Back to Journals » ClinicoEconomics and Outcomes Research » Volume 14
Quantifying the Value of Introducing an Oral Drug Delivery Option for Edaravone: A Review of Analyses Evaluating the Economic Impact of Oral versus Intravenous Formulations
Authors Ronquest NA , Paret K , Lucas A, Ciepielewska M, Hagan M
Received 12 February 2022
Accepted for publication 28 June 2022
Published 27 July 2022 Volume 2022:14 Pages 499—511
DOI https://doi.org/10.2147/CEOR.S359025
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Naoko A Ronquest,1 Kyle Paret,1 Aaron Lucas,1 Malgorzata Ciepielewska,2 Melissa Hagan2
1Health Economics, RTI Health Solutions, Research Triangle Park, Durham, NC, USA; 2Medical Affairs-HEOR/RWE/Publications, Mitsubishi Tanabe Pharma America, Inc, Jersey City, NJ, USA
Correspondence: Naoko A Ronquest, Health Economics, RTI Health Solutions, 3040 East Cornwallis Road, Research Triangle Park, Durham, NC, 27709, USA, Tel +1 919 597 5122, Fax +1 919 541 7222, Email [email protected]
Background: Drug formulation and route of administration can have an impact on not only patients’ quality of life and disease outcomes but also costs of care. It is essential for decision makers to use appropriate economic modeling methods to guide drug coverage policies and to support patients’ decision-making.
Purpose: To illustrate key cost considerations for decision makers in economic evaluation of innovative oral formulations as alternatives to intravenous medication.
Materials and Methods: A structured literature review was conducted using the PubMed database to examine methods used for quantifying the economic impact of introducing a new oral pharmaceutical formulation as an alternative to intravenous medication. To illustrate the methods described in this review, a cost-minimization analysis was conducted to quantify the impact of introducing an oral formulation of a medication originally developed as an intravenous treatment for amyotrophic lateral sclerosis.
Results: We identified 14 published evaluations of oral and intravenous formulations from 10 countries across a variety of disease areas. The identified studies used cost-effectiveness (n=10), cost-minimization (n=2), and cost-calculation (n=2) modeling approaches. All but one (13/14) reported outcomes from payers’ perspective, while societal perspectives were also incorporated in 3 of the reviewed evaluations. One study estimated costs from a public hospital’s perspective. Only a subset of the identified studies accounted for the effects of safety (n=6) or efficacy (n=8) differences on treatment costs when estimating the costs of a formulation choice. Many studies that omitted these aspects did not include rationales for their decisions.
Conclusion: We found significant design variations in published models that estimated the impact of an additional formulation option on the treatment costs to payers and the society. Models need to be accompanied with clear descriptions on rationales for their time horizons and assumptions on how different formulations may affect healthcare costs from the selected perspectives.
Keywords: cost-effectiveness analysis, cost-minimization, decision makers, formulation comparison, amyotrophic lateral sclerosis
Introduction
Drug formulation, dosing regimens, and route of administration can have an impact on patients’ quality of life, disease outcomes, and costs of care. Intravenous (IV), subcutaneous (SC), and intramuscular (IM) injections or infusions of a medication may be medically required or preferred by patients for a variety of reasons. However, due to the need for commitment to more frequent office visits for medication administrations, monitoring for potential injection-site reactions, and other infusion-/injection-specific side effects, many studies have reported patients’ preference for oral medications over medications delivered by other routes of administration.1–3 Because different medication formulations are typically associated with competing benefits and risks, it is critical that patients, providers, and formulary decision makers have a clear path for assessing the value of new formulations.
