Back to Journals » Drug Design, Development and Therapy » Volume 8
Pustular drug eruption due to Panax notoginseng saponins
Authors Yin Z, Ma L, Xu J, Xia J, Luo D
Received 30 April 2014
Accepted for publication 17 May 2014
Published 16 July 2014 Volume 2014:8 Pages 957—961
DOI https://doi.org/10.2147/DDDT.S67015
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
ZhiQiang Yin,1,* LiWen Ma,1,* JiaLi Xu,2,* JiPing Xia,1 Dan Luo1
1Department of Dermatology, 2Department of Oncology, First Affiliated Hospital of Nanjing Medical University, Jiangsu, People's Republic of China
*These authors contributed equally to this work
Abstract: Panax notoginseng saponins (PNS) are a patented product in the People's Republic of China, and have extensive effects on the cardiovascular system. Here we report on four elderly patients (one male and three female) with drug eruption induced by PNS injection. All developed a sudden skin rash with pruritus from head to foot, and subsequently accepted hospitalization. In each case, PNS had been used for less than 1 week before appearance of the rash. No specific short-term medications or changes in diet or exposure to environmental factors immediately prior to appearance of the rash were identified. These four patients had some interesting features in common, ie, pustules, fever, and elevated circulating neutrophil counts, which required high-dose, long-term glucocorticoid therapy. To our knowledge, this is the first report of pustular drug eruption induced by PNS and provides a useful reference and warning for clinicians.
Keywords: pustule, drug eruption, acute generalized exanthematous pustulosis, Panax notoginseng saponins
Introduction
Panax notoginseng saponins (PNS) constitute the principal ingredient extracted from the traditional herb medicine, P. notoginseng (Burk.) FH Chen (Chinese Sanqi), and the major effective components are; notoginsenoside R1, ginsenoside Rg1, ginsenoside Rb1, ginsenoside Rd, and ginsenoside Re.1 PNS has extensive effects on the cardiovascular system, along with other pharmacological effects, including antifatigue, immunological, anticancer, antioxidant, hepatoprotective, and renoprotective functions.2 PNS preparations are used widely by the general public, especially in the People’s Republic of China. Traditional medicine is very much welcomed by the Chinese public, especially the elderly, who believe that traditional Chinese medicine is natural and has no adverse effects. However, in recent years, we have observed many drug eruptions and other adverse reactions induced by a variety of traditional Chinese herbs and patented drugs. These adverse drug reactions, drug eruption in particular, have also been observed with PNS, but have been largely ignored. Here we report on four patients who developed drug eruption due to injection of PNS.
Case 1
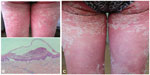
Case 1 was a 50-year-old woman who presented with a large area of erythema and hundreds of tiny nonfollicular pustules, and a 2-week history of itching and pain from head to foot (Figure 1A). She also had a fever of more than 38°C. On the day before the appearance of the skin rash, the patient had received her first injection of PNS (400 mg, intended for once-daily administration), and another injection of sulfotanshinone sodium (40 mg intended for once-daily administration, and used several times previously) as a treatment for dizziness. Histopathological examination of a biopsy from a pustule was consistent with a diagnosis of acute generalized exanthematous pustulosis (AGEP, Figure 1B). We treated this patient with once-daily intravenous infusion of methylprednisolone 80 mg, and the skin rash gradually improved. A single dose of intravenous methotrexate 7.5 mg was also administered. The patient’s condition improved further with gradually tapering doses of methylprednisolone (Figure 1C) during a hospital stay of 20 days.
Case 2
Case 2 (Figure 2A and B) was an 89-year-old man who presented with a 1-week history of generalized erythema, tiny pustules, and pruritus. He also had a fever of 37.6°C. One week before appearance of the rash, he had received his first injection of PNS (500 mg, intended for once-daily administration) and an injection of oxiracetam (4 g, intended for once-daily administration and received several times previously) for treatment of a cerebral infarct. The clinical diagnosis was AGEP; however, the patient refused a skin biopsy. We treated the patient with once-daily intravenous infusion of methylprednisolone 80 mg for 3 days. A new rash appeared on his face, so we changed the dosage of methylprednisolone to 40 mg twice daily for 4 days. The patient’s condition then came under control. During tapering of methylprednisolone, new rashes appeared on several occasions during his 23-day hospital stay. A single intravenous dose of methotrexate 7.5 mg was also administered.
