Back to Journals » International Journal of General Medicine » Volume 13
Pump Proton and Laryngeal H+/K+ ATPases
Authors Zhang Z, Bao YY, Zhou SH
Received 1 October 2020
Accepted for publication 20 November 2020
Published 14 December 2020 Volume 2020:13 Pages 1509—1514
DOI https://doi.org/10.2147/IJGM.S284952
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Zhe Zhang,1 Yang-Yang Bao,2 Shui-Hong Zhou2
1Department of Otolaryngology, Peoples Hospital of Yuyao City, Yuyao 315400, Zhejiang, People’s Republic of China; 2Department of Otolaryngology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang 310003, People’s Republic of China
Correspondence: Shui-Hong Zhou
Department of Otolaryngology, The First Affiliated Hospital, College of Medicine, Zhejiang University, 79 Qingchun Road, Hangzhou City 310003, People’s Republic of China
Tel +86-13868060120
Fax +86-571-87236895
Email [email protected]
Purpose: The presence of extra-gastric H+/K+ ATPases may explain the clinically significant effect of proton pump inhibitor (PPI) pharmacotherapy in patients with chronic laryngitis related to laryngopharyngeal reflux disease (LPRD) but without gastroesophageal reflux disease (GERD) symptoms. Given the need for a better understanding of GERD and LPRD, we review the various proton pumps with respect to their classification, function, and distribution. We then consider the potential role of the laryngeal H+/K+ ATPase pump in LPRD.
Methods: We searched databases of PubMed, EMBASE, and Web of Science to achieve related published before September 15, 2020.
Results: There were only seven English-literatures meeting inclusive criteria about laryngeal H+/K+ ATPases. Some studies provide convincing evidence of a laryngeal H+/K+ ATPase in normal laryngeal tissues but also suggest the potential role of the proton pump in the abnormal mucus secretion frequently seen in patients with chronic laryngitis.
Conclusion: A laryngeal H+/K+ ATPase expresses in normal laryngeal tissues. These findings question the current understanding of GERD and LPRD.
Keywords: laryngopharyngeal reflux disease, gastroesophageal reflux disease, larynx, proton pump, H+/K+ ATPases, chronic laryngitis
Introduction
Laryngopharyngeal reflux disease (LPRD) comprises a group of symptoms and signs caused by reflux of the gastric contents into the upper esophageal sphincter and is therefore considered an extra-esophageal variant of gastroesophageal reflux disease (GERD).1,2 However, whether the true pathogenesis of LPRD involves reflux of the gastric contents is unclear. The different symptoms of GERD and LPRD suggest that they are, at least clinically, two different diseases. For example, LPRD does not always include gastric reflux symptoms such as heartburn and eructation, and some patients report only the sensation of a foreign body in the throat and frequent throat clearing.3 Therefore, LPRD is not caused solely by gastric reflux but may involve other factors as well.
Recent studies of the etiology of LPRD have focused on exogenous factors affecting the laryngopharynx. The detection of salivary pepsin has also been performed to diagnose LPRD1,3–6 but is not applicable for all cases of LPRD.4 Thus, the search continues for endogenous factors of the laryngopharynx that participate in the pathogenesis of LPRD. Nonetheless, the proton pump is central to both LPRD and GERD.1–3,7,8 Whereas the gastric H+/K+ ATPase is a well-characterized proton pump mediating gastric acid secretion,7–9 other H+/K+ ATPases may be present in organs outside the stomach, such as the larynx. The presence of extra-gastric H+/K+ ATPases may explain the clinically significant effect of proton pump inhibitor (PPI) pharmacotherapy in patients with chronic laryngitis related to LPRD but without GERD symptoms.10,11 Given the need for a better understanding of GERD and LPRD, here we review the classification, function, and distribution of the various proton pumps, focusing on the laryngeal H+/K+ ATPase.
