Back to Journals » International Journal of General Medicine » Volume 9
Quality of life under oxycodone/naloxone, oxycodone, or morphine treatment for chronic low back pain in routine clinical practice
Authors Ueberall M , Eberhardt A, Mueller-Schwefe G
Received 18 August 2015
Accepted for publication 26 November 2015
Published 24 February 2016 Volume 2016:9 Pages 39—51
DOI https://doi.org/10.2147/IJGM.S94685
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Michael A Ueberall,1 Alice Eberhardt,2 Gerhard HH Mueller-Schwefe3
1Institute for Neurological Sciences, Nuernberg, Germany; 2Mundipharma GmbH, Limburg, Germany; 3Interdisciplinary Center for Pain and Palliative Care Medicine, Goeppingen, Germany
Objective: To compare the quality of life of patients with moderate-to-severe chronic low back pain under treatment with the WHO-step III opioids oxycodone/naloxone, oxycodone, or morphine in routine clinical practice.
Study design: Prospective, 12-week, randomized, open-label, blinded end-point study in 88 medical centers in Germany.
Patients and methods: A total of 901 patients requiring around-the-clock pain treatment with a WHO-step III opioid were randomized to either morphine, oxycodone, or oxycodone/naloxone (1:1:1). Changes from baseline to week 12 in quality of life were assessed using different validated tools (EuroQoL-5 Dimensions [EQ-5D], Short Form 12 [SF-12], quality of life impairment by pain inventory [QLIP]).
Results: EQ-5D weighted index scores significantly improved over the 12-week treatment period under all three opioids (P<0.001) with significantly greater improvements under oxycodone/naloxone (65.2% vs 49.6% for oxycodone and 48.2% for morphine, P<0.001). The proportion of patients without EQ-5D complaints was also significantly higher under oxycodone/naloxone (P<0.001). Although quality of life ratings with the QLIP inventory showed significant improvements in all the three treatment arms, improvements were significantly higher under oxycodone/naloxone than under oxycodone and morphine (P<0.001): 90.7% of all oxycodone/naloxone patients achieved ≥30% improvements in quality of life, 72.8% had ≥50%, and 33.2% ≥70% improvements. Similarly, both physical and mental SF-12 component scores showed significantly greater improvements under oxycodone/naloxone with both scores close to the German population norm after 12 weeks.
Conclusion: Treatment with morphine, oxycodone, or oxycodone/naloxone under routine daily practice conditions significantly improved state of health and quality of life of patients with moderate-to-severe low back pain over a 12-week treatment period. Comparison between the treatment groups showed significantly greater improvements for oxycodone/naloxone than for the other two opioids.
Keywords: chronic low back pain, quality of life, oxycodone/naloxone, oxycodone, morphine, routine clinical practice, EQ-5D, SF-12, QLIP
Introduction
In Germany, chronic nonmalignant pain is a major health care problem affecting 17% of the adult population.1 The majority (61%) suffers from chronic back pain.2 Debilitating and recurrent, this condition can result in substantial restrictions in patients’ daily activities. Globally, low back pain is the leading cause of years lived with disability.3 Depression, panic and anxiety disorders, and sleep disturbances are frequent comorbidities,4 and quality of life can be markedly diminished.5 The societal burden is also high with substantial health care and welfare resource utilization: in 2005, direct and indirect back pain-related costs corresponded to 2.2% of the German gross domestic product.6
In the majority of the cases, low back pain is nonspecific with no distinct disease etiology.5,7 It can comprise both nociceptive and neuropathic components,4 and thus necessitates a multimodal and individualized treatment approach.8
The practice guideline of the German Pain Association recommends paracetamol, metamizole, or nonsteroidal anti-inflammatory drugs (NSAIDs) as initial treatment followed by second-line treatment for nonresponders or patients with tolerability issues individually tailored according to pain intensity, presence of a neuropathic component, muscular pain, or inflammation pain.9 For patients with severe pain, second-line medications include both weak and strong opioids. Guidance regarding the use of opioids for chronic low back pain is provided by the interdisciplinary evidence- and consensus-based recommendations of the German LONTS (long-term use of opioids for non-tumor pain) guideline which states that opioids can be offered for a period of 4–12 weeks and for longer periods for treatment responders experiencing good tolerability.10 It should, however, be complemented by nonpharmacological measures. Based on a meta-analysis of 13 randomized controlled trials,11 the guideline concludes that there is no rationale for preference of one opioid and/or administration route (oral/transdermal) over another in the treatment of chronic nonmalignant pain.
We recently compared the effectiveness and tolerability of oxycodone/naloxone with oxycodone and morphine (all prolonged-release preparations) in the treatment of patients with moderate-to-severe nonmalignant chronic low back pain under clinical practice conditions. Oxycodone/naloxone had been developed as a strategy to prevent/treat opioid-induced constipation (and bowel dysfunction in general),12 experienced by ~40% of patients under opioid treatment.13 The administration of oxycodone/naloxone improved bowel dysfunction compared with oxycodone without compromise of analgesia in several randomized controlled trials.14–16 The present study was initiated to obtain real-life data regarding the benefits of oxycodone/naloxone vs a conventional opioid therapy with laxatives, as reimbursement authorities, health insurance companies, and physician organizations in Germany were not satisfied with the available data to recommend oxycodone/naloxone as a first-line opioid. Data of the intent-to-treat population of the study (for whom the randomized treatment recommendation was accepted) have been published17 and showed that 62% of the patients experienced a clinically relevant pain reduction of at least 50% accompanied by markedly improved functionality at the end of the 12-week treatment period. Stratification by opioid showed significantly better analgesic efficacy and gastrointestinal tolerability of oxycodone/naloxone compared to oxycodone and morphine. The study also obtained data regarding the quality of life under treatment with the different opioids, which are presented in this article.