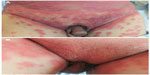  | Figure 2 (A) Erythema and lots of tiny pustules on the abdomen. (B) Many pustules on the basis of erythema on the lower abdomen. |
Case 3
Case 3 was a 62-year-old woman with a 5-day history of generalized erythema and papules with itching. She had a fever of 38.7°C. On the day before appearance of the skin rash, the patient had received her first injection of PNS (800 mg, intended for once-daily administration) and an injection of oxiracetam (6 g, intended for once-daily administration and received several times previously) for treatment of dizziness. This patient was treated with an intravenous infusion of methylprednisolone, 120 mg once daily. Four days later, many tiny pustules appeared on the trunk and lower limbs (Figure 3A) but disappeared on the same evening. We decreased the dose of methylprednisolone to 80 mg once daily on the following day because of her improving condition; however, the severe rash and pruritus returned, so we increased her methylprednisolone to 120 mg once daily. We subsequently tapered the methylprednisolone gradually. The hospital stay for this patient was 22 days.
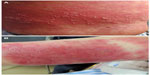  | Figure 3 (A) Lots of tiny pustules on the trunk. (B) Many tiny pustules and some scales on the upper limbs. |
Case 4
Case 4 was a 67-year-old woman with a 2-day history of generalized erythema and pruritus, who had a fever of 37.8°C. One week before appearance of the rash, the patient had received an injection of PNS (500 mg, intended for once-daily administration) for multiple lacunar infarction. This patient was started on an intravenous infusion of methylprednisolone 80 mg once daily. Three days later, many tiny pustules appeared on the upper limbs (Figure 3B), which disappeared on the following day. The patient’s condition gradually improved on a tapering regimen of methylprednisolone. Her hospital stay was 23 days.
All cases
Routine hematology showed a significantly increased neutrophil count in all four patients. Further tests did not identify any obvious common features in these patients. One patient had hepatic dysfunction. After effective treatment, all four patients were discharged from hospital, the rash disappeared, and the circulating neutrophil count and liver function returned to the normal range. Several follow-up visits did not reveal new rashes or other abnormalities.
Discussion
The PNS injections used by these patients were manufactured by different local manufacturers and were all produced as per the Good Manufacturing Practice standards. For each patient, the duration of continuous use of PNS before the appearance of the skin rash was less than 1 week. No other short-term special medication, diet, or exposure to environmental factors before the appearance of the rash was identified. Gallelli et al3 reported generalized urticaria in a young woman treated with clomipramine and after ingestion of codfish, indicating a probable causal relationship between the drug-food interaction and the skin rash. Our four patients were all elderly and paid close attention to their food intake. No specific dietary changes were associated with use of the drug and appearance of the skin rash, so a drug-food interaction was essentially excluded. Some of the patients had been using other long-term medications, such as antihypertensive and antidiabetic drugs for several years, which we thought were unrelated to the skin rash. For these reasons, we diagnosed the skin lesions in these patients as drug eruption caused by PNS injection. By using the World Health Organization’s Uppsala Monitoring Centre for standardized case causality assessment, the causality in our cases was not “certain”, but “probable/likely”, because all four patients refused rechallenge.4 Given the normal dose of PNS injected, the clinical manifestations of the rash, and effective treatment with methylprednisolone, the reaction was considered as a type of allergic drug eruption in all four patients. The informed consent of the patients was obtained.
These four patients with drug eruption, comprising one male and three females, all aged over 50 years, suddenly developed skin lesions with pruritus from head to foot, and then accepted hospitalization. After observation and treatment, we found that these patients had some interesting clinical features: a short incubation period (1–7 days, average 4 days); pustular skin lesions, AGEP, or transient local pustules; requirement for high-dose glucocorticoid therapy, comprising methylprednisolone given as an intravenous infusion of at least 80 mg once a day; long hospital stays (20–23 days, average 22 days); a significantly increased count and proportion of neutrophils in blood; and a good outcome. We also observed that new rashes continued to appear during tapering of methylprednisolone in two cases, so we believe that decrements in the glucocorticoid dose should be very slow during treatment and an immunosuppressant, such as methotrexate may be needed.
AGEP is a rare drug eruption presenting with acute extensive formation of nonfollicular sterile pustules on an erythematous and edematous base, and is typically accompanied by fever and leukocytosis. Only 18% of AGEP are not due to antibiotics.5 Schmid et al6 reported that drug-specific T-cells played an important role in the pathogenesis of AGEP, showing that secretion of interleukin-8 by T-cells and keratinocytes attracted neutrophils that filled the vesicles and transformed them into pustules. Whether the mechanism of this PNS-induced skin reaction is related to drug-specific T-cells and interleukin-8 needs further investigation. AGEP or pustular drug eruption can be induced by drugs other than antibiotics, including hydroxychloroquine,5,7 sorafenib,8,9 acetazolamide,10 gliclazide,11 recombinant interleukin-2,12 and ibuprofen.13 However, there are no reports in the literature on pustular drug eruption due to traditional Chinese medicine, including PNS.