A proton pump refers to a transmembrane integrated glycoprotein that transports hydrogen ions across the membrane against a concentration gradient. The energy fueling the proton pump and H+ efflux is provided by ATP hydrolysis.9 As a byproduct of the pump activity, a pH gradient forms on either side of the membrane. Four types of proton pumps with ATPase activity have been recognized: P-type,10–14 F-type,15–17 V-type,18–20 and ABC ATPases.18
Gastric Proton Pump H+/K+ ATPase and Its Functions
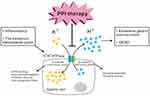
Gastric acid secretion is a physiological process common to all vertebrates. Its primary function is the digestion of food.9 H+ secretion is mediated by the gastric proton pump, H+/K+ ATPase, a member of the P2 ATPase family that also includes Ca2+ and Na+/K+ ATPases. The gastric H+/K+ ATPase mediates the transport of K+ ions in the extracellular fluid into the cell, through coupling of phosphorylation and dephosphorylation, while intracellular H+ ions are simultaneously pumped out of the cell against a 2.5-million-fold concentration gradient to complete ion transport and gastric acid secretion.9,21 H+/K+ ATPase activity is stimulated by inflammatory and other factors and by the bacterium Helicobacter pylori, leading to excessive gastric acid secretion and GERD.9,21 This is the mechanism targeted by PPI therapy.7,8,16 H+/K+ ATPases contain the catalytic α-subunit and non-catalytic glycosylation β-subunit. The α-subunit of the H+/K+ ATPases of different species is made up of 1035 amino acids and includes the catalytic center, which resides in the large cytoplasmic domain of the enzyme. ATP-binding, acyl-phosphorylation, inhibitor-binding, and ion-recognition sites are also included in the α-subunit.9,21 In addition to these functions, the α-subunit is important for the structure and function of H+/K+ ATPases, as it mediates ion transport and energy supply for transmembrane ion transport and confers stability to the holoenzyme. The 290-amino-acid β-subunit is responsible for enzyme assembly and plays a role in enzyme activity (Figure 1).9,21
Laryngeal H+/K+ ATPases
We searched databases of PubMed, EMBASE, and Web of Science to achieve related published before September 15, 2020. Search words included “laryngeal/larynx/head and neck” and “proton pump”; or “laryngeal/larynx/head and neck” and “H+/K+ ATPases”; or “laryngeal/larynx/head and neck” and “non-gastric H+/K+ ATPases”; or “non-gastric H+/K+ ATPases”;
There were only seven English-literatures meeting inclusive criteria about laryngeal H+/K+ ATPases. In 1995, H+/K+ ATPases were discovered outside the stomach, first in kidney tissues22 and subsequently in human lung mucus glands,23,24 rat and human kidney tissues,25–27 and rat rectum,28,29 uterus,29 heart,30 inner ear, vestibule,31–34 thymus,35,36 and prostate.37–41
In 2003, Altman et al used immunohistochemical staining to identify H+/K+ ATPase α- and β-subunits in the serous cells and ducts of the minor seromucinous glands of larynges from two human cadavers.42 In a follow-up study in 2005, those authors reported seromucinous glands in laryngeal surgical specimens without evidence of carcinoma and with an otherwise normal architecture on pathology. Of the 27 specimens obtained from 15 patients who underwent laryngeal surgery (11 total laryngectomies, 2 partial laryngectomies, 1 excision of a laryngeal mass, and 1 arytenoidectomy), the rates of positive staining of the α- and β-subunits were 96.3% (26/27) and 85.2% (23/27), respectively. The staining was observed mainly in the laryngeal seromucinous glands and ducts.43 In 2011, the same authors demonstrated that the immunohistochemical staining of α- and β-subunits in the larynx was consistent with the presence of a gastric proton pump (H+/K+ ATPase).43 Using immunohistochemical techniques and Western blotting, they also detected α- and β-subunits in three submandibular gland specimens, four normal laryngeal specimens (three benign and one invasive squamous cell carcinoma) without carcinoma and with an otherwise normal architecture confirmed by pathology, and three normal gastric specimens. Both the α- and β-subunits were identified in the submandibular gland ducts, together with high or strong expression in seromucinous glands and even stronger expression in seromucinous ducts. Although an anti-α-subunit antibody resulted in a