Patients and methods
Study design and patients
The study conformed to relevant national and local ethical and regulatory requirements. It was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice on behalf of the German Pain Association and the German Pain League by O.Meany MD&PM GmbH (Nürnberg, Germany) in 88 medical centers in Germany. Patients were enrolled between April and August 2013; all gave written informed consent prior to enrollment. The study is registered with the German pain study registry (DGS: 2012-0012-05) and with the European Medicines Agency (ENCEPP/SDPP/11035).
The study design has been described in detail elsewhere.17 Briefly, a prospective, randomized, open-label, blinded end point (PROBE) design was used where physicians and patients were aware of the study medication but were blinded to the study end points. This design reflects routine clinical practice conditions (a heterogeneous patient population, open-label dose adjustments, use of concomitant medications for opioid side effects as needed) with improved data analysis due to randomization and blinded end points. The collected data can be described as randomized real-life data. The study included adult patients in need of around-the-clock pain treatment with either morphine, oxycodone, or oxycodone/naloxone because of an insufficient response and/or unacceptable side effects to previous treatment with non-opioids or weak opioids for moderate-to-severe nonmalignant chronic low back pain. Detailed exclusion criteria have been published17,18 and include contraindications listed in the German prescribing information for the three opioids. Following baseline evaluation, patients were randomized using a computer-generated randomization scheme to receive prolonged-release preparations of either oxycodone/naloxone, oxycodone, or morphine (1:1:1) for 12 weeks. Physicians were not required to follow this recommendation; they were permitted to change the allocated medication twice (to each of the other two opioid options) in case of side effects, lack of efficacy, or economic reasons, before a patient was withdrawn from the study. Only data from the treatment period with the finally administered opioid were included in the study. All treatment decisions were made under routine clinical practice conditions solely by the participating physicians. All three opioids were prescribed according to the respective German summary of product characteristics; dose adjustments, prescriptions of analgesic co-medication, rescue medication, or laxatives were given according to the individual requirements of the patients.
Assessments
The participating physicians collected data at baseline, week 4, and week 12 (end of observation) using a web application providing standardized case report forms. They rated quality of life with an eleven-point numerical rating scale (NRS11) from 0= best possible to 10= worst possible quality of life, and a six-point numerical rating scale (NRS6) from 1= very good to 6= insufficient. The latter scale was also used to grade tolerability of the medication and patients’ compliance. Patients assessed their state of health with the EuroQoL-5 Dimensions-3 Levels (EQ-5D-3L) which includes the five dimensions mobility, self-care, usual activities, pain/discomfort, and anxiety/depression rated as “no problems”, “some problems”, or “extreme problems”.19 The ratings were then converted to a weighted EQ-5D index score from 0= worst possible state of health to 1= best possible state of health. Patients also rated their health on the EQ visual analog scale (EQ VAS) from 100 mm “best imaginable health state” to 0 mm “worst imaginable health state”. Additionally, physicians rated changes in the overall condition of the patients with the clinical global impression of change (CGI) seven-point scale from +3= very much better to –3= very much worse.20
Patient data were collected using conventional paper– pencil pain questionnaires from the German Pain Association.21 Patients independently answered these questionnaires without any influence from the treating physicians. At baseline evaluation (pretreatment documentation before randomization), patients completed the German Pain Questionnaire; thereafter, they received German Pain Diaries for documentation of weekly treatment-associated changes at the end of each week (12 diaries per patient). Both questionnaires contain validated instruments for the assessment of pain and pain-related impairments in daily activities and quality of life; data obtained from the following instruments are presented in this article:
- A visual analogue scale (VAS100) to assess pain intensity from 0= no pain to 100= worst imaginable pain.
- Assessment of pain severity from 1= low disability, low intensity to 4= high disability, severely limiting.22
- The quality of life impairment by pain inventory (QLIP) including the following items: QLIP-1, general well-being (0= very bad, 4= bad, 8= good, 12= very good); QLIP-2, sleep duration (0= not sufficient, 4= sufficient); QLIP-3, constant pain (0= yes, 4= no); QLIP-4, pain-related restrictions in activities, and QLIP-5, impairment of mood (0= very severe, 1= severe, 2= marked, 3= a little, 4= not at all); QLIP-6, patients’ influence on amelioration of pain (0= no, 1= a little, 2= marked, 3= strong, 4= very strong); QLIP-7, pain-related complaints (8= no complaints; for each mention of one of the predefined complaints, the score is reduced by 1: 8–x complaints = n complaints; 0= at least eight complaints). The QLIP sum score ranges from 0 to 40 points with 40= least affected. A sum score of ≤20 points indicates severe impairment.
- The Short Form 12 (SF-12) questionnaire, a shorter version of the SF-36 Health Survey, assessing quality of life in eight physical and mental domains.23 The domains are summarized in a physical and a mental component score. Higher scores indicate less impairment. Average scores for the German population norm are 49.6 for the physical and 52.3 for the mental component score.24
- The PainDETECT questionnaire25 to determine patients’ pain type (predominantly nociceptive, predominantly neuropathic, or mixed pain).
Data obtained from the questionnaire also permitted the determination of patients’ chronicity stage using the Mainz Pain Staging System26 (stage I = at risk of chronification, II = chronification, and III = marked chronification).