A variety of recently developed value assessment framework documents recommends that decision makers account for a new treatment’s ability to reduce regimen complexity as part of the contextual considerations for the drug’s benefits or disadvantages.4,5 However, the value of alternative formulations can also be measured quantitatively. For instance, as demonstrated in a review by Stewart et al,6 the impact of formulation alternatives on patient preference and willingness to pay for treatment has been assessed with the use of discrete choice experiments. Economic models have also played an essential role in guiding payers’ coverage policy and to support patients in making the best treatment choice.7–9 As rising healthcare costs and spending are global concerns to payers and the society, it is essential that decision makers have appropriate economic modeling methods to guide the development of drug coverage policies and support patients in making the most cost-effective treatment choice in a timely manner.
In this study, we conducted a structured literature review to identify and examine methods used in published health economic models that quantified the impact of introducing new pharmaceutical formulations. To ensure comparability across studies, our review focused on studies that compared administrations of an oral formulation with an alternative IV formulation.
We also present a case study to quantify the economic impact of introducing an oral formulation of edaravone (Radicava, approved by the Food and Drug Administration [FDA] in May of 2017), for amyotrophic lateral sclerosis (ALS) using a cost-minimization analysis that we developed. ALS is a fatal neurodegenerative disease with rapid progression of symptoms that directly result from degeneration in motor neurons in the spinal cord, brainstem and motor cortex.10–12 Despite the significant clinical, humanistic, and economic burden of this disease to patients and their caregivers,13–15 to date, riluzole and edaravone are the only drugs approved for the treatment of ALS in the United States (US). The case study supplements the literature review and serves as an example to highlight the value of incorporating key cost drivers and importance of describing underlying assumptions in the economic model. This work can inform health outcomes researchers and formulary decision makers regarding what cost attributes to consider when evaluating the value of new formulations.
Materials and Methods
An electronic keyword search of articles published from January 2000 to May 2021 was performed using the PubMed database. The literature search strategy targeted articles that included economic analyses comparing oral versus IV formulations of pharmacotherapy indicated for any condition. The following search terms were used to identify potential studies for inclusion: (“oral”[Title/Abstract] AND “versus”[Title/Abstract] AND “intravenous”[Title/Abstract] AND “cost”[Title/Abstract] AND “benefit”[Title/Abstract] OR “effectiveness”[Title/Abstract] OR “offset”[Title/Abstract] OR “minimization”[Title/Abstract]). From an initial review of the abstracts identified in the electronic searches, a subset of articles was selected for full review. The initial selection of studies was evaluated by another reviewer to confirm inclusion or exclusion. Review studies, articles published in a non-English language, and studies that did not explicitly estimate the economic impact of a treatment due to the formulation difference were excluded. The search strategy and each step of the search process are outlined in Figure 1.
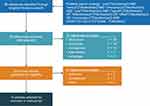 |
Figure 1 PRISMA flow chart and the literature search strategy. Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
In each of the studies, the design, perspective, interventions, and presence or absence of the key cost and healthcare resource measures were reported. Data were extracted from the identified studies, including information about publication year, country, disease area, study comparators, model type, model perspectives, time horizon, and key model outcomes. Outcomes related to healthcare resource use were further classified by the type of resource (eg, related to drug administration, IV maintenance, disease management, efficacy/adherence difference, or adverse events), and cost-related outcomes were grouped into categories of indirect and direct costs. To compare design features across the identified studies, we organized the data into two tables that present key study characteristics. The first table was organized by the methodology used to conduct the analyses and by the country of the study. The second table highlights a subset of studies that captured specific types of healthcare resources and unique types of cost drivers.
Case Study
As a case study illustrating methods described in the literature review, we developed a cost-calculator model that quantifies the effect of introducing an oral formulation of a marketed intravenous treatment for ALS, edaravone, on the costs to payers and the society. An IV form of edaravone was approved as a treatment for ALS by the US FDA in May of 201716 and an oral formulation was approved in May of 2022.17 This example was selected as a case study for several reasons. First, because similar efficacy outcomes between the oral and IV formulations were anticipated, the model could be simplified to assume equal efficacy between treatments, justifying a simple cost-minimization analysis (vs a cost-effectiveness analysis) as an appropriate approach. Second, to estimate the potential societal impact of the availability of a new formulation on patients and their caregivers, an ideal case study involved a situation where a currently available treatment required a substantial time commitment from patients and their caregivers. In the model, IV edaravone was administered for 14 consecutive days, 60-minutes each day, followed by a 2-week treatment-free period. Then, subsequent treatment cycles were repeated as a daily treatment for 10 of the next 14 days, followed by 2 weeks without treatment. We compared and contrasted the methods used to develop the ALS cost calculator to the methods identified in the literature.
