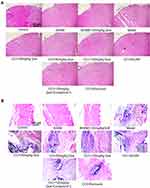
As shown in Figure 2A, HE staining results of the spinal dorsal horn showed that in the model there were many ruptures in the tissues of the spinal dorsal horn, suggesting the possibility of edema. Nissella bodies are rare, with obvious neuronal atrophy (the green box in the figure of the model group is the dorsal horn of the spinal cord), the number of neurons recovered after 120 mg/kg quercetin treatment. Compound C, on the other hand, showed obvious bulky nissella bodies in the gray matter of the spinal cord (cells shown as green arrows). Combined with the results of HE staining of sciatic nerve (Figure 2B), large areas of sciatic nerve necrosis were observed in the model, accompanied by a large number of inflammatory cell infiltration, and nerve bundles were arranged disorderly. After treatment with quercetin, the arrangement of nerve bundles was significantly improved compared with the model group, and inflammatory cell infiltration was reduced. The number of inflammatory cells was also significantly improved after treatment with AMPK agonists, and the etoricoxib group showed a good anti-inflammatory effect.
Quercetin Inhibits Inflammation by Inhibiting Microglial Activation in CCI Rats
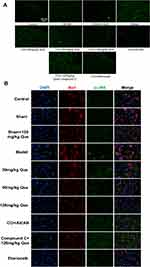
On day 28 after surgery, p-JNK was used as an indicator of immunofluorescence results (Figure 3A), which indicated that phosphorylated JNK was widely expressed in the MODEL. Meanwhile, after quercetin treatment, the level of JNK phosphorylation was negatively correlated with quercetin concentration. Subsequently, the phosphorylation levels of Iba1 and JNK in SCDH were investigated by immunofluorescence double antibody staining. It was found that IBA1 was widely distributed in SCDH, and the number of microglia positively expressing p-JNK in CCI + que group was lower than that in model group (Figure 3B). These data suggest that Iba1 is widely distributed in SCDH of rats. In the quercetin treated group, the phosphorylation level of JNK was inhibited, suggesting that quercetin can inhibit the phosphorylation level of JNK in the sciatic nerve.
Quercetin Down-Regulated the MAPK Pathway in CCI Rats
Western blot results are shown in Figure 4A. On the 28th day after surgery, p-JNK, p-38, and p-ERK in CCI model were significantly increased compared with the normal control group. Compared with the model group, quercetin significantly decreased the relative levels of p-JNK, p-38, and p-ERK in the spinal cord of CCI rats (P<0.05, Figure 4). The results show that quercetin can reduce the expression of inflammatory and pain-related molecules in the spinal cord of CCI rats.
Quercetin Ameliorated Inflammatory in CCI Rat
Compared with control group (Figure 5), the inflammation-related factors TNF-α, IL-6, and IL-1β in model increased significantly (P<0.01). Compared with model, adding 60 and 120 mg quercetin can significantly reduce inflammatory factors (P<0.05). Compared with the 120 mg treatment group, after adding Compound C, inflammatory factors increased significantly (P<0.01), and CCI +AICAR is significantly lower than the model group inflammatory factors, and pain threshold is significantly increased. It can be speculated that the cause of pain may be increased inflammatory factors. At the same time, quercetin has a certain effect of inhibiting inflammation (as seen from the comparison between sham+que and Control); compared with the model group, after adding different concentrations of quercetin to the CCI group, the inflammatory factors continued to decrease. After quercetin treatment, both MWT and TWL values increased, which further demonstrated the correlation between inflammatory factors and pain.
Discussion
Neuropathic pain is caused by neuropathy or disease, leading to abnormal spontaneous and induced pain, but there is a lack of effective treatment methods. In this study, we aimed to explore quercetin’s effect on neuropathic pain and its underlying mechanism.