Additionally, opioid-induced constipation was assessed with the three-item validated Bowel Function Index (BFI27,28) on a VAS from 0= freedom from the symptom to 100= maximum difficulty or most severe symptom. The BFI score is calculated as the mean of the three item scores retrospectively recorded by the patients for the previous 7 days. A mean BFI >28.8 is considered abnormal.28
Statistical analysis
Data management and statistical analysis were carried out by O.Meany MD&PM GmbH (Nürnberg, Germany) using the SPSS program. All data were checked for completeness, consistency, and plausibility.
Secondary effectiveness parameters described in this article were absolute and relative changes from baseline in different quality of life aspects for the entire study population and for each of the three opioid treatment arms. Missing data due to early discontinuation from the study were imputed using the “last observation carried forward” (LOCF) approach. The analysis was exploratory; 95% confidence intervals (CI) were added where appropriate. Student’s t-tests/Pearson’s chi-square tests were used for between-group comparisons, paired samples t-tests were performed for within-group comparison (change over time). All the statistical tests were carried out using a two-sided significance level of 0.05 adjusted for multiplicity (Holm–Bonferroni correction).
Results
A total of 901 patients with chronic low back pain (86.3% with nonspecific pain) were included in the study; they were treated by general practitioners (53.4%) or specialist physicians (46.6%). Mean age of the study population was 46.4±9.7 (95% CI 45.4–47.4) years with slightly more female patients (55.7%) and a mainly Caucasian background (93.9%). The majority (81.1%) had suffered from low back pain for more than 3 months with chronification stages mostly II (50.5%) and III (37.7%). Pain was predominantly nociceptive for 45.5%, predominantly neuropathic for 16.6%, and of a mixed type for 37.8% of the patients. At baseline evaluation, patients reported a mean average pain intensity of 47.2±20.5 (95% CI 45.2–49.2) and 43.8% presented with average pain intensity scores >50 mm VAS.
Patients were switched from weak (WHO-II) opioids (69.4%) or non-opioids (30.6%) to either oxycodone/naloxone, oxycodone, or morphine. Main reasons for switching were insufficient effectiveness (63.6%) or insufficient tolerability (15.2%) to the previous treatment. Half of the patients (50.3%; morphine 52.7%, oxycodone 50.3%, oxycodone/naloxone 47.8%) remained in their allocated treatment arm, 49.7% were switched (morphine 47.3%, oxycodone 49.7%, oxycodone/naloxone 52.2%). Baseline characteristics were comparable between the three treatment arms (Table 1).
Overall, mean study duration was 9.5±3.6 weeks, and 35.5% of all patients discontinued prematurely (morphine 43.0%, oxycodone 38.3%, oxycodone/naloxone 25.2%), mainly due to treatment-emergent adverse events (morphine 25.0%, oxycodone 24.7%, oxycodone/naloxone 7.3%) and/or insufficient tolerability (morphine 31.0%, oxycodone 31.3%, oxycodone/naloxone 21.3%; multiple answers permitted). For further details please refer to Ueberall et al.18
Treatment characteristics
The initial mean opioid dose was 28.7±10.8 mg morphine equivalent (MEQ, median 30) and had increased by study end to 107.8±37.2 mg MEQ (median 120). Starting doses of MEQ and the titration process were comparable between the treatment groups. Mean dosages for oxycodone/naloxone at study end were 112.9±34.2 mg MEQ (median 120, 95% CI 109.5–116.3) and slightly higher than those reported for oxycodone (106.6±37.4 mg MEQ, median 120, 95% CI 102.9–110.3; P=0.033) and morphine (103.8±39.3 mg, median 100, 95% CI 99.9–107.7; P=0.003).
The overall proportion of patients using laxatives increased from 28.6% at baseline to 56.8% at week 12. The proportion of patients receiving laxatives in response to gastrointestinal side effects of the administered opioid treatment was significantly lower with oxycodone/naloxone (20.6%, 44/214) than with oxycodone (46.7%, 99/212; P<0.001) and morphine (51.2%, 111/217; P<0.001).
Overall effects of treatment with WHO-step III opioids (entire study population)
Pain intensity
Under opioid treatment, mean average pain intensity improved from 47.2±20.5 mm VAS (median 48, 95% CI 45.2–49.2) to 25.2±21.5 mm VAS (median 20, 95% CI 23.1–27.3; P<0.001). In parallel, the proportion of patients reporting pain intensities of >50 mm VAS decreased from 43.8% to 13.3%. Vice versa, the proportion of patients who presented with average 24-hour pain intensity scores ≤30 mm VAS increased from 22.2% at baseline to 66% at week 12 (P<0.001).
Bowel function
Bowel function significantly worsened under opioid treatment. The proportion of patients with normal BFI scores (ie, ≤28.8 mm VAS) declined from 71.4% at baseline to 39% at study end (P<0.001). Mean BFI scores increased from 19.8±19.4 (median 16, 95% CI 17.9–21.7) at baseline to 43.9±32.3 (median 41, 95% CI 40.7–47.1) at week 12. The average number of complete spontaneous bowel movements per week was reduced from 4.3±1.7 (median 4) to 3.2±2.0 (median 3; P<0.001) over the 12-week treatment period.