Results
Figure 1 illustrates a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram of the search strategy, and the number of articles identified and/or excluded at each step in the search process. A total of 96 unique abstracts were retrieved from the PubMed database search. The dual review of these abstracts identified 26 articles that met the inclusion criteria for our review. After reviewing the full text of the selected 26 articles, 12 were excluded because of issues regarding the types of outcomes reported (n=7), comparisons made (n=3), and documentation of study methods and outcomes (n=2).
Table 1 presents a summary of the study characteristics, organized by study countries, interventions compared, and model design. Of the 14 studies reviewed, 5 were conducted in North America (3 in the US, 2 in Canada), and each of the remaining 9 studies were from different countries across Europe, Asia, and the Middle East (the United Kingdom, France, Italy, Taiwan, China, Japan, Thailand, and Qatar). Four of the 14 reviewed studies were in oncology, and 3 examined treatments of infections. The remaining 7 studies pertained to treatments for a rare autoimmune disease (antibody-associated vasculitis), anemia in patients with chronic kidney disease, anemia in patients with inflammatory bowel disease, a gastrointestinal disease (peptic ulcer hemorrhage), osteoporosis, a retinal disease (cytomegalovirus retinitis), and an infancy heart defect (patent ductus arteriosus).
 |
Table 1 Summary of Identified Studiesa |
All but one (13/14) study, which assessed three different formulations of the same drug (ie, intravitreal injection, IV or oral [or IV followed by oral], and intraocular implantation formulations), compared an oral drug with another drug in an IV formulation indicated for the same condition. With regard to the type of model selected, 8 studies used the decision analytic modeling approach to conduct a cost-effectiveness analysis, 2 studies conducted a cost-effectiveness analysis alongside clinical trials, and the remaining 4 studies used a cost-minimization (n=2) or cost-calculation (n=2) approach. Of the 14 studies, 10 evaluated the economic impact of a formulation choice in a time horizon within 1 year. One study reported outcomes at two different time horizons (at 1 year and at 15 years). The remaining 3 studies had a time horizon of 28 months, 25 years, and lifetime.
Approximately 20% of the studies reviewed (3/14) reported the societal costs of the formulation choice, and the rest of the studies focused on costs to third-party payers (10/14) or hospitals (1/14) only. All reviewed studies incorporated the costs of drug administrations and IV maintenance to estimate the impact of selecting oral versus alternative formulations on direct healthcare costs. Most studies accounted for the time for healthcare providers to administer the IV drug and the resources required for inserting and removing IV lines. Only a subset of the reviewed studies considered the costs of adverse events (n=6) or the different treatment or disease management costs due to differential efficacy (n=8). One study accounted for costs due to differences in treatment adherence based on the formulation choice.
Tables 2–4 summarize studies that included the societal perspective, studies that incorporated costs of treating adverse events, and studies that account for the impact of efficacy differences on direct costs, respectively. Of the three studies that examined results from the societal perspective, all accounted for the wages lost because of reduced productivity, while only two accounted for the cost of travel expenses (Table 2). One study reported the contribution of indirect costs to the overall incremental costs; in a cost-minimization analysis of patients with non-dialysis chronic kidney disease, Riccio et al9 compared differences in costs for treating patients with oral or IV iron supplements for anemia and found that approximately 69% of the total costs were non-direct medical costs (€65.75 [travel costs], €757.44 [productivity loss] vs €1191.25 [total costs]).