The pustular skin rash induced by streptococcal infection should be distinguished from pustular drug eruption. Patrizi et al14 reported a case of diffuse acute sterile pustular eruption after streptococcal infection. The antistreptolysin titer was found to be elevated and routine hematology tests showed leukocytosis. The pustular eruption resolved after an 8-day course of antibiotic therapy. In the present cases, no elevated antistreptolysin titer were found, and high-dose glucocorticoid therapy was required.
To our knowledge, this is the first report of the clinical features of PNS-induced drug eruption. Although there have been only four cases, we have observed the following common characteristics: pustules, fever, an elevated circulating neutrophil count, need for high-dose glucocorticoid therapy, and a long treatment duration, which would provide a useful reference and warning for clinicians. The documentation of further cases of drug eruption due to PNS would be beneficial.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81171518, 81301387), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (JX10231801).
Disclosure
The authors report no conflicts of interest in this work.
References
Wang L, Li Z, Zhao X, et al. A network study of Chinese medicine xuesaitong injection to elucidate a complex mode of action with multicompound, multitarget, and multipathway. Evid Based Complement Alternat Med. 2013;2013:652373. | |
Liu R, Qin M, Hang P, Liu Y, Zhang Z, Liu G. Effects of Panax notoginseng saponins on the activities of CYP1A2, CYP2C9, CYP2D6 and CYP3A4 in rats in vivo. Phytother Res. 2012;26(8):1113–1118. | |
Gallelli L, De Fazio S, Corace E, De Sarro G, Garcia CS, De Fazio P. Generalised urticaria in a young woman treated with clomipramine and after ingestion of codfish. Pharmacopsychiatry. 2006; 39(4):154–156. | |
Zaki SA. Adverse drug reaction and causality assessment scales. Lung India. 2011;28(2):152–153. | |
Bailey K, McKee D, Wismer J, Shear N. Acute generalized exanthematous pustulosis induced by hydroxychloroquine: first case report in Canada and review of the literature. J Cutan Med Surg. 2013;17(6):414–418. | |
Schmid S, Kuechler PC, Britschgi M, et al. Acute generalized exanthematous pustulosis: role of cytotoxic T cells in pustule formation. Am J Pathol. 2002;161(6):2079–2086. | |
Lateef A, Tan KB, Lau TC. Acute generalized exanthematous pustulosis and toxic epidermal necrolysis induced by hydroxychloroquine. Clin Rheumatol. 2009;28(12):1449–1452. | |
Maki N, Komine M, Takatsuka Y, Maekawa T, Murata S, Ohtsuki M. Pustular eruption induced by sorafenib in a case of psoriasis vulgaris. J Dermatol. 2013;40(4):299–300. | |
Pretel M, Iñarrairaegui M, Lera JM, Aguado L, Idoate MA. Acute generalized exanthematous pustulosis induced by sorafenib. JAMA Dermatol. 2014;150(6):664–666. | |
Benamara-Levy M, Haccard F, Jonville Bera AP, Machet L. Acute generalized exanthematous pustulosis due to acetazolamide: negative on patch testing and confirmed by delayed-reading intradermal testing. Clin Exp Dermatol. 2014;39(2):220–222. | |
Contreras-Steyls M, Vílchez-Márquez F, Mota A, Moyano B, Herrera-Ceballos E. Acute generalized exanthematous pustulosis induced by gliclazide: a case report. Int J Dermatol. 2013;52(12):1591–1593. | |
Gunawardane ND, Vaghani SP, Kuzel TM, Cotliar JA. Acute generalized exanthematous pustulosis in a patient receiving high-dose recombinant interleukin-2. J Am Acad Dermatol. 2013;69(4):e183–e184. | |
Arochena L, Zafra MP, Fariña MC, Del Pozo V, Fernández-Nieto M. Acute generalized exanthematic pustulosis due to ibuprofen. Ann Allergy Asthma Immunol. 2013;110(5):386–387. | |
Patrizi A, Savoia F, Giacomini F, Neri I, Ricci G. Diffuse acute pustular eruption after streptococcal infection – a new instance of pustulosis acuta generalisata. Pediatr Dermatol. 2007;24(3):272–276. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.