Quality of life
EQ-5D-3L
Mean weighted EQ-5D index score at baseline was 0.39±0.32 (95% CI 0.36–0.41) and improved under 12-week WHO-step III opioid treatment by 0.33±0.32 points (54.4%) to 0.72±0.27 (95% CI 0.70–0.74; P<0.001). A total of 62.2% of all patients reported an absolute EQ-5D index improvement beyond the minimal clinically important difference (MCID) of 0.14 index points,29 more than half of patients (51%) showed an improvement of twice the MCID, and almost three out of ten patients (29.3%) showed at least fourfold improvement. The overall proportion of patients without any complaints in the five EQ-5D dimensions increased significantly under opioid treatment (Figure 1).
  | Figure 1 Proportion of patients without any EQ-5D complaints during the course of 12 weeks of strong opioid treatment (n=901 patients). |
The corresponding EQ VAS health state score at baseline was 34.1±18.2 mm VAS (95% CI 24.2–44.0); at end of study, it had improved by 14.7±22.0 to 48.8±25.6 mm VAS (95% CI 34.9–62.7; P<0.001). The proportion of patients reporting EQ VAS scores in the upper range of the VAS scale (ie, ≥50 mm VAS) was 13.4% at baseline and 39.6% at study end (P<0.001).
QLIP inventory
Under opioid treatment, the QLIP sum score increased from 17.1±5.8 (median 17, 95% CI 16.5–17.7) at baseline to 28.1±5.8 (median 29, 95% CI 27.5–28.7; P<0.001). The proportion of low back pain patients presenting with a significant pain-related quality of life impairment (≤20 points) improved from 75.2% at baseline to 12.7% at study end (P<0.001). There were marked improvements in all QLIP items after 12 treatment weeks. The proportion of patients rating their general well-being (QLIP-1) as good/very good had increased to 60.3% from 33.4% at baseline. Sleep duration (QLIP-2) was regarded as adequate by the majority of patients (87.1%) compared to 43.5% at baseline. Constant pain (QLIP-3) was reported by 18.8% of the patients (baseline 59.7%). Nearly all patients (99.2% and 97%) had documented at least minor pain-related restrictions in activities (QLIP-4) and impairment of mood (QLIP-5), respectively, at baseline. This proportion was reduced to 70.7% and 66.7%, respectively. After 12 weeks, 48.1% of all patients felt strongly/very strongly that they had some influence on ameliorating their pain (QLIP-6). The occurrence of pain-related complaints (QLIP-7) decreased by 45.9% from 3.7±1.4 (95% CI 3.6–3.8) to 2.0±1.5 (95% CI 1.9–2.1). All complaints except constipation occurred less frequently, and 14.7% of the patients reported no complaints at all under opioid treatment (Table 2). Improvements from baseline in the QLIP sum score were already significant after 1 week of treatment (P<0.001) with an increase of 64.3% after 12 weeks.
  | Table 2 Occurrence of pain-related complaints |
SF-12
There was a larger burden of pain in the physical domains of quality of life than in the mental aspects: mean baseline physical component score of the SF-12 (37.5±7.3, median 37, 95% CI 36.8–38.2) was on average 12.1 points and mental component score (47.0±11.6, median 47, 95% CI 45.9–48.1) 5.3 points lower than the German population reference. Under opioid treatment, the physical component score continuously increased during the first 10 treatment weeks to 46.1±11.8 (median 45, 95% CI 44.9–47.3) points and remained on this value for the remaining 2 weeks (all time points P<0.001 vs baseline). Changes from baseline were also significant for all time points for the mental component score which improved continuously during the first 9 weeks of opioid treatment to 50.3±12.1 (median 52, 95% CI 49.1–51.5; P<0.001).
Tolerability, safety, and clinical global impression
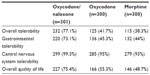
Physicians considered opioid treatment as at least sufficiently tolerated by the majority of the patients (93.3%; Figure 2); gastrointestinal tolerability was rated at least “satisfactory” for 81.6%. Good/very good overall quality of life and compliance was documented for 59.8% and 80.7% of the patients, respectively. At the end of observation, physicians stated a better, much better, or very much better clinical global impression for 70.5% of the patients.
Treatment effects of oxycodone/naloxone, oxycodone, or morphine
Pain intensity
Mean average pain intensity improved from 47.2±18.9 mm VAS (median 48, 95% CI 45.3–49.1) at baseline to 20.0±20.4 (median 12, 95% CI 18.0–22.0; P<0.001) under oxycodone/naloxone, from 46.8±21.2 (median 48, 95% CI 44.7–48.9) to 27.1±21.3 (median 23, 95% CI 25.0–29.2; P<0.001) under oxycodone, and from 47.7±21.4 (median 48, 95% CI 45.6–49.8) to 28.6±21.7 (median 24, 95% CI 26.5–30.7; P<0.001) under morphine. Improvements after 12 treatment weeks were significantly higher for oxycodone/naloxone (–27.5±20.7%/–57.9±46.4 mm VAS) compared to oxycodone (–20.0±22.2%/–35.5±68.2 mm VAS; P<0.001 for both) and morphine (–19.4±22.0%/–37.1±45.7 mm VAS; P<0.001 for both). Significantly more patients in the oxycodone/naloxone group (65.5%, 197/301) presented with at least 50% relief in average 24-hour pain intensity than under oxycodone (50.7%; P<0.001) and in particular under morphine (43.3%; P<0.001).
Bowel function
Treatment-related BFI changes with oxycodone/naloxone were significantly lower than those reported for the two other opioids. Mean BFI increase from baseline was 10.1±16.9 mm VAS (median 5, 95% CI 8.4–11.8) for oxycodone/naloxone compared to 28.3±26.9 (median 26, 95% CI 25.6–31.0; P<0.001) for oxycodone and 34.0±29.3 (median 32, 95% CI 31.1–36.9; P<0.001) for morphine. As a consequence, end-of-study BFI scores were significantly less for oxycodone/naloxone (30.0±26.2, median 25, 95% CI 27.4–32.6) compared to oxycodone (48.2±32.3, median 49, 95% CI 45.1–51.5; P<0.001) and morphine (53.6±33.1, median 54, 95% CI 50.3–56.9; P<0.001).