 |
Table 2 Studies That Incorporated a Societal Perspective (3 of 14) |
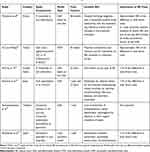 |
Table 3 Studies That Accounted for Costs of Adverse Events (6 of 14)a |
 |
Table 4 Studies That Accounted for Efficacy Differences (8 of 14) |
Less than half (6/14) of the reviewed studies accounted for the costs of adverse events (Table 1). Of the eight studies that did not account for adverse events, only one explained the study’s rationale to assume the equal or similar safety profiles between the treatments. In studies that incorporated the costs of treating adverse events, the proportion of the total costs that accounted for treatment of adverse events varied by studies, but it was typically less than 10% (Table 3). For instance, in a study comparing the costs of oral capecitabine and IV 5-fluorouracil in patients with stage III colorectal cancer in Japan, the researchers found an incremental cost from the treatment of adverse events associated with the oral formulation, but it was less than 1% of the total direct cost savings from the selection of the oral formulation.18 A similar outcome was reported in a US-based cost-effectiveness analysis of IV nivolumab versus oral everolimus for patients with advanced renal cell carcinoma.19 However, a Taiwanese study of stages II and III colorectal cancer reported that the cost savings from lower costs for adverse events were approximately 10% of the total cost savings from patients receiving oral uracil-tegafur.20 Finally, in a comparison of IV rituximab versus oral azathioprine, Montante et al8 estimated the total cost savings for patients who select oral azathioprine instead of IV rituximab for the treatment of antibody-associated vasculitis, but the incremental cost of treating serious adverse events because of an oral azathioprine was estimated to be approximately 30% of the total cost difference.
More than half (8/14) of the identified studies accounted for the effects of efficacy differences on treatment costs when estimating the costs of a formulation choice (Table 1). Among the studies that incorporated efficacy differences, some studies reported a sizable impact of such efficacy differences on the overall direct costs (Table 4). For instance, in a French study comparing IV rituximab with oral azathioprine in patients with antibody-associated vasculitis, 27% of the total costs were driven by efficacy differences.8 Furthermore, in a US-based analysis investigating the cost-effectiveness of an oral versus IV proton pump inhibitor in patients with peptic ulcer hemorrhage,21 two of the three model parameters that were reported to be drivers of the incremental cost-effectiveness ratios were the efficacy variables. Among the remaining studies (6/14) that did not account for efficacy differences, only four explicitly explained that they would be assuming the same efficacy between the comparators; two of these four studies indicated that the compared regimens were shown to have similar efficacy in previous studies.
Case Study – The Economic Impact of Introducing Oral Edaravone to Patients With ALS
In this case study, we illustrate a process of designing a cost-calculator model that estimates the costs of introducing an oral formulation of edaravone as a treatment for ALS over a 3-year time horizon. To estimate the differences in direct costs of treatment of ALS among patients receiving IV edaravone and oral edaravone, we first identified key resources required for the administrations and maintenance of IV edaravone. For IV administrations and maintenance (IV line replacement and removals), we accounted for the hours required for healthcare providers, patients, and their caregivers. Because the hours required for healthcare providers, patients, and their caregivers varied based on whether the IVs were administered at home or outpatient facilities, these resource requirements were separately identified. In addition, the resource requirements for IV removals and reinsertions vary by the methods of catheterization (a peripherally inserted central catheter [PICC] line, implantable port, or a peripheral line). Therefore, these resources were weighted by the distribution of patients who receive each type of IV treatment.