somewhat stronger immunostaining intensity, the staining pattern was identical in location and distribution with those of both the H+/K+ ATPase α- and β-subunits.40 Western blotting confirmed the presence of the H+/K+ ATPase α- and β-subunits in the laryngeal mucosa and submandibular gland. It was therefore suggested that symptoms of chronic laryngitis are not solely the result of acid produced by parietal cells of the stomach, but that acid-producing cells in the larynx also play a role.44 In 2015, Stevanović et al evaluated expression of the β-subunit of the proton pump in 50 cadaver larynges and 11 larynges from laryngectomies performed on patients with laryngeal carcinoma not treated with perioperative chemoradiotherapy.45 They found no β-subunit expression in the cadaver larynges, and 36% (4/11) of the laryngectomy specimens showed weak positivity in the seromucinous glands located in the supraglottis. The β-subunit was also evaluated in the chondrocytes of all surgical specimens and in most cadaver larynges evaluated.53 In cadavers, the rates of β-subunit positivity in chondrocytes in the supraglottis, glottis, and subglottis were 74%, 60%, and 74%, respectively.45 However, the role of H+/K+ ATPases in laryngeal chondrocytes is unclear.45 In 2015, Becker et al detected H+/K+ ATPases in biopsied laryngeal tissues from patients with LPRD symptoms who underwent Dx-pH and pH/MII measurements. The tissues were also analyzed by real-time RT-PCR and immunohistochemical techniques.46 Among the 20 patients, 14 had pathological Dx-pH results and 6 pathological pH/MII results, with 4 patients having pathological results on both tests. In the one patient with positive H+/K+ ATPase α- and β-subunit expression, as detected by immunohistochemistry, and pathological results on both the Dx-pH and pH/MII tests, PPI treatment was effective. PPI treatment was also effective in another patient positive for α- and β-subunit expression, as detected by real-time RT-PCR, and with pathological pH/MII test results. The low rate of H+/K+ ATPase positivity in that study can be explained by the fact that the proton pump is located mainly in the laryngeal seromucinous glands rather than in the squamous epithelium, whereas the biopsies were performed only in the superficial tissues, under endoscopy.46 Consequently, the submucosal glands, expressing the H+/K+ ATPase proton pump, were probably missed.54 Recently, McCormick CA et al detected alpha and beta subunits (ATP4A and ATP4B) of H+/K+ ATPase proton pump in the larynx of LPR and laryngeal carcinoma patients. They found that the positive expression of ATP4A and ATP4B was in 3/3 LPR, 4/8 laryngeal carcinoma-tumor and 3/8 laryngeal carcinoma-adjacent specimens. Although its small sample size and absence of a reflux and laryngeal cancer-free control cohort, they suggested that acid secretion by functional H+/K+ ATPase proton pumps expressed in laryngeal mucosa may elicit laryngeal mucosa cells and molecular changes associated with inflammation and cancerogenesis.47 However, Herrmann et al detected significant expression of the H+/K+ ATPase α- and β-subunits in the stomach among different human tissues evaluated, including the larynx.48
The above-cited studies provide convincing evidence of a laryngeal H+/K+ ATPase42–47 in normal laryngeal tissues but also suggest the potential role of the proton pump in the abnormal mucus secretion frequently seen in patients with chronic laryngitis. The discovery of a laryngeal proton pump may explain the pathologically acidic environment of the oropharynx in patients without gastroesophageal reflux, with profound implications for PPI therapy, as the larynx is one of the extragastric targets of these drugs.42–46 The discovery of a laryngeal H+/K+ ATPase may also explain some of the clinical controversies regarding LPRD or GERD,42–46 such as the inconsistent pH measurement in patients with significant LPRD symptoms,49 abnormal laryngeal seromucinous secretion, and reduced MUC5AC gene expression seen in patients with chronic laryngitis associated with LPRD,50 presence of pachydermia as a nonspecific finding associated with LPRD,4 efficacy of a placebo on LPRD,51 and high rate of failed laparoscopic Nissen fundoplication in patients with symptoms of LPRD.52
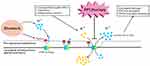