Through behavioral experiments, it was found that quercetin treatment can increase MWT and TWL, and the pathological results show that quercetin can reduce inflammatory cells. Quercetin is reported to have a number of beneficial properties, including antioxidant, analgesic and anti-inflammatory effects. The analgesic effects of quercetin have been demonstrated in several previous studies. Quercetin inhibits nocicidal responses in a mouse pain model induced by capsaicin, glutamate, and formalin (Filho et al). There is nociceptive response in the pain model. The authors suggest that these effects occur through regulation of GABAA, GABAB, and 5-HT receptors and endogenous release of glucocorticoids. Anyaneyulu et al reported that quercetin alleviated diabetic neuralgia in mice by regulating the opioid mechanism (Anjaneyulu et al). In Soner Civi’s report (Civi et al), it was also proved that quercetin alleviated mechanical tenderness and heat sensitivity in CCI rats, but its mechanism of action on quercetin has not been thoroughly studied. At the same time, this paper only verified the effect through the behavior of rats, and did not carry out the pathological analysis on the injury site and the tissue site responsible for nerve pain. In contrast, in this experimental study, the site of injury was also studied. As can be seen from the pathological staining of sciatic nerve from different groups, there were a large number of inflammatory cell infiltrates in the CCI model group. Characteristically, inflammation definitely plays an important role in chronic pain in CCI. Secondly, the spinal dorsal horn, which is responsible for regulating nerve sensation of lower limbs, was analyzed. It was obviously observed that the number of glial cells in the dorsal horn of the spinal cord of the model group was significantly less than that of the normal rats, and a large area of cracks in the green square of the model group was also observed. According to the previous experience analysis of tissue dehydration and section making, under the same dehydration condition, the water content of tissue cells may increase or edema, which may lead to great morphological changes of cells after dehydration, resulting in the phenomenon of broken tissue. From this, we infer that edema of the spinal cord may occur in the rats of the model group, but this still needs to be further analyzed by other experiments.
The core pathological mechanism leading to NPP may be central sensitization and neuronal synaptic plasticity changes after peripheral nerve injury.7,8 Simultaneously, the spinal cord dorsal horn and dorsal root ganglia pro-inflammatory factors released after activating neurons, microglia, and astrocytes are the most important factors,17,18 Our study also observed relevant results, and quercetin treatment reduced the spinal cord dorsal horn and p-JNK. We found that inflammatory factors were elevated in the model group, and quercetin treatment was able to reduce inflammatory factors. Besides, researchers found that TNF-α, IL-1β, IL-6, and microglia were significantly increased in the CCI model. Studies have shown that TNF-α, IL-1β, and IL-6 can induce and maintain hyperalgesia in a rat model.19 TNF-α mediates the central mechanism of neuropathic pain through the glial system to respond to nerve injury and inflammation.20 Microglia secretes pro-inflammatory cytokines, including TNF-α,21 which, through the p38-MAPK pathway, mediate its effect. The above research can explain that the pain of CCI model rats is affected by the above factors. After quercetin treatment, the stinging threshold and thermal pain threshold of rats increased significantly, and the inflammatory factors decreased significantly, indicating that quercetin can achieve the purpose of alleviating pain by reducing inflammatory factors.
The various clinical manifestations of chronic pain are ultimately determined by changes in gene expression.22 Studies have found that MAPK ranks first among the signals involved in NPP after spinal cord injury.23 Analyzing the SCDH gene profile and comparing it with normal rats, it was found that CCI rats have 63 gene expression changes, and all 7 down-regulated gene expressions are associated with MAPK inactivation.24 It can be seen that MAPK is an essential part of the pathological changes of NPP signal pathway. In the CCI model, p-ERK, p-38, and p-JNK were significantly increased, while quercetin treatment significantly reduced their levels. Quercetin has a significant impact on the maximum pain threshold, and the genes related to the maximum pain threshold are p-ERK, p-38, and p-JNK.
The literature shows that JNK and p-38 signal pathways mediate mechanical hyperalgesia induced by SNL or IL-6.25,26 Many active substances or drugs that inhibit NPP are related to inhibiting the activation of MAPK signaling pathways, but the specific MAPK family members involved are slightly different. For example, Resolvin D1 uses NF-κB/p65, ERK signaling pathways to inhibit microglia synthesis and release TNF-α and IL- 1β, thereby reducing NPP.27 Transforming growth factor-β1 (TGF-β1) inhibits ERK activation and p-38 in CCI rats, thereby inhibiting the activation of SCDH microglia and astrocytes, inhibiting neuroinflammatory responses, and producing analgesic effects.5 Catestatin polypeptide activates p-38 and ERK and aggravates the mechanical and thermal hyperalgesia of NPP.28 Curcumin exerts analgesic effects by inhibiting the activation of SCDH neuron astrocytes and ERK in CCI rats,29 paeoniflorin (PF) and albiflorin (AF) inhibit the spinal cord of CCI rats p-38 activity can reduce the synthesis and release of pro-inflammatory cytokines such as IL-1β and TNF-α in astrocytes, and can also significantly reduce the content of p-JNK in astrocytes.30 In short, the MAPK subfamily ERK, JNK, and p-38 signal pathways are closely related to the occurrence and development of NPP. However, there are still differences in the specific activation/inhibitory factors and the precise targets of analgesic drugs. Therefore, to study the ERK, JNK, and p-38 signaling pathways will help understand drug action.