The base-case analysis focused on costs associated with US payers only. However, patients who are prescribed the IV formulation of edaravone devote a significant amount of time to receiving IV infusions. Therefore, the model allowed for an option to account for the direct costs associated with the change in administration only as well as an option to incorporate a broader societal perspective, accounting for indirect costs from the lost productivity of patients and their unpaid caregivers. Figure 2 depicts the overall structure of this model. All key model inputs and their sources are presented in Supplementary Table S-1. Because of the 3-year time horizon, all cost results were undiscounted. We assumed both formulations provide similar efficacy outcomes given the oral formulation’s dose was selected to deliver a similar exposure to edaravone as the IV infusion (MTPA data on file, 2020). Additionally, only infusion-related safety events were differentiated between the formulations, as completed toxicology studies for the oral formulation demonstrated no new safety findings at the selected dose (MTPA data on file, 2020). The model accounted for indirect costs because of lost wages as a result of travel and appointment time for IV administrations and maintenance only.
 |
Figure 2 ALS case study model design. Abbreviations: ALS, amyotrophic lateral sclerosis; IV, intravenous. |
As presented in Figure 3, the cost-calculator model estimated oral edaravone would save each patient and his or her caregivers approximately $13,700 in direct costs in year 1. Including indirect costs, the cost savings in year 1 were approximately $16,000. The number of IV administrations (approximately 134, 130, and 130 infusions in years 1, 2, and 3) drove the economic burden of administrations in office and hospital settings. Although the cost of each insertion was lower with peripheral line compared with PICC line and implantable port, the number of reinsertions each year (approximately 27 in year 1 and 26 each year in years 2 and 3) drove the costs of peripheral-line maintenance. In addition to administration and insertion/removal, IV-related adverse events also had a small contribution to the total cost of selecting the IV formulation compared with switching to the oral formulation.
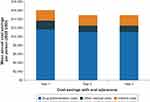 |
Figure 3 Mean average cost savings per person with oral edaravone. Abbreviation: USD, United States dollars. |
Discussion
In our review of studies that quantified the economic impact of introducing an oral formulation drug as an alternative regimen to an IV formulation, we confirmed that all 14 studies we identified accounted for the costs of providers’ time to administer IV medications. Most studies used a simple model structure with a time horizon of 1 year or less, estimating the high-level economic impact of choosing a different formulation. Most studies we reviewed (11 of 14) accounted for costs that may stem from differences in safety or efficacy profiles associated with changes in formulations. However, when studies did not account for these differences, few (1 of 8 that omitted safety considerations, 2 of 6 that omitted efficacy considerations) explained the reasons why the study assumed similar efficacy or safety outcomes between treatments. In studies that incorporated efficacy and/or safety differences between treatments, these differences often contributed to the economic impact of selecting a certain formulation over another. Therefore, to ensure economic models to be reliable sources for a variety of stakeholders to estimate the value of a new formulation, a transparent explanation on the rationale for key assumptions on efficacy and safety between treatments is critical.
There was heterogeneity across studies in terms of how and whether researchers accounted for costs of IV insertions and removals. This may be due to differences in the way these resources are reimbursed in different countries. For future studies, we recommend researchers ensure that their analyses account for all resources used for drug administrations and maintenance paid by key stakeholders relevant to the selected perspective of the analyses and include a rationale for excluding costs from the analysis when this occurs.
Only 3 of the 14 studies we reviewed accounted for how a formulation choice may affect the indirect costs of treatment. In these published models and in our case study, although the total indirect costs were relatively small compared with the direct costs, the estimated impact of a formulation selection on patients and their caregivers’ ability to work was sizable. For instance, our case study estimated that over 600 hours would be lost for patients and their family members due to IV administration and maintenance per year. Two studies also highlighted the potential barrier to nonoral treatments due to the cost of traveling.9,22 Therefore, although these outcomes are typically not required for inclusion in analyses reviewed by third-party payers, given how these factors are important determinants of quality of life among patients and their family members, payers may be interested in reviewing a co-analysis that accounts for these societal outcomes and costs to make coverage decisions based on patient-centered outcomes goals.