The function of the laryngeal H+/K+ ATPase proton pump is unclear. However, Altman et al suggested that H+/K+ ATPases are transmembrane enzymes mainly responsible for the transport of ions, similar to V-type ATPases.42–44 A similarity of the laryngeal proton pump H+/K+ ATPase to the V-type ATPase in rodent seromucinous glands has also been reported.42–45 These findings may be of clinical significance, given the indispensable role of the seromucinous glands in the normal and pathological functions of the larynx. For example, in some pathophysiological conditions, especially those associated with acidic environments, proton pumps in the laryngeal seromucinous glands and ducts may be responsible for regulating seromucinous secretion.42–45 These conditions include (1) direct acid reflux in LPRD, (2) first-stage reflux, when reactivation during subsequent acid exposure leads to pepsin binding and thus to tissue autodigestion, which together with cellular necrosis regulates the pH of the interstitium and may lead to laryngopharyngeal reflux-induced acid exposure. These findings imply that the laryngeal H+/K+ ATPase is activated by laryngopharyngeal reflux (similar to esophageal Na+/H+ exchangers) and other infectious or inflammatory stimuli (similar to tracheal epithelial proton secretion) to maintain the intracellular pH and cell viability.53,54 Thus, the symptoms of chronic laryngitis may not be caused solely by the acid produced by gastric parietal cells but rather also that produced by laryngeal seromucinous glands and ducts positive for H+/K+ ATPase. The larynx is susceptible to an altered pH. Thus, we suggest that the laryngeal H+/K+ ATPase secretes low levels of acid, similar to acid release in the stomach. This low level of acid in the larynx may induce laryngeal damage and, subsequently, chronic laryngitis or other laryngeal diseases. It may also explain why patients without apparent LPRD are sometimes responsive to PPI pharmacotherapy (Figure 2).
Conclusions and Expectations
The detection of a H+/K+ ATPase proton pump in laryngeal nononcological patients suggests a mechanism for LPRD attributable to the larynx alone. However, the function of the laryngeal H+/K+ ATPase remains unclear. Further studies are needed to determine whether its activities include acid secretion, and whether there is a correlation between laryngeal H+/K+ ATPase and laryngeal chronic laryngitis. The PPI binding site in laryngeal H+/K+ ATPases also remains to be identified.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lechien JR, Saussez S, Karkos PD. Laryngopharyngeal reflux disease: clinical presentation, diagnosis and therapeutic challenges in 2018. Curr Opin Otolaryngol Head Neck Surg. 2018;26(6):392–402. doi:10.1097/MOO.0000000000000486
2. Wong MW, Bair MJ, Chang WC, et al. Clinical and psychological characteristics in gastroesophageal reflux disease patients overlapping with laryngopharyngeal reflux symptoms. J Gastroenterol Hepatol. 2019;34(10):1720–1726. doi:10.1111/jgh.14651
3. Lechien JR, Akst LM, Hamdan AL, et al. Evaluation and management of laryngopharyngeal reflux disease: state of the art review. Otolaryngol Head Neck Surg. 2019;160(5):762–782. doi:10.1177/0194599819827488
4. Tan JJ, Wang L, Mo TT, et al. Pepsin promotes IL-8 signaling-induced epithelial-mesenchymal transition in laryngeal carcinoma. Cancer Cell Int. 2019;19(1):64. doi:10.1186/s12935-019-0772-7
5. Sasaki CT, Toman J, Vageli D, Sethi G. The in vitro effect of acidic-pepsin on nuclear factor kappa B activation and its related oncogenic effect on normal human hypopharyngeal cells. PLoS One. 2016;11(12):e0168269. doi:10.1371/journal.pone.0168269
6. Sereg-Bahar M, Jerin A, Hocevar-Boltezar I. Higher levels of total pepsin and bile acids in the saliva as a possible risk factor for early laryngeal cancer. Radiol Oncol. 2015;49(1):59–64. doi:10.2478/raon-2014-0020
7. Diaz D, Clarke RJ. Evolutionary analysis of the lysine-rich N-terminal cytoplasmic domains of the gastric H(+), K(+)-ATPase and the Na(+), K(+)-ATPase. J Membr Biol. 2018;251(5–6):653–666. doi:10.1007/s00232-018-0043-x
8. Miyazaki Y, Ichimura A, Sato S, et al. The natural flavonoid myricetin inhibits gastric H(+), K(+)-ATPase. Eur J Pharmacol. 2018;820:217–221. doi:10.1016/j.ejphar.2017.12.042
9. Sakai H, Fujii T, Takeguchi N. Proton-potassium (H(+)/K(+)) ATPases: properties and roles in health and diseases. Met Ions Life Sci. 2016;16:459–483.