Quality of life
EQ-5D-3L
At baseline, EQ-5D weighted index scores were comparable for all treatment groups (morphine 0.388±0.319, oxycodone 0.391±0.321, oxycodone/naloxone 0.385±0.315) and improved significantly over the 12-week treatment course (P<0.001; Figure 3). EQ-5D index scores at week 12 were 0.68±0.30 for morphine, 0.69±0.28 for oxycodone, and 0.79±0.23 for oxycodone/naloxone. The mean improvement from baseline to week 12 was significantly greater for oxycodone/naloxone (0.40±0.32 mm VAS, 65.2% improvement) than for oxycodone (0.30±0.30 mm VAS, 49.6% improvement; P<0.001) and morphine (0.29±0.32 mm VAS, 48.2% improvement; P<0.001) with significantly more patients in the oxycodone/naloxone group (70.3%) with an EQ-5D index improvement beyond the MCID compared to oxycodone (58.7%; P=0.003) and morphine (57.7%; P=0.002). The proportion of patients without any complaints was – evaluated independently of the EQ-5D dimensions – significantly higher under oxycodone/naloxone than under the other two opioids (P<0.001 for each comparison; Figure 4).
  | Figure 4 Proportion of patients without any EQ-5D complaints after 12 weeks of opioid treatment (morphine n=300, oxycodone n=300, oxycodone/naloxone n=301). |
Corresponding data of the EQ VAS score improved significantly under all three opioids: from 33.7±17.1 at baseline to 45.5±25.0 at week 12 for morphine (P<0.001), from 34.5±18.1 to 47.5±25.8 for oxycodone (P<0.001), and from 34.2±19.2 to 53.5±25.4 for oxycodone/naloxone (P<0.001). Related changes from baseline were significantly better for oxycodone/naloxone (19.3±22.6, 29.3% improvement) compared to oxycodone (13.0±21.1, 19.9% improvement; P<0.001) and morphine (11.8±21.5, 17.8% improvement; P<0.001). The proportion of patients reporting EQ VAS scores of ≥90 mm VAS (close to the “best imaginable health state”) at end of study was 14.3% for oxycodone/naloxone compared to 3% for oxycodone (P<0.001) and 2.3% for morphine (P<0.001).
QLIP inventory
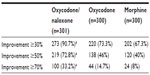
The proportion of patients with a clinically relevant pain-related quality of life impairment was significantly reduced under all three opioids: from 75.7% at baseline to 16.3% at end of study in the oxycodone group (P<0.001), from 73.7% to 15.7% under morphine (P<0.001), and from 76.4% to 6% in the oxycodone/naloxone group (P<0.001) with significantly greater improvements under oxycodone/naloxone (P<0.001 vs oxycodone and morphine). Significant improvements were also observed under all three opioids in the QLIP sum score: from 17.1±5.6 (median 17, 95% CI 16.5–17.7) at baseline to 30.6±4.9 (median 32, 95% CI 30.1–31.1; P<0.001) for oxycodone/naloxone, from 17.1±5.7 to 27.5±5.8 (P<0.001) for oxycodone, and from 17.2±5.9 to 26.4±5.9 (P<0.001) for morphine. Figure 5 shows the relative changes from baseline in the QLIP sum score with significantly greater improvements in the oxycodone/naloxone group compared to morphine after 2 weeks and compared to oxycodone after 3 weeks of treatment.
All seven items of the QLIP inventory showed significantly greater improvements in oxycodone/naloxone patients compared to oxycodone and morphine (P≤0.001). Most of the patients in the oxycodone/naloxone group (95%) reported adequate sleep duration after 12 treatment weeks (oxycodone 83.3%, morphine 83%); 88% were no longer in constant pain (oxycodone 79.9%, morphine 76%). At baseline, the proportion of patients with pain-related complaints was comparable between the groups; after 12 weeks of treatment, this proportion was significantly lower for oxycodone/naloxone compared to the other two opioids (P<0.001, Table 2). Although constipation complaints increased under all three treatments, the number of complaints was significantly lower for oxycodone/naloxone (29.6% vs 56.7% for morphine and 55.3% for oxycodone, P<0.001).
Nearly all patients under oxycodone/naloxone (90.7%) achieved ≥30% improvements in quality of life from baseline. The proportion of patients with improvements was significantly greater than under oxycodone and morphine (Table 3).
SF-12
Both the physical and mental component scores increased significantly from baseline in all the three treatment groups after 12 treatment weeks (P<0.001; Figure 6). Reported end-of-study changes for the physical/mental subscale to baseline were significantly higher under oxycodone/naloxone (10.4±13.6/5.0±12.4; median 9/5 points) in comparison to oxycodone (7.9±15.1/2.5±10.0, median 5/0, P=0.033/0.007), and morphine (7.7±12.1/2.3±10.8, median 6/1; P=0.011/0.005). For the physical component score, significantly greater achievements under oxycodone/naloxone were already observed at the end of week 4 compared to morphine (P=0.021) and at the end of week 6 compared to oxycodone (P=0.017). For the mental aspects, treatment with oxycodone/naloxone was significantly better compared to morphine at the end of week 5 (P=0.021) and compared to oxycodone at the end of week 6 (P=0.042).