Furthermore, the three identified studies that incorporated indirect costs did not differentiate the estimates for lost wages by patients’ demographics. In the case study, it was critical for the model to reflect the expected earnings among people in the typical age group of patients with ALS because ALS often affects older patients who typically have higher earning potentials but may be nearing the age to retire from the workforce. Researchers who are developing a model that incorporates indirect costs should consider how the treatment may impact the earning potentials of the population with the typical demographic characteristics associated with the condition of their interest.
There are several limitations to this study. First, our literature review was targeted to understand the designs used to quantify the economic impact of using an oral formulation compared with a different formulation. However, our review of the literature was structured with a search strategy that has been documented and is fairly reproducible. Second, we reviewed only English-language articles; despite this limitation, we identified 14 studies from 10 different countries. Readers who are interested in understanding how to conduct an economic analysis of switching from an IV to an oral formulation in a non-English speaking country may want to conduct further analysis including articles written in that country’s native language. Finally, although we focused on reviewing approaches used for estimating the economic impact of formulation choice, there are many other factors beyond cost that are important to patients when they are choosing between different formulations. For instance, factors such as patients’ preference, distance to healthcare providers, level of independence, and disease severity may have a strong impact on how patients value certain formulations over others. Although this is outside the scope of the current study, further research on the value of new formulations is warranted.
Key limitations of the modeled case study analyses pertain primarily to assumptions required because of a lack of data. Specifically, there may be potential indirect costs associated with IV administrations other than lost earnings from IV administrations and maintenance, such as lost earnings from time required to manage IV-related adverse events, interrupted educational pursuits for provider visits, or costs for making different living arrangements to care for family members. Further, published data on the frequency of edaravone IV reinsertions is limited. The assumptions made to estimate IV maintenance costs may need to be updated as new data become available. Additionally, the current evidence on hours lost because of IV administration and maintenance in this population is limited. Finally, researchers should be careful not to use the cost-minimization approach unless there is a strong clinical foundation that justifies an assumption of equal efficacy across treatment.
Conclusion
We examined published studies to compare methods for assessing the economic value of additional formulation options from a variety of perspectives. We found significant variations in the impact of an additional formulation option on treatment efficacy, safety, and ease of use by patients, depending on the treatments of comparison across all analyses. Models need to be accompanied by clear descriptions of the rationales for the model time horizon and assumptions on how different formulations may affect healthcare costs according to selected perspectives in the model. Additionally, while the economic consequence of the societal burden due to selecting a certain formulation may not be sizable, we found it is important to consider and report the societal burden such as time spent by patients and caregivers. A standardized approach to formulation comparisons may help improve such comparisons in the future.
Abbreviations
5-FU, 5-fluorouracil; AE, adverse event; ALS, amyotrophic lateral sclerosis; BIA, budget-impact analysis; CEA, cost-effectiveness analysis; CMA, cost-minimization analysis; FDA, Food and Drug Administration; GI, gastrointestinal; HCRU, healthcare resource use; ICER, incremental cost-effectiveness ratio; IM, intramuscular; IV, intravenous; LV, leucovorin; MRSA, methicillin-resistant staphylococcus aureus; ND-CKD, non-dialysis chronic kidney disease; NHS, National Health Service; PICC, peripherally inserted central catheter; PPI, proton pump inhibitor; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SC, subcutaneous; US, United States; UK, United Kingdom.
Data Sharing Statement
Only published data were used in this study; all references reviewed are presented in Table 1. All sources for model inputs are reported in Supplementary Table S-1.
Acknowledgments
The authors wish to thank Steven Apple of Mitsubishi Tanabe Pharma America for providing expert knowledge in IV edaravone administrations and John Forbes of RTI Health Solutions for providing medical editing support.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This manuscript was supported by consultancy fees from Mitsubishi Tanabe Pharma America, Inc.