10. Palmgren MG, Nissen P. P-type ATPases. Annu Rev Biophys. 2011;40(1):243–266. doi:10.1146/annurev.biophys.093008.131331
11. Dyla M, Terry DS, Kjaergaard M, et al. Dynamics of P-type ATPase transport revealed by single-molecule FRET. Nature. 2017;551(7680):346–351. doi:10.1038/nature24296
12. Grønberg C, Sitsel O, Lindahl E, et al. Membrane anchoring and ion-entry dynamics in P-type ATPase copper transport. Biophys J. 2016;111(11):2417–2429. doi:10.1016/j.bpj.2016.10.020
13. Lekeux G, Crowet JM, Nouet C, et al. Homology modeling and in vivo functional characterization of the zinc permeation pathway in a heavy metal P-type ATPase. J Exp Bot. 2019;70(1):329–341. doi:10.1093/jxb/ery353
14. Gibbons A, Bell L, Udawela M, et al. mRNA expression of the P5 ATPase ATP13A4 is increased in Broca’s area from subjects with schizophrenia. World J Biol Psychiatry. 2020;21(5):402–408. doi:10.1080/15622975.2018.1548781
15. Kühlbrandt W. Structure and mechanisms of F-type ATP synthases. Annu Rev Biochem. 2019;88(1):515–549. doi:10.1146/annurev-biochem-013118-110903
16. Akanuma G, Tagana T, Sawada M, et al. C-terminal regulatory domain of the ε subunit of F(o) F(1) ATP synthase enhances the ATP-dependent H(+) pumping that is involved in the maintenance of cellular membrane potential in Bacillus subtilis. Microbiologyopen. 2019;e815.
17. Blum TB, Hahn A, Meier T, et al. Dimers of mitochondrial ATP synthase induce membrane curvature and self-assemble into rows. Proc Natl Acad Sci USA. 2019;116(10):4250–4255. doi:10.1073/pnas.1816556116
18. Pedersen PL. Transport ATPases into the year 2008: a brief overview related to types, structures, functions and roles in health and disease. J Bioenerg Biomembr. 2007;39(5–6):349–355. doi:10.1007/s10863-007-9123-9
19. Beyenbach KW, Wieczorek H. The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J Exp Biol. 2006;209(4):577–589. doi:10.1242/jeb.02014
20. Muench SP, van der Laan M. No longer hidden secrets of proton pumping: the resolution revolution enlightens V-ATPases. Mol Cell. 2018;69(6):921–922. doi:10.1016/j.molcel.2018.02.031
21. Abe K, Irie K, Nakanishi H, et al. Crystal structures of the gastric proton pump. Nature. 2018;556(7700):214–218. doi:10.1038/s41586-018-0003-8
22. Ahn KY, Kone BC. Expression and cellular localization of mRNA encoding the “gastric” isoform of the H -K-ATPase in rat kidney. Am J Physiol. 1995;268:F99–109.