AMPK plays a crucial role in regulating the homeostasis of systemic energy metabolism. Studies have found that the AMPK agonist metformin inhibits SCDH microglia activation by activating the AMPK pathway and effectively reduces NPP in SNL rats. Ge et al found that metformin can effectively reverse the content of p-AMPK and p-STAT3 in SCDH of CCI rats, and the activity of microglia and astrocytes is excessively enhanced. Resveratrol can reduce the hyperalgesia performance of trigeminal neuralgia rats lazily, and at the same time, reduce the content of TNF-α, IL1-β, and other inflammatory factors in the spinal trigeminal nucleus (STN). The activities of glial cells and microglia are inhibited. In vivo and in vitro experiments have found that resveratrol produces anti-inflammatory effects and improves NPP by activating AMPK and inhibiting MAPK activity pathways.31 Clinically, ozone (ozone) used for NPP treatment has a good analgesic effect on CCI rats by activating the AMPK pathway.32 By inhibiting the activation of JNK and ERK, and promoting the phosphorylation of AMPK at the same time, urolithin B inhibits lipopolysaccharide (LPS)-induced microglial activation to produce an important signaling pathway mechanism,33 which can also be allosteric. AMPK is activated to achieve analgesic effect.34
The relationship between AMPK and MAPK is also seen in various pains. ERK, MAPK family member, can activate various ion channels closely related to pain such as Kv4.2, Cav2.2, and Nav1.7. Activation of AMPK can inhibit ion channels. Activity further reduces the excitability of neuronal cells.35 After the intragastric administration of quercetin, the plasma inflammatory cytokine IL-1β, IL-6, and TNF-α levels decreased in rats.36 Quercetin also inhibits the phosphorylation levels of NF-κB, p-38, ERK 1/2, and SAPK/JNK in prostate tissue, indicating that the NF-κB/MAPKs signaling pathway is partly involved in the anti-inflammatory and antioxidant effects of quercetin. Quercetin has a significant analgesic effect on NPP and can increase the threshold of mechanical and thermal hypersensitivity pain in CCI rats.37 And the analgesic effect of NPP is related to the inhibition of DRG glial cell activity.38 Studies have found that the MAPK signaling pathway is involved in the analgesic effect of quercetin. For example, Ji et al39 found that quercetin can reduce the phosphorylation level of MAPK family members TAK1, IKK, and JNK, and TAK1 mediates the inhibitory effect of quercetin on NF-κB activity, and the p-38 MAPK pathway is involved. Thus the analgesic effect of quercetin on diabetic NPP rats.40
Therefore, we speculated that quercetin indirectly affected the expression of related proteins in the MAPK pathway by stimulating the AMPK pathway, thereby inhibiting NPP in CCI rats. In addition, inflammation was involved in the pathological process of NPP, and inflammatory factors (IL-1β, IL-6, and TNF-α) were significantly increased in CCI model rats. We demonstrated that inhibiting the production of inflammatory factors could alleviate NPP. This study showed that quercetin has anti-inflammatory effects, could reduce inflammatory factors caused by CCI injury, and reduce the expression of pain-related proteins. Quercetin is widely sold and used as a safe supplement. In a rodent trial, quercetin supplementation at doses ranging 3–3000 mg/day for 28 days showed no significant changes in standard toxicological parameters and was not found in histopathological examinations of various organs (María et al). In conclusion, quercetin treatment could significantly inhibit inflammation of sciatic nerve in CCI model rats, increase the threshold of TWL and MWT, and alleviate sciatic nerve pain. Quercetin can be used as a supplementary food drug with high safety, and may play an important role in the future treatment of NPP.
Acknowledgments
This project is funded by the Natural Science Foundation of Ningbo (No.2019A610281).
Disclosure
There are no conflicts of interest in this study.