Tolerability, safety, and clinical global impression
Overall tolerability, gastrointestinal and central nervous system (CNS) tolerability, as well as quality of life were rated significantly better under oxycodone/naloxone compared to oxycodone and morphine treatment at the end of the 12-week period (P<0.001 for all four parameters). The proportion of patients with “good” or “very good” ratings was over 90% for CNS tolerability in all the three treatment groups (Table 4). “Good” or “very good” ratings for overall tolerability, gastrointestinal tolerability, and quality of life were also documented for well over 70% of the oxycodone/naloxone patients, but only for 38.3%–55.3% of the patients in the two other groups. There was also a significant difference in physicians’ clinical global impression of the oxycodone/naloxone group compared to oxycodone and morphine patients (P<0.001): 90.7% of all oxycodone/naloxone patients were rated “better”, “much better” or “very much better” (oxycodone 62%, morphine 58.7%). Only three patients (1%) under oxycodone/naloxone were considered worse.
Discussion
The majority of the patients participating in this study presented with nonspecific, moderate-to-severe chronic low back pain that substantially impaired their daily life. Chronic low back pain placed a greater burden on the physical aspects of quality of life than on the mental domains: the mean baseline physical component score of the SF-12 was 12.1 points and the mental component score was 5.3 points below the German population norm. Fifty percent of the patients reported severe restrictions in daily activities, 57% regarded their sleep duration as being insufficient, and 75% considered their quality of life severely diminished. Previous treatments with non-opioids or weak opioids had been inadequate or were not tolerated. The substantial impact of pain (notably severe daily pain) on quality of life had been a key finding of the analysis of German data from the National Health and Wellness Survey (an internet-based survey on health care attitudes, behaviors, and characteristics of the adult population of several countries).24 The contribution of pain far outweighed the impact of sociodemographic factors, health risk factors such as obesity, alcohol, or smoking, or comorbidity status.30
In the present study, 12 weeks of treatment with a WHO-step III opioid provided a clinically relevant reduction in pain intensity of at least 50% for the majority of the patients and was accompanied by significant improvements of pain-related restrictions in daily activities.17 These improvements resulted in a markedly better state of health and quality of life of the patients who rated their pain-related quality of life impairments already significantly improved after the first treatment week. At the end of the 12-week treatment, the majority considered their sleep duration adequate, anxiety and/or depression occurred less often, the occurrence of pain-related complaints with the exception of constipation was reduced, and physicians rated the overall clinical state of the majority of the patients as improved. All opioid analgesics are associated with gastrointestinal and central nervous system side effects which can lead to inadequate compliance and/or treatment withdrawal.31 Interestingly, the proportion of patients reporting pain-related gastric complaints, nausea, or dizziness declined during opioid treatment in this study, and physicians considered gastrointestinal tolerability at least satisfactory in the majority of patients. Overall compliance was also good. It is noteworthy that patients rated their quality of life significantly improved despite an increase in constipation complaints and significantly worsened bowel function (which in turn might cause pain). For the majority of the patients, the benefits of pain relief, better quality of sleep, and fewer restrictions in daily activities seemed to balance the disadvantage of opioid-induced constipation and other gastrointestinal side effects.
Although systematic reviews of randomized controlled trials found only small/medium effect sizes for pain relief and improvement of functionality when comparing opioids to placebo in the treatment of chronic low back pain,32,33 our data suggest that the use of strong opioids for up to 12 weeks has a place in the treatment of chronic low back pain in routine clinical practice. Some quality of life data were used in the analysis of functionality (physical component score ratings of the SF-12 or SF-36), but mental component scores or other quality of life data were either not available or not included in randomized trials.33 These are frequently assessed in observational studies. In these studies under routine clinical practice conditions, short- or long-term treatment with different strong opioids provided – often significant – improvements in quality of life with generally good tolerability in patients suffering mainly from low back pain.34–38
In contrast to the recent meta-analysis of 13 randomized controlled trials which concluded that there were no significant differences between different opioids and between oral or transdermal application in the treatment of chronic nonmalignant pain,11 our clinical practice data showed significant and clinically relevant differences between the three WHO-step III opioids oxycodone/naloxone, morphine, and oxycodone regarding pain relief and improvement of functionality17 and regarding improvement of state of health and quality of life. Ratings by both physicians and patients using several different assessment instruments showed significantly greater quality of life improvements and significantly better tolerability under oxycodone/naloxone than under the other two opioids. After 12 weeks, treatment with oxycodone/naloxone had resulted in a ≥50% improvement in quality of life for more than 70% of the patients, whereas only 46% and 40% of patients under oxycodone and morphine, respectively, achieved this improvement (QLIP questionnaire). Oxycodone/naloxone patients had fewer pain-related complaints, more adequate sleep duration, and fewer were in constant pain. The QLIP results are supported by the ratings with the SF-12: both the physical and mental component scores showed significantly greater improvements under oxycodone/naloxone with both scores close to the German population norm after 12 weeks. The overall clinical state was considered improved for 91% of oxycodone/naloxone patients compared to 62% of oxycodone and 59% of morphine patients.
Although constipation complaints increased and bowel function worsened in all three opioid groups, this was observed less often in oxycodone/naloxone patients: the proportion of constipation complaints was nearly half the proportion in the other groups (29.6% vs 56.7% for morphine and 55.3% for oxycodone) and relative changes in BFI were 51% compared to 171% for morphine and 140% for oxycodone. A recent post hoc analysis of the complete data set of this study found a significantly lower risk of opioid-induced constipation under oxycodone/naloxone compared to the other two opioids.18 The findings were not unexpected as the fixed-dose combination of oxycodone/naloxone improves bowel dysfunction compared with oxycodone without compromise of analgesia in patients with chronic pain.12,14 As constipation has a negative influence on quality of life,39 the markedly better gastrointestinal tolerability profile might be considered as one of the reasons for the significantly better quality of life outcomes for oxycodone/naloxone patients in this study.