23. Shah VS, Meyerholz DK, Tang XX, et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351(6272):503–507. doi:10.1126/science.aad5589
24. Tamura S, Oshiman K, Nishi T, et al. Sequence motif in control regions of the H+/K+ ATPase α and β subunit genes recognized by gastric specific nuclear protein(s). FEBS Lett. 1992;298(2–3):137–141. doi:10.1016/0014-5793(92)80040-N
25. Crambert G. H-K-ATPase type 2: relevance for renal physiology and beyond. Am J Physiol Renal Physiol. 2014;306(7):F693–700. doi:10.1152/ajprenal.00605.2013
26. Salhi A, Lamouroux C, Pestov NB, et al. A link between fertility and K+ homeostasis: role of the renal H,K-ATPase type 2. Pflugers Arch. 2013;465(8):1149–1158. doi:10.1007/s00424-013-1252-x
27. Codina J, Opyd TS, Powell ZB, et al. pH-dependent regulation of the α-subunit of H+-K+ -ATPase (HKα 2). Am J Physiol Renal Physiol. 2011;301(3):F536–543. doi:10.1152/ajprenal.00220.2011
28. Codina J, Kone BC, Delmas-Mata JT, et al. Functional expression of the colonic H+,K+ -ATPase α-subunit. J Biol Chem. 1996;271(47):29759. doi:10.1074/jbc.271.47.29759
29. Crowson MS, Shull GE. Isolation and characterization of a cDNA encoding the putative distal colon H+,K(+)-ATPase. Similarity of deduced amino acid sequence to gastric H+,K(+)-ATPase and Na+,K(+)-ATPase and mRNA expression in distal colon, kidney, and uterus. J Biol Chem. 1992;267:13740–13748.
30. Beisvag V, Falck G, Loennechen JP, et al. Identification and regulation of the gastric H+/K+ -ATPase in the rat heart. Acta Physiol Scand. 2003;179(3):251–262. doi:10.1046/j.0001-6772.2003.01191.x
31. Takumida M, Takumida H, Anniko M. Gastric-type H+,K+ -ATPase in mouse vestibular end organs. Acta Otolaryngol. 2017;137(5):455–459. doi:10.1080/00016489.2016.1245865
32. Miyazaki H, Wangemann P, Marcus DC. The gastric H,K-ATPase in stria vascularis contributes to pH regulation of cochlear endolymph but not to K secretion. BMC Physiol. 2016;17(1):1. doi:10.1186/s12899-016-0024-1
33. Mori N, Miyashita T, Inamoto R, et al. Ion transport its regulation in the endolymphatic sac: suggestions for clinical aspects of Meniere’s disease. Eur Arch Otorhinolaryngol. 2017;274(4):1813–1820. doi:10.1007/s00405-016-4362-1
34. Shibata T, Hibino H, Doi K, et al. Gastric type H+,K+ -ATPase in the cochlear lateral wall is critically involved in formation of the endocochlear potential. Am J Physiol Cell Physiol. 2006;291(5):C1038–1048. doi:10.1152/ajpcell.00266.2006
35. Cantó E, Vidal S, Rodríguez-Sánchez JL. HK-ATPase expression in the susceptible BALB/c and the resistant DBA/2 strains of mice to autoimmune gastritis. Autoimmunity. 2003;36(5):275–283. doi:10.1080/0891693031000152679
36. Alderuccio F, Gleeson PA, Berzins SP, et al. Expression of the gastric H/K-ATPase alpha-subunit in the thymus may explain the dominant role of the beta-subunit in the pathogenesis of autoimmune gastritis. Autoimmunity. 1997;25(3):167–175. doi:10.3109/08916939709008023
37. Streif D, Iglseder E, Hauser-Kronberger C, et al. Expression of the non-gastric H+/K+ ATPase ATP12A in normal and pathological human prostate tissue. Cell Physiol Biochem. 2011;28(6):1287–1294. doi:10.1159/000335860
38. Pestov NB, Korneenko TV, Shakhparonov MI, et al. Loss of acidification of anterior prostate fluids in Atp12a-null mutant mice indicates that nongastric H-K-ATPase functions as proton pump in vivo. Am J Physiol Cell Physiol. 2006;291(2):C366–374. doi:10.1152/ajpcell.00042.2006
39. Pestov NB, Korneenko TV, Radkov R, et al. Identification of the beta-subunit for nongastric H-K-ATPase in rat anterior prostate. Am J Physiol Cell Physiol. 2004;286(6):C1229–1237. doi:10.1152/ajpcell.00393.2003
40. Modyanov N, Pestov N, Adams G, et al. Nongastric H,K-ATPase: structure and functional properties. Ann NY Acad Sci. 2003;986:183–187.