References
1. Bannister K, Sachau J, Baron R, Dickenson AH. Neuropathic pain: mechanism-based therapeutics. Annu Rev Pharmacol Toxicol. 2020;60(1):257–274. doi:10.1146/annurev-pharmtox-010818-021524
2. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599–1606. doi:10.1097/j.pain.0000000000000492
3. Binder A, Baron R. The pharmacological therapy of chronic neuropathic pain. Dtsch Arztebl Int. 2016;113(37):616–625. doi:10.3238/arztebl.2016.0616
4. Price TJ, Dussor G. AMPK: an emerging target for modification of injury-induced pain plasticity. Neurosci Lett. 2013;557 Pt A:9–18. doi:10.1016/j.neulet.2013.06.060
5. Chen NF, Chen WF, Sung CS, et al. Contributions of p38 and ERK to the antinociceptive effects of TGF-beta1 in chronic constriction injury-induced neuropathic rats. J Headache Pain. 2016;17(1):72. doi:10.1186/s10194-016-0665-2
6. Moy JK, Khoutorsky A, Asiedu MN, et al. The MNK-eIF4E signaling axis contributes to injury-induced nociceptive plasticity and the development of chronic pain. J Neurosci. 2017;37(31):7481–7499. doi:10.1523/JNEUROSCI.0220-17.2017
7. Trang T, Beggs S, Salter MW. ATP receptors gate microglia signaling in neuropathic pain. Exp Neurol. 2012;234(2):354–361. doi:10.1016/j.expneurol.2011.11.012
8. Yang KY, Bae WS, Kim MJ, et al. Participation of the central p38 and ERK1/2 pathways in IL-1beta-induced sensitization of nociception in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:98–104. doi:10.1016/j.pnpbp.2013.07.004
9. Carling D. AMPK signalling in health and disease. Curr Opin Cell Biol. 2017;45:31–37. doi:10.1016/j.ceb.2017.01.005
10. Marin-Aguilar F, Pavillard LE, Giampieri F, Bullon P, Cordero MD. Adenosine monophosphate (AMP)-activated protein kinase: a new target for nutraceutical compounds. Int J Mol Sci. 2017;18(2):288. doi:10.3390/ijms18020288
11. Oboh G, Ademosun AO, Ogunsuyi OB. Quercetin and its role in chronic diseases. Adv Exp Med Biol. 2016;929:377–387.
12. Singh AK, Kumar S, Vinayak M. Recent development in antihyperalgesic effect of phytochemicals: anti-inflammatory and neuro-modulatory actions. Inflamm Res. 2018;67(8):633–654.
13. Massi A, Bortolini O, Ragno D, et al. Research progress in the modification of quercetin leading to anticancer agents. Molecules. 2017;22(8):1270. doi:10.3390/molecules22081270
14. Qiu L, Luo Y, Chen X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed Pharmacother. 2018;103:1585–1591. doi:10.1016/j.biopha.2018.05.003
15. Britti D, Crupi R, Impellizzeri D, et al. A novel composite formulation of palmitoylethanolamide and quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain models. BMC Vet Res. 2017;13(1):229. doi:10.1186/s12917-017-1151-z
16. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107. doi:10.1016/0304-3959(88)90209-6
17. Popiolek-Barczyk K, Mika J. Targeting the microglial signaling pathways: new insights in the modulation of neuropathic pain. Curr Med Chem. 2016;23(26):2908–2928. doi:10.2174/0929867323666160607120124
18. Tsuda M, Koga K, Chen T, Zhuo M. Neuronal and microglial mechanisms for neuropathic pain in the spinal dorsal horn and anterior cingulate cortex. J Neurochem. 2017;141(4):486–498. doi:10.1111/jnc.14001
19. Huang PC, Tsai KL, Chen YW, Lin HT, Hung CH. Exercise combined with ultrasound attenuates neuropathic pain in rats associated with downregulation of IL-6 and TNF-alpha, but with upregulation of IL-10. Anesth Analg. 2017;124(6):2038–2044. doi:10.1213/ANE.0000000000001600
20. Gruber-Schoffnegger D, Drdla-Schutting R, Honigsperger C, Wunderbaldinger G, Gassner M, Sandkuhler J. Induction of thermal hyperalgesia and synaptic long-term potentiation in the spinal cord lamina I by TNF-alpha and IL-1beta is mediated by glial cells. J Neurosci. 2013;33(15):6540–6551. doi:10.1523/JNEUROSCI.5087-12.2013
21. Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21(5):570–579. doi:10.1097/ACO.0b013e32830edbdf
22. Ray P, Torck A, Quigley L, et al. Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research. Pain. 2018;159(7):1325–1345. doi:10.1097/j.pain.0000000000001217