Disclosure
Melissa Hagan and Malgorzata Ciepielewska have received personal compensation for serving as full-time employees of Mitsubishi Tanabe Pharma America, Inc. Naoko Ronquest, Kyle Paret, and Aaron Lucas are employees of RTI Health Solutions, which received consultancy fees from Mitsubishi Tanabe Pharma America, Inc. to conduct this study. The authors report no other conflicts of interest in this work.
References
1. Aurilio G, Gori S, Nolè F, et al. Oral chemotherapy and patient perspective in solid tumors: a national survey by the Italian association of medical oncology. Tumori. 2016;102(1):108–113. doi:10.5301/tj.5000383
2. Ciruelos EM, Díaz MN, Isla MD, et al. Patient preference for oral chemotherapy in the treatment of metastatic breast and lung cancer. Eur J Cancer Care. 2019;28(6):e13164. doi:10.1111/ecc.13164
3. Geba D, Mohd Sani J, Gascon M, Hahn R, Aggarwal K, Rosselli J. Hereditary angioedema patients would prefer newer-generation oral prophylaxis. J Drug Assess. 2021;10(1):51–56. doi:10.1080/21556660.2020.1863699
4. Institute for Clinical and Economic Review. 2020–2023 value assessment framework; 2020. Available from: https://icer.org/wp-content/uploads/2021/03/ICER_2020_2023_VAF_013120-4-2.pdf.
5. Seidman J, Gaur P, Boler C The patient-perspective value framework: short- and long-term recommendations to influence value assessment methodology; 2019. Available from: https://avalere.com/wp-content/uploads/2019/05/20190530_PPVF-Working-Group-Paper_Final.pdf.
6. Stewart KD, Johnston JA, Matza LS, et al. Preference for pharmaceutical formulation and treatment process attributes. Patient Prefer Adherence. 2016;10:1385–1399. doi:10.2147/PPA.S101821
7. McMeekin N, Geue C, Briggs A, et al. Cost-effectiveness of oral versus intravenous antibiotics (OVIVA) in patients with bone and joint infection: evidence from a non-inferiority trial. Wellcome Open Res. 2019;4:108. doi:10.12688/wellcomeopenres.15314.3
8. Montante A, Le Bras A, Pagnoux C, Perrodeau E, Ravaud P, Terrier B. Cost-effectiveness of rituximab versus azathioprine for maintenance treatment in antineutrophil cytoplasmic antibody-associated vasculitis. Clin Exp Rheumatol. 2019;37(Supl 117):S137–S143.
9. Riccio E, Sabbatini M, Capuano I, Pellegrino AM, Petruzzelli LA, Pisani A. Oral Sucrosomial® iron versus intravenous iron for recovering iron deficiency anaemia in ND-CKD patients: a cost-minimization analysis. BMC Nephrol. 2020;21(1):1–8. doi:10.1186/s12882-020-01716-w
10. Mehta P, Kaye W, Bryan L, et al. Prevalence of amyotrophic lateral sclerosis - United States, 2012–2013. MMWR Surveill Summ. 2016;65(8):1–12. doi:10.15585/mmwr.ss6508a1
11. Salameh JS, Brown RH Jr, Berry JD. Amyotrophic lateral sclerosis: review. Semin Neurol. 2015;35(04):469–476. doi:10.1055/s-0035-1558984
12. Miller RG, Jackson CE, Kasarskis EJ, et al. Practice parameter update; the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the quality standards subcommittee of the American Academy of neurology. Neurology. 2009;73:1218–1226. doi:10.1212/WNL.0b013e3181bc0141
13. Brizzi KT, Bridges JFP, Yersak J, et al. Understanding the needs of people with ALS: a national survey of patients and caregivers. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21(5–6):355–363. doi:10.1080/21678421.2020.1760889
14. Lillo P, Mioshi E, Hodges JR. Caregiver burden in amyotrophic lateral sclerosis is more dependent on patients’ behavioral changes than physical disability: a comparative study. BMC Neurol. 2012;7(12):156. doi:10.1186/1471-2377-12-156
15. Jennum P, Ibsen R, Pedersen SW, Kjellberg J. Mortality, health, social and economic consequences of amyotrophic lateral sclerosis: a controlled national study. J Neurol. 2013;260(3):785–793. doi:10.1007/s00415-012-6706-0
16. U.S. Food and Drug Administration. Novel drug approvals for 2017; 2017. Available from: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2017.