41. Pestov NB, Korneenko TV, Adams G, et al. Nongastric H-K-ATPase in rodent prostate: lobe-specific expression and apical localization. Am J Physiol Cell Physiol. 2002;282(4):C907–916. doi:10.1152/ajpcell.00258.2001
42. Altman KW, Haines GK
43. Altman KW, Waltonen JD, Hammer ND, et al. Proton pump (H+/K+-ATPase) expression in human laryngeal seromucinous glands. Otolaryngol Head Neck Surg. 2005;133(5):718–724. doi:10.1016/j.otohns.2005.07.036
44. Altman KW, Kinoshita Y, Tan M, et al. Western blot confirmation of the H+/K+ -ATPase proton pump in the human larynx and submandibular gland. Otolaryngol Head Neck Surg. 2011;145(5):783–788. doi:10.1177/0194599811415589
45. Stevanović S, Radić R, Kačarević ŽP, et al. Proton pump (H+/K+-ATPase) expression in human larynx. Auris Nasus Larynx. 2015;42(6):458–462. doi:10.1016/j.anl.2015.04.013
46. Becker V, Drabner R, Graf S, et al. New aspects in the pathomechanism and diagnosis of the laryngopharyngeal reflux-clinical impact of laryngeal proton pumps and pharyngeal pH metry in extraesophageal gastroesophageal reflux disease. World J Gastroenterol. 2015;21(3):982–987. doi:10.3748/wjg.v21.i3.982
47. McCormick CA, Samuels TL, Battle MA, et al. H+/K+ATPase expression in the larynx of laryngopharyngeal reflux and laryngeal cancer patients. Laryngoscope. 2020. doi:10.1002/lary.28643
48. Herrmann M, Selige J, Raffael S, et al. Systematic expression profiling of the gastric H+/K+ ATPase in human tissue. Scand J Gastroenterol. 2007;42(11):1275–1288. doi:10.1080/00365520701405579
49. Noordzij JP, Khidr A, Desper E, et al. Correlation of pH probe-measured laryngopharyngeal reflux with symptoms and signs of reflux laryngitis. Laryngoscope. 2002;112(12):2192–2195. doi:10.1097/00005537-200212000-00013
50. Johnston N, Bulmer D, Gill GA, et al. Cell biology of laryngeal epithelial defenses in health and disease: further studies. Ann Otol Rhinol Laryngol. 2003;112(6):481–491. doi:10.1177/000348940311200601
51. Steward DL, Wilson KM, Kelly DH, et al. Proton pump inhibitor therapy for chronic laryngo-pharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg. 2004;131(4):342–350. doi:10.1016/j.otohns.2004.03.037
52. Spechler SJ, Lee E, Ahnen D, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease. Follow-up of a randomized controlled trial. JAMA. 2001;285(18):2331–2338. doi:10.1001/jama.285.18.2331
53. Roussa E, Thévenod F, Sabolic I, et al. Immunolocalization of vacuolar-type H+-ATPase in rat submandibular gland and adaptive changes induced by acid-base disturbances. J Histochem Cytochem. 1998;46:91–100.
54. Layden TJ, Agnone LM, Schmidt LN, et al. Rabbit esophageal cells possess an Na+,H+ antiport. Gastroenterology. 1990;99(4):909–917. doi:10.1016/0016-5085(90)90606-2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.