Marked differences in favor of oxycodone/naloxone compared to other strong opioids were also observed in the treatment of low back pain under clinical practice conditions for both physical and mental SF-36 component scores.37 In addition, the quality of life benefits of oxycodone/naloxone have been shown in a large observational study in patients with severe chronic pain of various etiologies, wherein the study reported a quality of life improvement of 43% over the 4-week treatment period.40 These results support previous findings from randomized trials.41 Furthermore, patients previously treated with non-opioids or weak opioids indicated their preference for oxycodone/naloxone treatment with respect to quality of life outcomes.42 Direct and indirect treatment costs for oxycodone/naloxone were also ~13% lower compared to other strong opioids for chronic low back pain patients in Germany; the cost-effectiveness analysis indicates better health economic benefits for oxycodone/naloxone.37
Compared to double-blind randomized controlled trials, the open-label aspect of our study design carries the risk of bias, as physicians were able to address effectiveness and tolerability problems with concomitant treatment options chosen in the knowledge of which opioid analgesic the patient was receiving. However, randomized treatment recommendations and blinded study end points permitted an improved data analysis of these real-life data. Moreover, the open-label design in our study allowed individualized dose titrations to optimize the analgesic effect and to address tolerability and safety issues. In addition to the patient-reported outcomes, this approach offered a much deeper insight into efficacy and tolerability of WHO-step III opioids under real-world conditions and their differential impact on quality of life beyond pure analgesia.
Conclusion
Treatment with the WHO-step III opioids morphine, oxycodone, or oxycodone/naloxone under routine daily practice conditions significantly improved state of health and quality of life of patients with moderate-to-severe low back pain over a 12-week treatment period. The comparison between the treatment groups showed significantly greater improvements for oxycodone/naloxone than for the other two opioids. This result and the previously reported significantly higher effectiveness in pain relief and significantly better tolerability suggest oxycodone/naloxone as an alternative option to conventional WHO-step III opioids in the treatment of nonmalignant low back pain.
Acknowledgments
Writing assistance was provided by Elke Grosselindemann and Birgit Brett and was funded by Mundipharma GmbH.
Data of this study were presented at the World Pain Congress of the International Association for the Study of Pain (IASP), October 6–11, 2014, Buenos Aires, Argentina.
Disclosure
The concept for this study was developed by the Institute for Neurological Sciences on behalf of the German Pain Association and the German Pain League. The study was realized by an independent contract research organization and partly (<49%) sponsored by an unrestricted scientific grant from Mundipharma, Germany. MAU and GHHM-S are physicians and independent of any significant/relevant financial or other relationship to the sponsor, except for minor reimbursements for occasional lecture or consulting fees. AE is an employee of Mundipharma Germany. The authors report no other conflicts of interest in this work.
References
Wolff R, Clar C, Lerch C, Kleijnen J. Epidemiologie von nicht tumorbedingten chronischen Schmerzen in Deutschland [Epidemiology of chronic non-malignant pain in Germany]. Schmerz. 2011;25:26–44. | |
Müller-Schwefe GHH. European survey of chronic pain patients: results for Germany. Curr Med Res Opin. 2011;27:2099–2106. | |
Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. | |
Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185–190. | |
Morlion B. Chronic low back pain: pharmacological, interventional and surgical strategies. Nat Rev Neurol. 2013;9:462–473. | |
Wenig CM, Schmidt CO, Kohlmann T, Schweikert B. Costs of back pain in Germany. Eur J Pain. 2009;13:280–286. | |
Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344:363–370. | |
Morlion B. Pharmacotherapy of low back pain: targeting nociceptive and neuropathic pain components. Curr Med Res Opin. 2011;27:11–33. | |
German Pain Association. DGS-Praxisleitlinie Kreuzschmerz [Practice guideline low back pain]. Available from: http://www.dgs-praxisleitlinien.de/. Accessed December 14, 2014. | |
Häuser W, Bock F, Engeser P, Tölle T, Willweber-Strumpf A, Petzke F. Clinical practice guideline: long-term opioid use in non-cancer pain. English version available from: http://www.awmf.org/leitlinien/detail/ll/145-003.html. Accessed January 25, 2015. | |
Lauche R, Klose P, Radbruch L, Welsch P, Häuser W. Opioide bei chronischen nicht-tumorbedingten Schmerzen – gibt es Unterschiede? Systematische Übersicht und Metaanalyse der Wirksamkeit, Verträglichkeit und Sicherheit in randomisierten Direktvergleichen von Opioiden über mindestens 4 Wochen. [Opioids in chronic noncancer pain–are opioids different? A systematic review and meta-analysis of efficacy, tolerability and safety in randomized head-to-head comparisons of opioids of at least four week’s duration]. Schmerz. 2015;29:73–84. English version available under dx.doi.org/10.1007/s00482-014-1432-4, supplementary material. | |
Mueller-Lissner S. Fixed combination of oxycodone with naloxone: a new way to prevent and treat opioid-induced constipation. Adv Ther. 2010;27:581–590. | |
Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol. 2011;106:835–842. | |