17. U.S. Food and Drug Administration. FDA approves oral form for the treatment of adults with amyotrophic lateral sclerosis (ALS); 2022. Available from: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-oral-form-treatment-adults-amyotrophic-lateral-sclerosis-als.
18. Shiroiwa T, Fukuda T, Shimozuma K, Ohashi Y, Tsutani K. Cost-effectiveness analysis of capecitabine compared with bolus 5-fluorouracil/l-leucovorin for the adjuvant treatment of colon cancer in Japan. Pharmacoeconomics. 2009;27(7):597–608. doi:10.2165/11310110-000000000-00000
19. McCrea C, Johal S, Yang S, Doan J. Cost-effectiveness of nivolumab in patients with advanced renal cell carcinoma treated in the United States. Exp Hematol Oncol. 2018;7:4. doi:10.1186/s40164-018-0095-8
20. Hsu TC, Wang CC. Cost minimization comparison of oral UFT/leucovorin versus 5-fluorouracil/leucovorin as adjuvant therapy for colorectal cancer in Taiwan. J Comp Eff Res. 2019;8(2):73–79. doi:10.2217/cer-2018-0078
21. Spiegel BM, Dulai GS, Lim BS, Mann N, Kanwal F, Gralnek IM. The cost-effectiveness and budget impact of intravenous versus oral proton pump inhibitors in peptic ulcer hemorrhage. Clin Gastroenterol Hepatol. 2006;4(8):988–997. doi:10.1016/j.cgh.2006.05.019
22. Teerawattananon K, Iewsakul S, Yenjitr C, et al. Economic evaluation of treatment administration strategies of ganciclovir for cytomegalovirus retinitis in HIV/AIDS patients in Thailand. Pharmacoeconomics. 2007;25(5):413–428. doi:10.2165/00019053-200725050-00005
23. Zhang L, Hu P. Cost-effectiveness analysis of oral versus intravenous drip infusion of levofloxacin in the treatment of acute lower respiratory tract infection in Chinese elderly patients. Clin Interv Aging. 2017;12:673–678. doi:10.2147/CIA.S127009
24. Ferko NC, Borisova N, Airia P, Grima DT, Thompson MF. How rebates, copayments, and administration costs affect the cost-effectiveness of osteoporosis therapies. Manag Care. 2012;21(11):44–52.
25. Pettigrew M, Thirion DJ, Libman M, Zanotti G. Cost comparison of linezolid versus vancomycin for treatment of complicated skin and skin-structure infection caused by methicillin-resistant Staphylococcus aureus in Quebec. Can J Infect Dis Med Microbiol. 2012;23(4):187–195. doi:10.1155/2012/585603
26. Hillner BE, Agarwala S, Middleton MR. Post hoc economic analysis of temozolomide versus dacarbazine in the treatment of advanced metastatic melanoma. J Clin Oncol. 2000;18(7):1474–1480. doi:10.1200/JCO.2000.18.7.1474
27. Abushanab D, Rouf PA, Al Hail M, et al. Cost-effectiveness of oral versus intravenous Ibuprofen therapy in preterm infants with patent ductus arteriosus in the neonatal intensive care setting: a cohort-based study. Clin Ther. 2021;43(2):336–348. doi:10.1016/j.clinthera.2020.12.004
28. You R, Zhang Y, Wu DB, et al. Cost-effectiveness of zoledronic acid versus oral alendronate for postmenopausal osteoporotic women in China. Front Pharmacol. 2020;(11):456. doi:10.3389/fphar.2020.00456
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