23. Zhang G, Yang P. Bioinformatics genes and pathway analysis for chronic neuropathic pain after spinal cord injury. Biomed Res Int. 2017;2017:6423021. doi:10.1155/2017/6423021
24. Du H, Shi J, Wang M, An S, Guo X, Wang Z. Analyses of gene expression profiles in the rat dorsal horn of the spinal cord using RNA sequencing in chronic constriction injury rats. J Neuroinflammation. 2018;15(1):280. doi:10.1186/s12974-018-1316-0
25. Polo S, Diaz AF, Gallardo N, Leanez S, Balboni G, Pol O. Treatment with the delta opioid agonist UFP-512 alleviates chronic inflammatory and neuropathic pain: mechanisms implicated. Front Pharmacol. 2019;10:283. doi:10.3389/fphar.2019.00283
26. Ding CP, Guo YJ, Li HN, Wang JY, Zeng XY. Red nucleus interleukin-6 participates in the maintenance of neuropathic pain through JAK/STAT3 and ERK signaling pathways. Exp Neurol. 2018;300:212–221. doi:10.1016/j.expneurol.2017.11.012
27. Liu ZH, Miao GS, Wang JN, Yang CX, Fu ZJ, Sun T. Resolvin D1 inhibits mechanical hypersensitivity in sciatica by modulating the expression of nuclear factor-kappab, phospho-extracellular signal-regulated kinase, and pro- and antiinflammatory cytokines in the spinal cord and dorsal root ganglion. Anesthesiology. 2016;124(4):934–944. doi:10.1097/ALN.0000000000001010
28. Deng Z, Li C, Du E, et al. Catestatin enhances neuropathic pain mediated by P2X4 receptor of dorsal root ganglia in a rat model of chronic constriction injury. Cell Physiol Biochem. 2018;51(2):812–826. doi:10.1159/000495334
29. Ji FT, Liang JJ, Liu L, Cao MH, Li F. Curcumin exerts antinociceptive effects by inhibiting the activation of astrocytes in spinal dorsal horn and the intracellular extracellular signal-regulated kinase signaling pathway in rat model of chronic constriction injury. Chin Med J (Engl). 2013;126(6):1125–1131.
30. Zhou J, Wang L, Wang J, et al. Paeoniflorin and albiflorin attenuate neuropathic pain via MAPK pathway in chronic constriction injury rats. Evid Based Complement Alternat Med. 2016;2016:8082753. doi:10.1155/2016/8082753
31. Yang YJ, Hu L, Xia YP, et al. Resveratrol suppresses glial activation and alleviates trigeminal neuralgia via activation of AMPK. J Neuroinflammation. 2016;13(1):84. doi:10.1186/s12974-016-0550-6
32. Lu L, Pan C, Chen L, et al. AMPK activation by peri-sciatic nerve administration of ozone attenuates CCI-induced neuropathic pain in rats. J Mol Cell Biol. 2017;9(2):132–143. doi:10.1093/jmcb/mjw043
33. Lee G, Park JS, Lee EJ, Ahn JH, Kim HS. Anti-inflammatory and antioxidant mechanisms of urolithin B in activated microglia. Phytomedicine. 2019;55:50–57. doi:10.1016/j.phymed.2018.06.032
34. Asiedu MN, Han C, Dib-Hajj SD, Waxman SG, Price TJ, Dussor G. The AMPK activator A769662 blocks voltage-gated sodium channels: discovery of a novel pharmacophore with potential utility for analgesic development. PLoS One. 2017;12(1):e0169882. doi:10.1371/journal.pone.0169882
35. Stanimirovic J, Obradovic M, Panic A, et al. Regulation of hepatic Na(+)/K(+)-ATPase in obese female and male rats: involvement of ERK1/2, AMPK, and Rho/ROCK. Mol Cell Biochem. 2018;440(1–2):77–88. doi:10.1007/s11010-017-3157-z
36. Meng LQ, Yang FY, Wang MS, et al. Quercetin protects against chronic prostatitis in rat model through NF-kappaB and MAPK signaling pathways. Prostate. 2018;78(11):790–800. doi:10.1002/pros.23536
37. Civi S, Emmez G, Dere UA, Borcek AO, Emmez H. Effects of quercetin on chronic constriction nerve injury in an experimental rat model. Acta Neurochir (Wien). 2016;158(5):
38. Muto N, Matsuoka Y, Arakawa K, et al. Quercetin attenuates neuropathic pain in rats with spared nerve injury. Acta Med Okayama. 2018;72(5):457–465. doi:10.18926/AMO/56243
39. Ji C, Xu Y, Han F, et al. Quercetin alleviates thermal and cold hyperalgesia in a rat neuropathic pain model by inhibiting Toll-like receptor signaling. Biomed Pharmacother. 2017;94:652–658. doi:10.1016/j.biopha.2017.07.145
40. Yang R, Li L, Yuan H, et al. Quercetin relieved diabetic neuropathic pain by inhibiting upregulated P2X4 receptor in dorsal root ganglia. J Cell Physiol. 2019;234(3):2756–2764. doi:10.1002/jcp.27091
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.