Clemens KE, Mikus G. Combined oral prolonged-release oxycodone and naloxone in opioid-induced bowel dysfunction: review of efficacy and safety data in the treatment of patients experiencing chronic pain. Expert Opin Pharmacother. 2010;11:297–310. | |
Blagden M, Hafer J, Duerr H, et al; Long-term evaluation of combined prolonged-release oxycodone and naloxone in patients with moderate-to-severe chronic pain: pooled analysis of extension phases of two phase III trials. Neurogastroenterol Motil. 2014;26:1792–1801. | |
Ahmedzai SH, Leppert W, Janecki M, et al; Long-term safety and efficacy of oxycodone/naloxone prolonged-release tablets in patients with moderate-to-severe chronic cancer pain. Support Care Cancer. 2015;23:823–830. | |
Ueberall MA, Mueller-Schwefe GHH. Safety and efficacy of oxycodone/naloxone vs oxycodone vs morphine in the treatment of chronic low back pain: results of a 12-week prospective, randomized, open-label blinded endpoint streamlined study with prolonged-release preparations. Curr Med Res Opin. 2015;31:1413–1429. | |
Ueberall MA, Mueller-Schwefe GHH. Development of opioid-induced constipation: post hoc analysis of data from a 12-week prospective, open-label, blinded-endpoint streamlined study in low-back pain patients treated with prolonged-release WHO step III opioids. J Pain Res. 2015;8:459–475. | |
The EuroQol Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. | |
Guy W, editor. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare; 1976. | |
German Pain Association. Der Deutsche Schmerzfragebogen. [German Pain Questionnaire]. Available from: http://www.dgss.org/deutscher-schmerzfragebogen/. Accessed November 22, 2014. | |
von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. | |
Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. | |
Gandek B, Ware JE, Aaronson NK, et al; Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA project. J Clin Epidemiol. 1998;51:1171–1178. | |
Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–1920. | |
Schmitt N, Gerbershagen HU. The Mainz Staging System (MPSS) for chronic pain. Pain. 1990;41(Suppl 5):S484 (abstract). | |
Rentz AM, Yu R, Müller-Lissner S, Leyendecker P. Validation of the bowel function index to detect clinically meaningful changes in opioid-induced constipation. J Med Econ. 2009;12:371–383. | |
Ueberall MA, Müller-Lissner S, Buschmann-Kramm C, Bosse B. The bowel function index for evaluating constipation in pain patients: definition of a reference range for a non-constipated population of pain patients. J Int Med Res. 2011;39:41–50. | |
Parker SL, Adogwa O, Mendenhall SK, et al; Determination of minimum clinically important difference (MCID) in pain, disability, and quality of life after revision fusion for symptomatic pseudoarthrosis. Spine J. 2012;12:1122–1128. | |
Langley PC. The societal burden of pain in Germany: health-related quality-of-life, health status and direct medical costs. J Med Econ. 2012;15:1–15. | |
Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105−S120. | |
Chaparro LE, Furlan AD, Deshpande A, Mailis-Gagnon A, Atlas S, Turk DC. Opioids compared to placebo or other treatments for chronic low-back pain. Cochrane Database Syst Rev. 2013;8:CD004959. | |
Petzke F, Welsch P, Klose P, Schaefert R, Sommer C, Häuser W. Opioide bei chronischem Kreuzschmerz - Systematische Übersicht und Metaanalyse der Wirksamkeit, Verträglichkeit und Sicherheit in randomisierten, placebokontrollierten Studien über mindestens 4 Wochen [Opioids in chronic low back pain. A systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration]. Schmerz. 2015;29:60–72. English version available under dx.doi.org/10.1007/s00482-014-1449-8, supplementary material. | |
Kosinski MR, Schein JR, Vallow SM, et al; An observational study of health-related quality of life and pain outcomes in chronic low back pain patients treated with fentanyl transdermal. Curr Med Res Opin. 2005;21:849–862. | |
Allan L, Richarz U, Simpson K, Slappendel R. Transdermal fentanyl versus sustained release oral morphine in strong-opioid naïve patients with chronic low back pain. Spine. 2005;30:2484–2490. | |
Wallace M, Thipphawong J. Open-label study on the long-term efficacy, safety, and impact on quality of life of OROS hydromorphone ER in patients with chronic low back pain. Pain Med. 2010;11:1477–1488. | |
Rychlik R, Viehmann K, Daniel D, Kiencke P, Kresimon J. Pain management and costs of a combination of oxycodone + naloxone in low back pain patients. In: Raez GB, Noe CE, editors. Pain Management–Current Issues and Opinions. Rijeka: InTech; 2012. Available from: http://www.intechopen.com/books/pain-management-current-issues-and-opinions. Accessed June 10, 2015. | |
Strick V. Management of severe chronic pain with tapentadol prolonged release – long-term data from pain specialists. Curr Med Res Opin. 2014;30:2085–2092. | |
Penning-van Beest FJ, van den Haak P, Klok RM, Prevoo YF, van der Peet DL, Herings RM. Quality of life in relation to constipation among opioid users. J Med Econ. 2010;13:129–135. | |
Schutter U, Grunert S, Meyer C, Schmidt T, Nolte T. Innovative pain therapy with a fixed combination of prolonged-release oxycodone/naloxone: a large observational study under conditions of daily practice. Curr Med Res Opin. 2010;26:1377–1387. | |
Morlion B, Clemens KE, Dunlop W. Quality of life and healthcare resource in patients receiving opioids for chronic pain: a review of the place of oxycodone/naloxone. Clin Drug Investig. 2015;35:1–11. | |
van Dongen VC, Vanelderen PJ, Koopmans-Klein G, van Megen YJ, Van Zundert J, Huygen FJ. Patient preference with respect to QoL and reduction in opioid-induced constipation (OIC) after treatment with prolonged-release (PR) oxycodone/naloxone compared with previous analgesic therapy [PREFER study]. Int J Clin Pract. 2014;68:1364–1375. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.